Antioxidant, antimicrobial, cytotoxic and analgesic activities of ethanolic extract of Mentha arvensis L.
Nripendra Nath Biswas, Subarna Saha, Mohammed Khadem Ali
1School of Chemistry, University of New South Wales, NSW-2052, Sydney, Australia
2Pharmacy Discipline, Life science school, Khulna University, Khulna-9208, Bangladesh
3Department of Biotechnology and Genetic Engineering, Life Science School, Khulna University, Khulna-9208, Bangladesh
Antioxidant, antimicrobial, cytotoxic and analgesic activities of ethanolic extract of Mentha arvensis L.
Nripendra Nath Biswas1,2, Subarna Saha2, Mohammed Khadem Ali3*
1School of Chemistry, University of New South Wales, NSW-2052, Sydney, Australia
2Pharmacy Discipline, Life science school, Khulna University, Khulna-9208, Bangladesh
3Department of Biotechnology and Genetic Engineering, Life Science School, Khulna University, Khulna-9208, Bangladesh
PEER REVIEW
Peer reviewer
Md. Mofizur Rahman, Assistant Professor, Department of Pharmacy, Bangladesh University, Bangladesh.
Tel: +88-01911605139
E-mail: rmfi02@yahoo.com
Comments
This is a valuable research work in which authors have made the study interesting by evaluating the antioxidative, analgesic, cytotoxic and antibacterial effects of M. arvensis L. extract in association with phytochemical screening. Materials and methods are well planned. Findings are attention-grabbing and the discussion section contains scientifically interpretation.
Details on Page 786
Objective:To investigate potential antioxidant, antimicrobial, cytotoxic and analgesic activities of ethanolic extract of Mentha arvensis L. in different in vivo and in vitro experimental models.
Antioxidant, Antibacterial, Cytotoxic, Analgesic, Mentha arvensis L.
1. Introduction
Mentha arvensis(M. arvensis) belonging to the family of Lamiaceae, is a small to moderate sized perennial herb, commonly known as pudina, corn mint or wild mint in Bangladesh and India. It is widely cultivated in Bangladesh, Nepal, India, Srilanka, Thailand, and Japan for its use as a food seasoner, household remedy, and industrial purposes. The plant has been reported to possess a large number of different chemicals like α-menthol, neomenthol, menthofuran, d-menthone, isomenthol, isomnethone, menthylacetate, cineol, phellandrene, p-cymene, aromadendrene, limonine, piperitone, carvomenthone, pinene, carvacrol, α-pinene, α-phellandrene, dipentene, cadinene, thujone, menthofuran, carvone, linalool, linalyl acetate and piperitenone oxide which are usedin pharmaceutical, food, flavour, cosmetics, beverages and allied industries[1-4]. The plant leaf and oil contain acetaldehyde, amyl alcohol, methyl esters, limonene, β-pinene, β-phellandrene, cadinene, dimethyl sulphide, and traces of α-pinene, sabinene, terpinolene,transocimene, g-terpinene, fenchene, α-thujone, β-thujone, citronellol and luteolin-7-O-rutinoside[5]. It also possesses the flavonoids like quercetin, menthoside, and isorhoifolin, vitamin K, eugenol and thymol[2]. According to several reports the plant contains 90% mint oil. It contains monoterpenes (menthone, menthofuran, methyl acetate cineole and limonene), sesquiterpenes (viridiflorol), flavonoids (luteolin, menthoside, isorhoifolin, rutin hesperidin), phenolic acids (caffeic acid, chlorogenic and rosmarinic), triterpenes (squalene, a-amyrin, urosolic acid and sitosterol), phytol, tocopherols, carotenoids, choline, betaine, cyclenes, rosmarinic acid, tannin and minerals[6-8]. More recently, linarin (acacetin-7-O-β-D-rutinoside) was extracted from the flower of the plant[6].
M. arvensisL. is used as a carminative, antispasmodic, anti-peptic ulcer agent, and has been given to treat indigestion, skin diseases, coughs and colds in folk medicine. Different parts of the plant have been reported to possess diverse medicinal properties. The leaves are stimulant, acrid, thermogenic, antispasmodic, antihelmenthic, anodyne, vulnerary, deodorant, sudorific, dentrific, febrifuge, contraceptive, carminative, digestive, expectorant, cardiotonic, diuretic and hepatalgia[7]. It is also used for the treatment of liver and spleen disease, jaundice and asthma. The infusion of leaves is used to treat indigestion, rheumatism, infantile troubles, vomiting in pregnancy, hysteria and as remedy for inflamed joints[9]. The plant is used in small amount in the mixtures of lotions, ointments and creams to treat skin disorders. It also acts as an antipruritic, a counterirritant, an antiseptic, a stimulant and an anaesthetic in treating dermatological cases. The entire plant, apart from the root, is used to treat coryza, fever, headache, rhinitis, cough, pharyngitis, arthralgia, neuralgia, abdominal colic, nausea, vomiting, dyspepsia, diarrhea and prurigo. It is also claimed to be an emmenagogue[7]. The dried plant is used as an antiseptic, carminative, stomachic, refringent, stimulant, emmenagogue and diuretic[7]. The aerial part is used in Chinese medicine as a cooling remedy for colds, influenza, headache, sore throat and eye inflammation. It is also used as a liver stimulant[7].
Recent investigations have confirmed that the plant extract possesses hepatoprotective, anti-oxidant, anti-allergic, anti-inflammatory, sedative-hypotonic and antimicrobial effect[7,8-10]. The traditional uses claim thatM. arvensisL. is a potential folk medicine but very few phytochemical and biological works have been conducted on this plant. This issue is particularly crucial for medicinal interest and, to the authors’ knowledge, has not been resolved thus far. The present experimental study was carried out to evaluate the pharmacological basis for the use of the plant in folk medicine by using established scientific method.
2. Materials and methods
2.1. Plant material
The plantM. arvensisL. was collected from Badargonj Upazilla of Rangpur district, Bangladesh during the month of January 2011 at day time. The plants were mounted on paper and the sample was identified by the experts of Bangladesh National Herbarium, Mirpur, Dhaka (DACB Accession Number-3678).
2.2. Extraction preparation
The collected plants were washed by fresh water, cut into small pieces, and shed dried for two weeks. The dried plant material was grounded into fine powdered form. The plant extract was collected by cold extraction method by taking 200 mg powders in 700 mL ethanol in a glass container for 14 d. The extract was separated from the plant debris by filtration using Whatman filter paper. The extract was concentrated in evaporation (initially by open air and finally by water bath) process. The amount of yield in the extract was 8.77%.
2.3. Animals
Swiss albino mice of either sex (20-29 g body weight) were collected from animal resources. Mice of random sex (Swiss-webstar strain, 19-40 g body weight) were collected from animal resources branch of the International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR, B) and were used for the experiments. The animals were kept at animal house (Pharmacy Discipline, Khulna University, Khulna) for adaptation after collection under standard laboratory conditions (relative humidity 55%-65%, room temperature (25±2) °C and 12 h light: dark cycle) and fed with standard diets (ICDDR, B formulated) for period of 14 d prior to performing the experiments.
2.4. Chemicals
Diclofenac sodium was collected from Square Pharmaceuticals Ltd., Bangladesh. Glacial acetic acid and ascorbic acid were purchased from Sigma Chemicals, USA. All other chemicals were of analytical grade.
2.5. Phytochemical group test
The preliminary phytochemical group test was carried out by following standard procedure[1]. The extract was screened for the presence of alkaloids, reducing sugar, flavonoids,gums, and tannins.
2.6. Determination of in vitro antioxidant activity (DPPH free radical scavenging activity)
2.6.1. Qualitative assay
Antioxidant activity was determined on the basis of their scavenging activity of the stable DPPH free radical[11]. Commercially available thin layer chromatography TLC plate was used. A suitably diluted stock solutions were spotted on pre-coated silica gel TLC plates and the plates were developed in solvent systems of different polarities (polar, medium polar and non-polar) to resolve polar and non-polar components of the extract. The plates were dried at room temperature and were sprayed with 0.02% DPPH in ethanol. Bleaching of DPPH by the resolved bands was observed for 10 min and the color changes (yellow on purple background) were noted. DPPH forms deep pink color when it is dissolved in ethanol. When it is sprayed on the chromatogram of the extract, it forms pale yellow or yellow color which indicates the presence of antioxidants.
2.6.2. Quantitative assay
Stock solution of the extract was prepared in ethanol, from which serial dilutions were carried out to obtain concentration of 1, 8, 16, 32, 64, 128, 256 µg/mL. Then 2 mL from each diluted solution was added to 2 mL of a 0.004% ethanolic solution of DPPH, mixed and allowed to stand for 30 min for reaction to occur. The absorbance was determined at 517 nm for each concentration, and from this values corresponding percentage of inhibition were calculated. IC50value was determined from % inhibitionv.s.log concentration graph. Ascorbic acid was used as positive control. The formula used for % inhibition ratio was as follows:

2.7. Determination of antimicrobial activity by the disc diffusion method
Antimicrobial activity of the ethanolic extract ofM. arvensisL. was determined by disc diffusion method[12]. Nutrient agar and Muellar-Hinton agar were sterilized in a flask and cooled to 45-50 °C and then taken in sterilized Petri dishes with a diameter of 120 mm. The filter paper discs (6 mm in diameter) were impregnated with the crude extract at the concentration of 250 and 500 µg/disc and then placed onto the agar plates previously inoculated with the tested microorganisms. The tested microorganisms includedSalmonella typhi, Salmonella paratyphi, Shigella boydii, Escherichia coli, Shigella flexneri, Streptococcus pyogenus,andStreptococcus aureus.The Petri dishes were kept at 4 °C for 2 h. The plates were incubated at 37 °C for 16 h to allow the growth of the microorganisms. The diameters of the zones of inhibition were measured in millimeters using a calibrated scale. All the tests were repeated triplicate. Kanamycin was used as standards at the dose of 30 µg/disc.
2.8. Determination of cytotoxic activity by brine shrimp lethality bioassay
In vitrolethality bioassay of the ethanolic extract ofM. arvensisL. was exploited to detect cytotoxicity activity following the method described by Meyeret al[13]. A total of 38 g of sea salt was weighed accurately, dissolved in distilled water to make one liter and then filtered off to get a clear solution. Sea water was taken in the small tank and brine shrimp eggs were added and incubated at 28 °C in front of a lamp. The shrimps were allowed for 24 h to hatch and mature as nauplii (larvae). A solution of 5 µg/µL of the extract was prepared by using dimethyl sulfoxide (DMSO). For this purpose 24 clean test tubes were taken, 12 of which were for the samples in six concentrations (2 test tubes for each concentration) and 12 for control test. Then 5 mL of sea water was given to each of the test tubes. Then with the help of the micropipette specific volumes (10, 20, 40, 80, 160 and 320 µL) of samples were transferred from the stock solutions to the test tubes to get final sample concentrations of 5, 10, 20, 40, 80, and 160 µg/mL respectively. The concentration of DMSO in these test tubes did not exceed 10 µL/mL. For controlling, same volumes of DMSO (as in the sample test tubes) were taken in the rest of the 12 test tubes. Finally, with the help of a Pasteur pipette 10 living shrimps were kept to each of the test tubes. After 24 h the test tubes were observed and the number of survived nauplii in each test tube was counted and the results were noted. From this, the percentage of lethality of brine shrimp nauplii was calculated at each concentration for each sample. Then percent mortality was plotted against log concentration on the graph paper to produce an approximate linear correlation between them graphically.
2.9. Determination of analgesic activity by acetic acid induced writhing method
The analgesic activity of the sample was studied using acetic acid induced writhing model in mice[14]. The experimental animals were randomly divided into four groups: control, positive control, test group I and test group II consisting of five mice in each group. Control group received 1% (v/v) Tween-80 in water at the dose of 10 mL/ kg of body weight. In the animals of positive control group standard drug diclofenac sodium was used at the dose of 25 mg/kg of body weight. Test group I and group II were treated with the sample at a dose of 250 and 500 mg/kg body weight respectively. Control vehicle, standard drug and extracts were administered orally, 30 min prior to the intraperitoneal injection of 0.7% acetic acid. An interval of 5 min was given for absorption of acetic acid and the number of writhing was counted for 15 min. The incomplete writhing was takenas half-writhing, so two half-writhing were taken as full writhing. The number of writhing in the control was taken as 100% and percent inhibition was calculated as follow:

2.10. Statistical analysis
The results were expressed as the mean±SEM. The study was analyzed by using student’st-test. The data were presented as mean±SEM (n=5). Results were analysed by One-way analysis of variance (ANOVA) followed by Dunnett’st-test for multiple comparisons. For the comparison between two groups, student’st-test was employed. The significant difference was considered atP<0.05.
3. Results
3.1. Phytochemical group test
Phytochemical analysis of ethanolic extract ofM. arvensisL. indicated the presence of alkaloids, gum, flavonoids, tannin, glycosides, and reducing sugar.
3.2. Determination of in vitro antioxidant activity (DPPHfree radical scavenging activity)
In the TLC-based qualitative antioxidant assay using DPPH assay, extract showed free radical scavenging properties indicated by the presence of yellow color on a purple background on the TLC plate (data not shown). However, in the quantitative assay, the extract demonstrated free radical scavenging activity in the DPPH assay (IC50=41 µg/ mL approximately) which is comparable to that of ascorbic acid (IC50=19 µg/mL approximately), a well known standard antioxidant (Figure 1).

Figure 1. DPPH scavenging properties of M. arvensis extract (% inhibition v.s. log concentration).
3.3. Determination of antimicrobial activity by the disc diffusion method
The extract showed significant zone of inhibition at 14 mm and 27 mm with Gram negativeSalmonella typhiat the dose of 250 µg/disc and 500 µg/disc respectively, whereas the standard drug kanamycin showed zone of inhibition at 32 mm at the dose of 30 µg/disc. In case of the bacteriaSalmonella paratyphiandShigella boydiithe extract produced zone of inhibition 17 mm (250 µg/disc), 34 mm (500 µg/disc) and 8 mm (250 µg/disc), 15 mm (500 µg/disc) respectively, whereas the standard drug kanamycin showed zone of inhibition 23 mm and 28 mm respectively at the dose of 30 µg/disc. But in case of bacteriaEscherichia coliandShigella flexnerithe extract produced no zone of inhibition, whereas the standard drug kanamycin showed zone of inhibition 10 mm and 30 mm respectively at the dose of 30 µg/disc. Similarly with the Gram positive bacterial strain, zone of inhibition produced by the extract were 10 mm and 18 mm forStreptococcus pyogenusat the dose of 250 µg/ disc and 500 µg/disc respectively, and 8 mm and 17 mm forStreptococcus aureusat the dose of 250 µg/disc and 500 µg/ disc respectively, whereas the standard drug kanamycin showed zone of inhibition 20 mm and 22 mm respectively at the dose of 30 µg/disc.
3.4. Determination of cytotoxic activity by brine shrimp lethality bioassay
The mortality rate of brine shrimp was found to be increased with increasing concentration of the sample and plot of percent mortality versus log concentration on the graph paper produced an approximate linear correlation between them. The concentrations at which 50% mortality (LC50) of brine shrimp nauplii occurred were found to be 40 µg/mL for the crude extract (Figure 2). The 90% mortality (LC90) values were 160 µg/mL (Figure 2). It is clearly showed that the concentrations at which LC50and LC9of brine shrimp nauplii occurred were obtained by extrapolation.
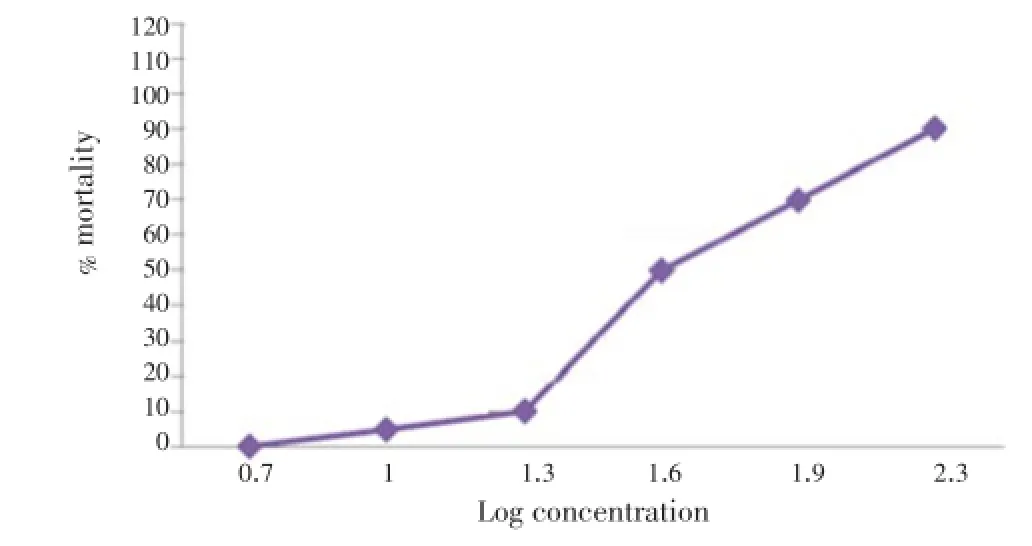
Figure 2. Cytotoxic effects of ethanolic extract of Mentha arvensis L. LC50=40 µg/mL; LC90=160 µg/mL.
3.5. Determination of analgesic activity by acetic acid induced writhing method
The extract produced 45.61% and 63.85% writhing inhibition in mice at oral doses of 250 mg/kg and 500 mg/kg body weights of mice respectively while the standard drug diclofenac sodium exhibited inhibition of 76.69% at a dose of 25 mg/kg body weight (Table 1).

Table 1 Effects of M. arvensis L on acetic acid-induced writhing in mice (mean±SEM, n=5).
4. Discussion
In the TLC-based qualitative antioxidant assay using DPPH assay, extract showed the free radical scavenging properties indicated by the presence of moderate yellow spot on a purple background on the TLC plate. It led to the quantitative antioxidant activity. In the quantitative assay, extract displayed free radical scavenging activity in the DPPH assay (IC50~41 µg/mL) which is comparable to that of ascorbic acid (IC50~19 µg/mL), a well-known standard antioxidant. Plant antioxidants have generally phenolic moiety. Phenolic compounds can easily donate electrons to reactive radicals because of the resonance stability of phenoxy radical and thus retard radical chain reactions[15]. The flavonoids from plant extracts have been found to possess antioxidants, antimicrobial and antiinflammatory properties in various studies[16,17]. Several plant components like tannins are responsible for showing antioxidant property[18]. As in the phytochemical test,M. arvensisshowed the presence of flavonoids and tannins, its antioxidant and anti-inflammatory properties may be observed due to the presence of these chemical components. The DPPH antioxidant assay is based on the ability DPPH, a stable free radical, to decolorize in the presence of antioxidants[19].
Antimicrobial assessment demonstrates whether any species under investigation possess inhibitory activity against microbial species. In this experiment the ethanol crude extract was found to have prominent inhibitory property against several pathogenic microbial species related to standard drug kanamycin. This property indicates the presence of one or several chemical moieties in the crude extract having antimicrobial property. However, further research on this plant is crucial to identify and isolate the responsible compounds for discovery of each of their specific action mechanism.
Furthermore, the results also showed that the plant extract has potent cytotoxic activity. It is depicted that the extract may contain antitumor, antibacterial or pesticidal compounds. However since a broad range of phytocompounds are capable of exhibiting nonspecific cytotoxicity, plant extracts with significant cytotoxic activity should be further investigated using animal models to confirm antitumour activity, and/or a battery of various cell lines to detect specific cytotoxicity. This step is necessary to eliminate cytotoxic compounds with little value for further investigation as anticancer agents[20].
Pain is an unpleasant sensation, usually evoked by external or internal noxious stimulus. An analgesic selectively relieves pain by acting in the central nervous system or on peripheral pain mechanisms, without significantly altering consciousness. Acetic acid is a pain inducing agent. Intraperitoneal administration of acetic acid (0.7%) causes the release of free arachidonic acid from tissue phospholipid by the action of phospholipase A2and other acyl hydrolases[21]. There are three major pathways in the synthesis of the eicosanoids from arachidonic acid. All the eicosanoids with ring structures that is the prostaglandins, thromboxanes and prostacyclines are synthesized via the cyclooxygenase pathway. The leucotrienes, hydroxy eicosatetraenoic acids and hydroperoxy eicosatetraenoic acids are hydroxylated derivatives of straight-chain fatty acids and are synthesized via the lipooxygenase pathway. The released prostaglandins, mainly prostacyclinand prostaglandin-E have been reported to be responsible for pain sensation by exciting the A δ-fibers. Activity in the A δ-fibers causes a sensation of sharp well localized pain[21,22]. In preliminary phytochemical investigation, the extract showed the presence of alkaloids, gum, flavonoids, tannin, and glycosides. So the observed analgesic activity may be attributed to these compounds. Moreover, recent studies suggest that the inflammatory tissue damage is due to liberation of reactive oxygen species from phagocytes invading the inflammation site[19].
According to the above mentioned results it can be concluded that the ethanolic calyx extract ofM. arvensisshowed significant antioxidant, anti-bacterial, cytotoxic and analgesic activities that supports this plant for the treatment of traditional medicine. This study also suggest further investigation to isolate most bioactive compounds responsible for the uses of this plant as traditional medicine.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
Authors are grateful to the authority of the Khulna University for giving the opportunity to conduct such experiment and providing necessary chemical, instrument and utility support. Authors also like to express their cordial thanks to the experts of Bangladesh National Herbarium who helped for the identification of the plant.
Comments
Background
In traditional therapeutic system, several plants of medicinal properties are useful for antioxidant, antibacterial, cytotoxicity and analgesic purposes. However,M. arvensisL. is one of the widely used species in folk medicine. This study aimed to evaluate the pharmacological basis for the use of the plant in folk medicine by using established scientific method.
Research frontiers
The authors have demonstrated antioxidant, antimicrobial, cytotoxic and antinociceptive properties in the plant using bioactivities test as well as phytochemical screening with crude ethanolic extract in differentin vivoandin vitroexperimental models.
Related reports
Although, some studies have carried out the test with different solvents and different strains for antioxidant and antimicrobial test, same activities have been acknowledged for the plant species.
Innovations and breakthroughs
This elegant study showed that the ethanolic extract of this plant has significant antioxidant, analgesic, cytotoxic and antibacterial activities. The phytochemical screening also confirms the presence of alkaloids, gum, flavonoids, tannin, glycosides, and reducing sugar.
Applications
This scientific study support and suggest that the plants may be used strongly as a local source of antioxidant, analgesic, cytotoxic and antibacterial agents.
Peer review
This is a valuable research work in which authors have made the study interesting by evaluating the antioxidative, analgesic, cytotoxic and antibacterial effects ofM. arvensisL. extract in association with phytochemical screening. Materials and methods are well planned. Findings are attention-grabbing and the discussion section contains scientifically interpretation.
[1] Ghani A. Medicinal plants of Bangladesh: chemical constituents and uses. 2nd ed. Dhaka: Asiatic Society of Bangladesh; 2003, p. 1-16, 381.
[2] Satyavati GV, Gupta AK, Tandon N. Medicinal plants of India, Vol 2. New Delhi: Indian Council of Medical Research; 1987, p. 230-239.
[3] Council of Scientific and Industrial Research. Wealth of India: raw materials series. IX. New Delhi: Council of Scientific and Industrial Research; 1972, p. 337-346.
[4] Verma RS, Rahman L, Verma RK, Chauhan A, Yadav AK, Singh A. Essential oil composition of menthol mint (Mentha arvensis) and peppermint (Mentha piperita) cultivars at different stages of plant growth from Kumaon Region of western Himalaya. Open Access J Med Aromat Plants 2010; 1: 13-18.
[5] Rastogi RP, Mehrotra BN, Sinha S, Seth R. Compendium of Indian medicinal plants. New Delhi: Central Drug Research Institute and Publications & Information Directorate; 1990, p. 388-389.
[6] Oinonen PP, Jokela JK, Hatakka AI, Vuorela PM. Linarin, a selective acetylcholinesterase inhibitor from Mentha arvensis. Fitoterapia 2006; 77: 429-434.
[7] Institute for Medical Research, Herbal Medicine Research Centre. Compendium of Medicinal Plants Used in Malaysia. Kuala Lumpur: Institute for Medical Research, Herbal Medicine Research Centre; 2002, p. 136.
[8] Rajesh K, Swamy AH, Inamdar SS, Joshi V, Kurnool AN. Hepatoprotective and antioxidant activity of ethanol extract of Mentha arvensis leaves against carbon tetrachloride induced hepatic damage in rats. Int J Pharm Tech Res 2013; 5: 426-430.
[9] Malik F, Hussain S, Sadiq A, Parveen G, Wajid A, Shafat S, et al. Phyto-chemical analysis, anti-allergic and anti-inflammatory activity of Mentha arvensis in animals. Afr J Pharm Pharmacol 2012; 6: 613-619.
[10] Verma SM, Arora H, Dubey R. Anti-inflammatory and sedativehypnotic activity of the methanolic extract of the leaves of Mentha arvensis. Anc Sci Life 2003; 23: 95-99.
[11] Prakash A, Rigelhof F, MIller E. Antioxidant activity. Minneapolis: Medallion Labs; 2001. [Online] Available from: http://www.medlabs. com/downloads/antiox_acti_.pdf [Accessed on 23th October, 2013]
[12] Ahmed F, Das AK, Islam MA, Rahman KM, Rahman MM, Selim MS. Antibacterial activity of Cordyline terminalis Kunth. leaves. J Med Sci 2003; 3(5): 418-422.
[13] Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 1982; 45(5): 31-34.
[14] Ahmed F, Selim MS, Das AK, Choudhuri MS. Anti-inflammatory and anti-nociceptive activities of Lipia nodiaflora Linn. Pharmazie 2004; 59: 329-330.
[15] Özgen U, Mavi A, Terzi Z, Kazaz C, Asçı A, Kaya Y, et al. Relationship between chemical structure and antioxidant activity of luteolin and its glycosides isolated from Thymus sipyleus subsp. sipyleus var. sipyleus. Planta Med 2010; doi: 10.1055/s-0030-1264257.
[16] Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 2008; 8: 634-646.
[17] Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 2009; 9: 31-59.
[18] Zhang LL, Lin YM. Tannins from Canarium album with potent antioxidant activity. J Zhejiang Univ Sci B 2008; 9(5): 407-415.
[19] Hasan SM, Jamila M, Majumder MM, Akter R, Hossain MM, Mazumder ME, et al. Analgesic and antioxidant activity of the hydromethanolic extract of Mikania scandens (L.) Willd. leaves. Am J Pharmacol Toxicol 2009; 4(1): 1-7.
[20] Ali AM, Mackeen MM, Saleh H, Ei-Sharkawy SH, Abdul Hamid J, Ismail NH, et al. Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J Trop Agric Sci 1996; 19: 129-136.
[21] Ranolds JE, Prasad AB. Martindale: the extra pharmacopoeia. 28th ed. London: The Pharmaceutical Press; 1982, p. 245.
[22] Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 2nd ed. UK: Churchill Livingstone; 1993, p. 706-711.
10.12980/APJTB.4.2014C1298
*Corresponding author: Mohammed Khadem Ali, Department of Biotechnology and Genetic Engineering, School of Life Science, Khulna University, Khulna-9208, Bangladesh.
Tel: +88-01914-827647
E-mail: khadem_bge05@yahoo.com
Foundation Project: Supported by Pharmacy Discipline, Life Science School, Khulna University (Grant No. KU: PHRM: 2012/April-081134).
Article history:
Received 9 Dec 2013
Received in revised form 13 Mar, 2nd revised form 20 Mar, 3rd revised form 25 Mar 2014
Accepted 20 Apr 2014
Available online 26 Aug 2014
Methods:In vitro DPPH radical scavenging assay was used to evaluate the antioxidant activity of the plant extract. In vivo analgesic activity was carried out by acetic acid-induced writhing test in Swiss albino mice. All studies in mice were undertaken at the doses of 250 and 500 mg/kg body weight. Antibacterial activity was studied by disk diffusion assay against some Gram-positive and Gram-negative bacterial strains. Brine shrimp lethality assay was used to investigate cytotoxicity effects of the plant extract.
Results:The extract showed free radical scavenging activity in the DPPH assay (IC50~41 µg/mL) compared to the standard antioxidant ascorbic acid (IC50~19 µg/mL). The extract also produced prominent antimicrobial activity against Salmonella typhi, Salmonella paratyphi, Shigella boydii, Streptococcus pyogenes and Streptococcus aureus compared to standard drug kanamycin at the dose of 30 µg/disc. The extract exhibited lethality against the brine shrimp nauplii with the LC50values of 40 µg/mL, and also 90% mortality (LC90) value was found to be 160 µg/mL. In analgesic test, the extract demonstrated statistically significant (P<0.01) analgesic effect in acetic acid induced writhing in white albino mice at both dose levels.
Conclusions:These results suggest that the ethanolic extract of Mentha arvensis L. has potential antioxidant, antibacterial, cytotoxic and analgesic activities that support the ethnopharmacological uses of this plant.
 Asian Pacific Journal of Tropical Biomedicine2014年10期
Asian Pacific Journal of Tropical Biomedicine2014年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Bartonella species in small mammals and their potential vectors in Asia
- Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae)
- In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts
- In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus
- Production and purification of a bioactive substance against multi-drug resistant human pathogens from the marine-sponge-derived Salinispora sp.
- Calcinosis circumscripta in a captive African cheetah (Acinonyx jubatus)
