In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts
Nadira Binte Samad, Trishna Debnath, Michael Ye, Md. Abul Hasnat, Beong Ou Lim
Department of Applied Biochemistry, Konkuk University, Chungju 380-701, Korea
In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts
Nadira Binte Samad, Trishna Debnath, Michael Ye, Md. Abul Hasnat, Beong Ou Lim*
Department of Applied Biochemistry, Konkuk University, Chungju 380-701, Korea
PEER REVIEW
Peer reviewer
Tae-Il Jeon, Ph.D, Assistant Professor, Department of Animal Science, Chonnam National University, Korea. Tel: +82-62-530-2127, E-mail: tjeon@ jnu.ac.kr
Co-reviewer: Jae-Hak Moon, Gwangju, Korea.
Comments
Authors reported that extracts of Korean blueberries significantly reduced free radicals generation and DNA damage. Moreover, the extracts have a potent anti-inflammatory activity. Therefore, the extracts can be useful for the amelioration of oxidative stress and inflammation.
Details on Page 814
Objective:To investigate in vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.).
Phenolics, Flavonoids, DPPH radical scavenging, Nitrite scavenging, DNA damage, Antiinflammatory activity
1. Introduction
Oxidation is the process in living organisms through which they produce energy. In the regular pathway of aerobic metabolism, different reactive oxygen species (ROS) or reactive nitrogen species are formed as intermediates. ROS and reactive nitrogen species include free radicals which oxidize the membrane lipids. As a result, many cellular components including proteins, lipids and DNA become damaged[1]. Thus different diseases such as age-related degenerative brain disorders, atherosclerosis, cirrhosis, cancer, arthritis, diabetes, hemorrhagic shock,etc.are caused[2]. To neutralize free radicals, most of all organisms have evolved highly complex antioxidant protection mechanisms. The mechanism also includes different diet-derived antioxidants such as vitamin E, carotenoids, ascorbic acid and polyphenols[3]. That is why discovery of natural antioxidants derived from food sources and food processing by-products are receiving much attention[4,5].
Fruits and vegetables are found to be a rich source of antioxidants that may reduce symptoms of many chronic diseases by acting on various components of the immune system[5-7]. Varieties of berries are known to possess significant antioxidant activity[5,8]. Blueberry(Vaccinium corymbosumL.) is a well-known edible fruit of the Ericaceae family and it has been considered to be one of the fruits with the highest antioxidant potentials[9]. Many studies have documented to exhibit their potential biological activities due to possessing of high antioxidant activity. Phenolic compounds from blueberries inhibit colon cancer cell proliferation and protect cells damaged by excess H2O2[10].
USA, Australia and Canada are prominent in their productions and researches of blueberry. South Korea is one of the leaders in blueberry productions in Asia. The demand for blueberries is booming rapidly in South Korea. South Korea exhibits traits of a substantial net importer of blueberries for years. Considering its high profile usage, Korea has started the cultivation of blueberry locally few years ago. One thousand and five hundred tons of blueberries had been harvested in 2010. In spite of its great demand, there are only a few researches found on Korean blueberry to study its antioxidant properties and some other disease preventing activities. Moreover, Korean blueberries are cultivated in an artificial moist condition by using moss; therefore the antioxidative profiles need to be examined to determine its quality.
So, the objective of this study was to determine the different free radical scavenging activities and antiinflammatory activities of water and ethanol extracts of locally grown (Gangwon-do province in Korea) fresh blueberries (Vaccinium corymbosum). We measured the total phenol and flavonoid content, scavenging activity of different free radicals including 2,2-diphenyl-picrylhydrazyl hydrate (DPPH), hydroxyl, superoxide, nitric oxide, superoxide dismutase (SOD) like activity, catalase activity, preventive activity on oxidative DNA damage and anti-inflammatory activity using lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells.
2. Materials and methods
2.1. Materials and chemicals
Gallic acid, Folin-Ciocalteu reagent, DPPH, linoleic acid, N-(1-Naphthyl) ethylene diamine dihydrochloride and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1,2,2-Azinobis-(3-ethylbenzothiazoline-6-sulfonicacid) (ABTS), trichloroacetic acid and potassium per sulfate were from Sigma-Aldrich (Steinheim, Germany). Phosphoric acid and potassium ferricyanide were from Daejung Chemicals and Metals (Seoul, Korea). Ammonium thiocyanate and sulfanilicacid were from Sigma-Aldrich (Tokyo, Japan). Iron trichloride was from Wako Pure Chemical Industries (China). All chemicals and solvents used in this study were of commercial or analytical grade. The pBR322 DNA and 6× DNA loading dye were purchased from Fermentas Inc. (Cromwell Park, Glen Burnie, USA). Agarose A was purchased from Bio Basic Inc. (Ontario, Canada).
2.2. Preparation of extracts
Water and ethanol extracts of blueberry were prepared according to a modified method of Simet al[11]. Both samples (300 g) were extracted three times with 700 mL of distilled water and 700 mL of ethanol. This experiment was done each time for 3 h in a water bath at 90 °C. Then the extracts were filtered through Whatman No.1 (GE Healthcare UK Limited, UK) filter paper. These extracts were evaporated using a rotary evaporator under vacuum condition. The residual crude distilled extracts were dried in freeze-drier and weighed. The samples were stored at -20 °C until further analysis.
2.3. Measurement of total phenolics
The amount of total phenolics was measured using Folin-Ciocalteu method[1]. In this method gallic acid was used as a standard and a calibration curve was obtained. Initially, 0.4 mL aliquot of diluted extracts, 0.2 mL of 1 mol/L Folin-Ciocalteu reagent and 0.6 mL of Na2CO3(20%, w/v) were mixed. Mixtures were kept for 30 min in dark at room temperature to complete the reaction. The absorbance at 700 nm was measured by using a UV-vis spectrophotometer (Sunrise Basic Tecan, Austria). The content of phenolics thus obtained was multiplied by the dilution factor and the results were expressed as the equivalent to milligrams of gallic acid equivalent per gram (mg GAE/g) of fresh weight.
2.4. Determination of total flavonoid content
Total flavonoid content was determined following a colorimetric method where catechin was used as a standard. A calibration curve was obtained with solutions of 0.05 to 0.5 mg/mL of this compound which was described previously by Samadet al[1]. Briefly, 0.025 mL of the extracts (10 mg/mL) or catechin standard solution (0.05 to 0.50 mg/mL) was mixed with 0.125 mL of distilled water in 96-well plate and afterward 0.08 mL of a 5% w/v sodium nitrite solution was added. Aluminum chloride solution of 0.015 mL of a 10% w/v was added after 5 min. The mixture was kept for another 6 min. Then 0.05 mL of 1 mol/L sodium hydroxide and 0.027 mL distilled water were added to the mixture. The final solution was mixed vigorously with a vortex mixer and the absorbance of the final solution was measured immediately at 510 nm using a UV-vis spectrophotometer. The results were expressed as the equivalent to milligrams catechin per gram (mg CE/g) of fresh weight.
2.5. DPPH radical scavenging activity
Hydrogen donating ability of the extracts was determined by using a method based on the reduction of a methanolic solution of the colored free radical DPPH to the non radical form[12,13]. Briefly, stock solutions of the extracts (10 mg/mL) were prepared in distilled water and ethanol, respectively. The synthetic standard antioxidant butylatedhydroxytoluene (BHT) and ascorbic acid (10 mg/mL) were prepared in dehydrated alcohol and water, respectively. Dilutions were made to obtain concentrations ranging from 0.3 to 3.0 mg/mL. The diluted solution 0.08 mL was mixed with 0.08 mL of freshly prepared 0.24 mol/L DPPH-methanol solution. The solution was then kept for 30 min in dark at room temperature for its reaction. The absorbance of the solutions was determined by a UV-vis spectrophotometer at 517 nm. The percentage inhibition of free-radical DPPH was calculated as:
Inhibition (%)=[(Ablank-Asample)/Ablank]×100
Where Ablankis the absorbance of the control reaction (containing all reagents except the test compound); and Asampleis the absorbance of the test compound.
2.6. ABTS radical cation (ABTS·+) scavenging activity
ABTS·+ scavenging activity of the extracts were measured using ABTS·+ assay as described by Salemet al[1]. Scavenging activity was measured based on the ability of the antioxidant molecules to quench the ABTS·+. ABTS·+ is a blue-green chromophore with characteristic absorption at 734 nm and compared with that of BHT and ascorbic acid. After addition of 0.970 mL of ABTS·+ solution to 0.03 mL of blueberry extracts and both standards (0.6 to 3.0 mg/mL) and the decrease in absorbance at 734 nm was monitored. The ability to scavenge ABTS·+ was as:
Scavenge ABTS·+ ability (%)=[(Ablank-Asample)/Ablank]×100
Where Ablankis the absorbance of the control reaction (containing all reagents except the test compound); and Asampleis the absorbance of the test compound.
2.7. Determination of reduced power
The reduced power of the extracts was estimated by the method of Samadet al[1]. The extracts of 0.02 mL were mixed with 2.5 mL of 0.2 mol/L phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The reaction mixture was incubated for 30 min at 50 °C. Trichloroacetic acid (10%) of 2.5 mL was then added to the mixture and centrifuged for 10 min at 3 000 r/min in a refrigerated centrifuge. The uppermost layer of the supernatant (2.5 mL) was collected and was mixed with 2.5 mL of distilled water. Afterward ferric chloride (0.5 mL, 0.1%) and the absorbance was measured at 700 nm in UV-vis spectrophotometer. Increase in absorbance of the reaction mixture indicated the reduced power.
2.8. Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of the extracts was determined according to the Haber-Weiss reaction[14]. Hydroxyl radical was generated rapidly from this reaction and was reacted with nitrone spin trap DMPO. The resultant DMPO-OH was detected with an electron spin resonance (ESR) spectrometer. Briefly, 0.2 mL of each samples’ extracts were mixed with 0.2 mL of DMPO (0.3 mol/L), 0.2 mL of FeSO4(10 mmol/L). A volume of 0.2 mL of H2O2(10 mmol/L) in a phosphate buffer solution (pH 7.2) was added to the solution. After 2.5 min reaction, the mixture was transferred to a 0.1 mL teflon coated capillary tube. An ESR spectrum of the mixture was recorded through a JES-FA ESR spectrometer (JEOL Ltd., Tokyo, Japan). Experimental conditions were as follows: central field, 3475 G; microwave power, 1 mW; modulation frequency, 100 kHz; modulation amplitude, 2 G; gain, 6.3× 105; and temperature, 25 °C.
2.9. Ferric thiocyanate (FTC) method
The antioxidant activities of blueberry extracts were determined according to the FTC method with some modifications[5]. Firstly, 2.5 mL of each extracts samples were dissolved in 1 mL phosphate-buffered saline (PBS). Distilled water (0.5 mL) was added with the solution. Then 1 mL linoleic acid (50 mmol/L in methanol) was added to the mixture. Afterwards, the mixtures were incubated at 40 °C in dark. After incubation of 3 min, 2.35 mL methanol (75%) and 0.05 mL NH4SCN (30%, as a color reagent) were added to 0.05 mL of the mixture. Precisely after the addition of 0.05 mL of FeCl2(0.002 mol/L) in HCl (3.5%) to the reaction mixture for 3 min, the absorbance of the resulting red color was measured at 500 nm using UV-vis spectrophotometer. Absorbance was measured every 24 h until the absorbance of the control reached maximum value.
2.10. Measurement of nitric oxide scavenging ability
The nitric oxide scavenging ability of extracts were determined according to a method by using Griess reagent[15]. Firstly, 1 mL of extracts was mixed with 1 mL of 1 mmol/L sodium nitrite. Thereafter, 8 mL of 0.2 mol/L citrate buffer (pH 4.2) was added to the mixture. After the mixtures had been incubated at 37 °C for 1 h, 1 mL of this was withdrawn and added to a mixture of 2 mL acetic acid (2%) and 0.4 mL of Griess reagent (1% sulfanilic acid and 1% of N-(1-naphthyl) ethylene diamine dihydrochloride solution). Solution was vigorously mixed with a vortex mixer and placed at room temperature for 15 min. Then the absorbance of the final solution was measured at 520 nm in the UV-vis spectrophotometer. The nitrite scavenging activity was calculated with the equation:
Nitric oxide scavenging activity (%)=[1-(A-C)/B]×100
Where A is the absorbance of the treated sample; C is the absorbance of extracts; and B is the absorbance of 1 mmol/L NaNO2.
2.11. SOD-like scavenging activity assay
The SOD-like scavenging activities of water and ethanol extracts of blueberry were determined following the method described by Tianet al. with a few modifications[16]. Briefly, 200 µL of different sample solutions (0.125-2.000 mg/mL) were mixed with 200 µL of pyrogallol solution (7.2 mL mol/L in water) and 3 mL of 50 mL mol/L Tris-HCl buffer (pH 8.5) containing 10 mmol/L EDTA. After 10 min of incubation, 1 mL of 1 mol/L HCl was added to the above mixture to stop thereactions. Ascorbic acid was used (0.125-2.000 mg/mL) as a positive control. The absorbance was taken at 420 nm. SOD-like activity was calculated by using the following equation:
Scavenging activity(%)= 100-[(A-B)/C]×100
Where A is the absorbance of sample/standard with reagent; B is the absorbance of sample/standard without reagent; and C is the absorbance of the control.
2.12. Catalase activity assay
Enzymatic activity in the peroxidation function of catalase was investigated using Cayman’s catalase assay kit and the assay was performed according to the method followed by Debnathet al[17]. Briefly, 100 µL of assay buffer and 30 µL of methanol were mixed. The sample solution (3 mg/mL) was mixed with the above mixture. For standard curve calibration, 20 µL of different formaldehyde solutions was also added in the mixture separately to make final concentrations of formaldehyde of 0, 5, 15, 30, 45, 60 and 75 µmol/L, afterward 20 µL of H2O2was added to each solution to start the reaction and the solutions were incubated on a shaker for 20 min at room temperature. Then 30 µL of potassium hydroxide and purpald was added to each well and incubated for 10 min. Thereafter, 10 µL potassium periodate was added. The mixture was kept for 5 min and the absorbance was measured at 540 nm. Catalase activity was expressed as nmol/min/mL.
2.13. Prevention of oxidativeDNAdamage
The prevention of oxidative DNA damaged by H2O2was described by Tianet alwith some minor modifications[18]. Briefly, 1 µL of plasmid pBR 322 DNA (0.5 µg/µL) was treated with 4 µL of 30% H2O2(v/v), 3 µL of FeSO4(0.08 mmol/L), 3 µL distilled water and 2 µL test sample extracts with different concentrations (1 and 3 mg/mL). Then the mixture was incubated at 37 °C for 1 h. Then, 2 µL of 6× DNA loading dye were added with the mixture. Finally, the mixture was subjected to 0.8% agarose gel electrophoresis at 70 V at room temperature. Ethidium bromide was used to stain the DNA bands (supercoiled, linear and open circular). Gels were scanned with the help of gel documentation system (Nextep, Korea) and bands were quantified using nextep analysis software. Antioxidant effects on DNA were evaluated based on the increase or loss percentage of supercoiled monomer, compared with the control value.
2.14.RAW 264.7cell line and sample treatment
2.14.1. Cell line and cell culture
RAW 264.7 cell lines of murine macrophage were grown in Dulbecco’s modified Eagle’s medium. The medium was supplemented with 10% heat inactivation of fetal bovine serum, penicillin (100 units/mL) and streptomycin sulfate (100 units/mL). Cells were cultured in a humidified atmosphere and incubated at 37 °C in 5% CO2. Cells were cultured in 96 well plates and seeded as 5×104per well. The extracts were dissolved in PBS and applied to the cell cultures alone or with 10 µg/mL of LPS.
2.14.2.MTTassay
Cell viability was evaluated following a conventional MTT [1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan] assay[19]. A total of 20 µL of the MTT solution (5 mg/mL in a PBS, pH 7.4) was added to each well at 24 h prior to culture termination. The cells were continuously cultured for 4 h. Then 100 µL of dimethyl sulfoxide was added for solubilization. Absorbance was measured at 594 nm in the UV-vis spectrophotometer.
2.14.3. Nitric oxide determination
The level of nitric oxide (NO) production in cell culture supernatants was determined based on the Griess reaction using a colorimetric assay[19]. Aliquots of 100 µL of supernatants were mixed with 100 µL Griess reagent (50 µL of 1% sulfanilamide in 5% phosphoric acid and 50 µL of 0.1% napthyl-ethylenediamine dihydrochloride). After 10 min incubation at room temperature, the absorbance was determined at 594 nm in the UV-vis spectrophotometer.
2.15. Statistical analysis
Data were presented as mean±SD. All analyses were carried out in triplicates. Statistical analyses were performed by One-way analysis of variance. Significant differences between groups were determined atP<0.05 andP>0.05. Microsoft Excel 2007 were used for the graphical and statistical evaluations.
3. Results
3.1. Extraction yield, total phenolic and flavonoid content
Plant phenolics and flavonoids compounds are found to be shown antioxidative activities[1,16]. So the content of both compounds were investigated in blueberry extracts. Phenolic contents were found to be (115.0±3.0) and (4.2± 3.0) mg GAE/100 g fresh mass for both extracts, respectively. Flavonoid contents were found to be (1 942.8±7.0) and (1 292.1 ±6.0) mg CE/100 g fresh mass for water and ethanol extracts, respectively.
3.2.DPPHandABTS(ABTS·+) radical scavenging activity
The percentage of DPPH radical scavenging capacity of the blueberry extracts was shown in Figure 1a. Both positive controls (BHT and ascorbic acid) and blueberry extracts displayed higher free radical scavenging activity with an increase in concentration. Furthermore, the water extract had greater scavenging activity at (80.07±1.20)% while ethanol extract had (73.27±1.60)% scavenging activity at 3.0 mg/mL with IC50values of (1.80±0.03) mg/mL and (2.05±0.06) mg/mL, respectively. At a concentration range of 0.1 to 0.5 mg/mL, the blueberry extracts had lower scavenging activity than the reference standards BHT and ascorbic acid. However, theextracts showed significant amount of scavenging activity at higher concentrations.
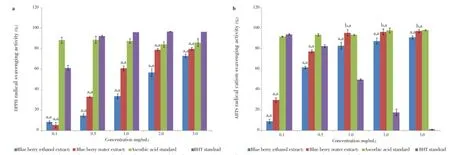
Figure 1. The ability of the water and ethanol extracts of blue berry, ascorbic acid and BHT to scavenge DPPH and ABTS radicals (0.1-3.0) mg/mL.a: DPPH radical svavenging activity; b: ABTS radicals svavenging activity. All data are expressed as mean±SD, n=3. The experimental results are statistically analyzed in comparison with ascorbic acid and BHT standards, represented as x, y respectively, whereaP<0.05,bP>0.05.
On the other hand, the ABTS·+ scavenging activity of the extracts was determined at a concentration range of 0.1 to 3.0 mg/mL for both blueberry extracts. Both the extracts displayed strong scavenging activity since the water extract had (97.2±1.5)% scavenging activity comparing with ethanol at (91.1±3.4)%. Nevertheless, the IC50values were (1.5±0.02) mg/ mL and (1.6±0.03) mg/mL in the water and ethanol extracts, respectively. Ascorbic acid was used as a positive control for which scavenging activity was the highest [(98.1±0.0)% at 3.0 mg/mL and IC50value of (1.5±2.7) mg/mL)] though BHT showed gradual concentration wise decrease in activity (Figure 1b).
3.3. Estimating reduced power
Reduced power or the reductive ability of antioxidants was measured by the transformation of Fe (III) to Fe (II) in the presence of the blueberry extracts[1]. In this assay, an increase in absorbance indicates an increase in reduction of Fe (III) to Fe (II).
At a concentration range of 0.1 to 3.0 mg/mL, BHT and ascorbic acid had much higher reductive activity than blueberry (Figure 2).

Figure 2. Reducing power of ascorbic acid, BHT, water and ethanol extracts of blueberry.All data are expressed as mean±SD, n=3. For the experimental results,aP<0.05.
3.4. Inhibition of linoleic acid oxidation
Huda-Faujanet al. reported that antioxidant capacity and total phenolic content in linoleic acid emulsion are positively correlated[5]. The absorbance of the blueberry extracts (3.0 mg/mL) was plotted as a function of time (Figure 3). Before the experiment the samples had been allowed to be oxidized for up to six days in dark at 40 °C. Unlike ascorbic acid, BHT and blueberry inhibited linoleic acid oxidation at similar rates and the activity gradually increases over time.
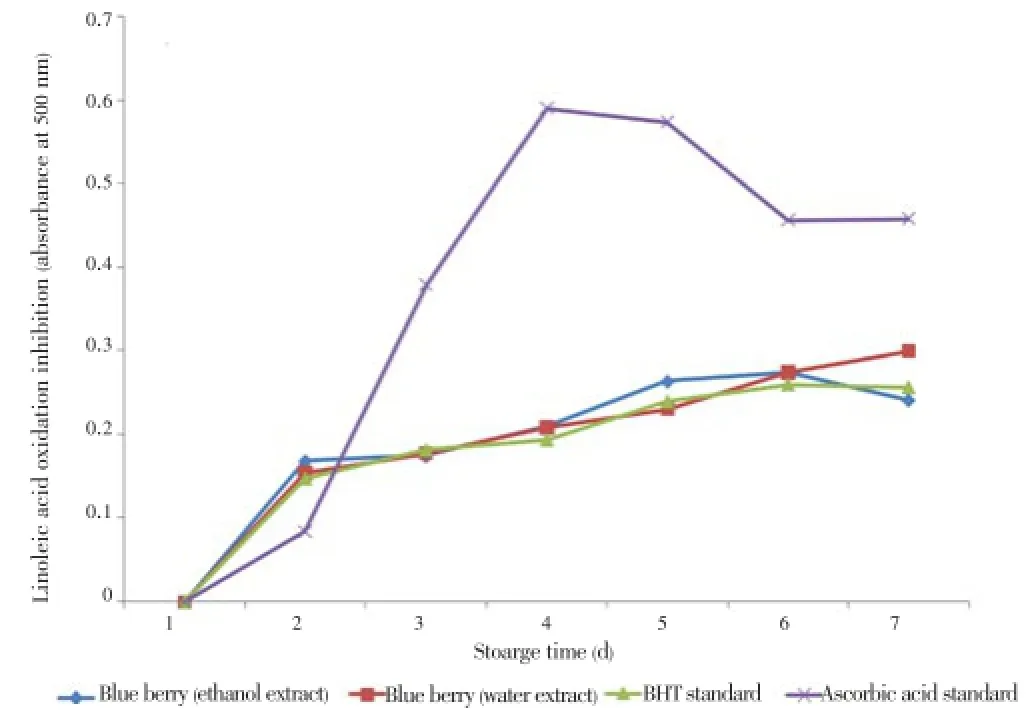
Figure 3. Inhibition of linoleic acid oxidation by the water and ethanol extracts of blueberry at 3 mg/mL.BHT and ascorbic acid were used as standard. All data are expressed as mean±SD, n=3. Statistical comparisons were made between the extracts and standards (P>0.05).
3.5. Nitrite scavenging activity
Nitrite is a toxic substance which is available in proteinrich foods or medicines. Phenolic compounds have nitrite scavenging activities at low pH[20,21]. Therefore, the nitrite scavenging activity of the blueberry extracts were examined. The nitrate scavenging activity of the extracts increased with an increase in blueberry extract concentration and showed greater activity than the control reagents (Table 1). The ethanol extract of blueberry was the most active nitrite scavenger of (99.5±0.1)% followed by the water extract of (87.6 ±0.7)%.
3.6. Hydroxyl radical scavenging activity
The ESR technique was used to determine hydroxyl free radical scavenging activity of the blueberry extracts. The ethanol extract of blueberry (3.0 mg/mL) showed similar activity to BHT where ascorbic acid was able to scavenge (93.5 ±2.5)% of the hydroxyl radicals (Table 1). In contrast, the water extract of blueberry had low hydroxyl radical scavenging activity (18.8±1.0)% at the same concentration.
3.7.SOD-like and catalase activity
A method for assaying SOD-like activities were widely used to predict antioxidant capability based on inhibiting the autooxidation of pyrogallol. In our experiments, both blueberry extracts had similar SOD-like activity (Table 1). However, ascorbic acid was found to be more efficient at inhibiting auto-oxidation of pyrogallos (95.0%). The catalase activity of ethanol extract was slightly higher (0.67 nmol/min/mL) than the water extract (0.64 nmol/min/mL).
3.8. Effects of blueberry on free radical-inducedDNAstrand breaks
Effects of blueberry in water and ethanol extracts on the free radical-induced pBR322 DNA damage were investigated as shown in Figure 4. Antioxidant effects were investigated by using a free radical-inducedin vitroplasmid DNA breaks system. Here,-OH is produced during Fenton’s reaction. The generated-OH attacks the supercoiled plasmid DNA (Lane 1) which afterwards are broken into supercoiled and open circular forms (Lane 2). Both the extract shows similar prevention of-OH induced DNA breaks (Lane 3 and 4).
3.9. Assessment of cell viability and inhibitionNOproduction inLPS-stimulatedRAW 264.7cells
To investigate the cell viability of the blueberry extracts, RAW 264.7 cells were incubated with 5 and 50 µL/mL for 24 h. Blueberry extracts protected the RAW 264.7 cells from LPS-induced apoptosis (Figure 5). Ninety-nine percent of cells were viable after stimulation with LPS for 24 h. However, after treatment with blueberry the viability of the RAW cells increased about 100% with the treatment of 50 µg/mL of the both blueberry extracts.
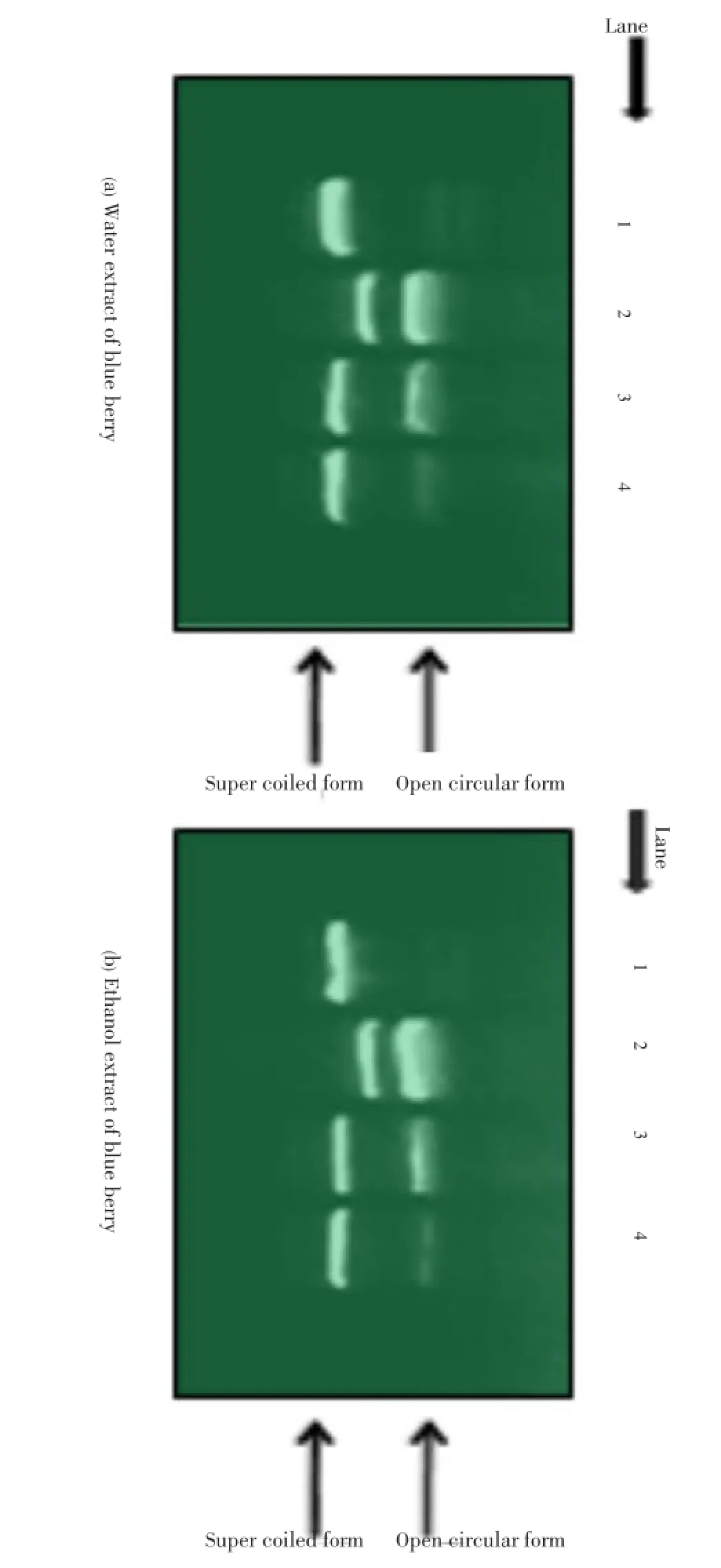
Figure 4. Agarose gel electrophoretic patterns of plasmid DNA breakage.Lane 1: No addition (plasmid DNA); Lane 2: FeSO4and H2O2(DNA damage control); Lanes 3-4: FeSO4and H2O2in the presence of blueberry water (a) and ethanol (b) extracts with concentrations of 1 mg/mL and 3 mg/mL, respectively. All data are expressed as mean±SD, n=3.
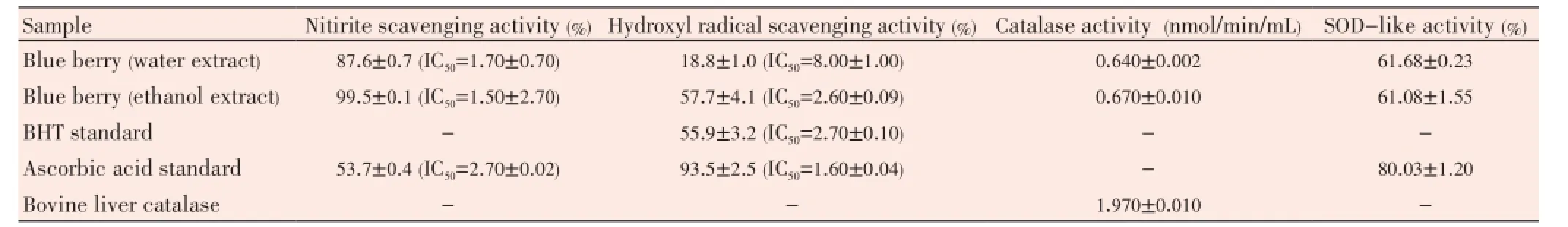
Table 1 Nitrite, hydroxyl radical scavenging, catalase and SOD-like activity at 3 mg/mL of blueberry extracts.
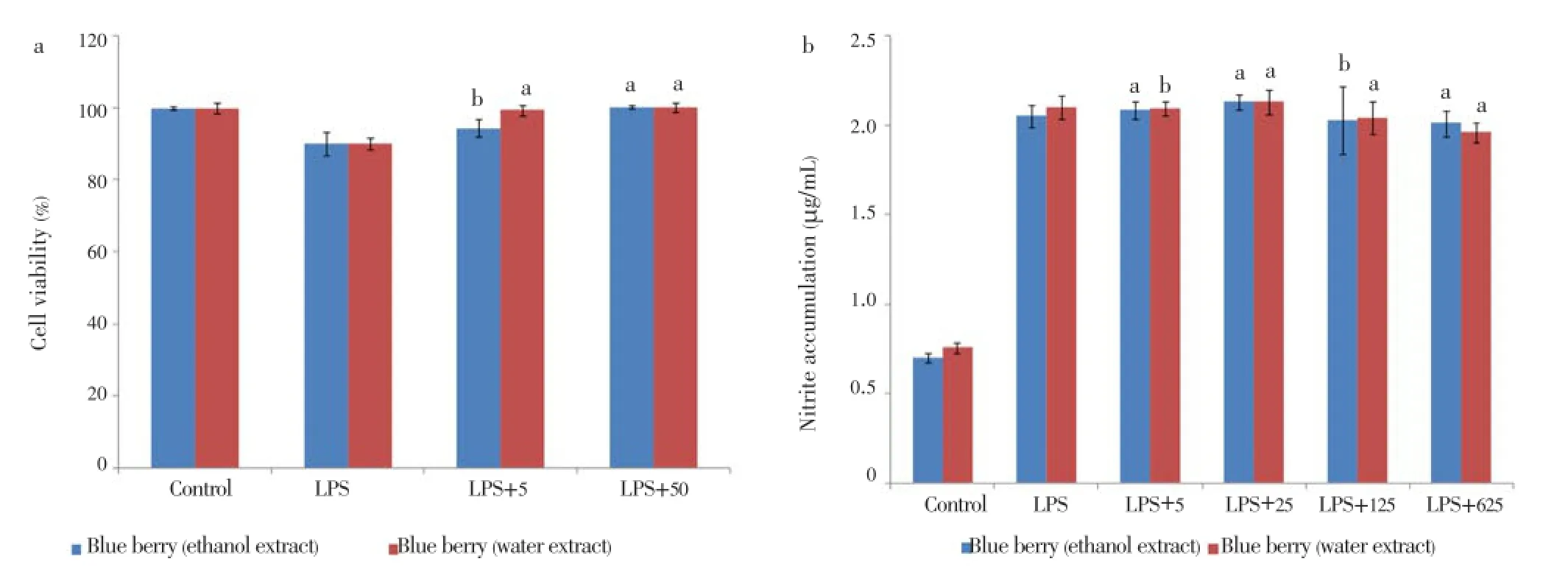
Figure 5. Anti-inflammatory activity of water and ethanol extract of blueberry.a: Cell viability of RAW 264.7 cells treated with blueberry extracts and 10 µg/mL of LPS; b: Inhibition NO production of RAW 264.7 cells treated with blueberry extracts and 10 µg/mL of LPS. All data are expressed as mean±SD, n=3. The experimental results are statistically analyzed in comparison with ascorbic acid and BHT standards, represented as x, y respectively, whereaP<0.05,bP>0.05.
The effect of the blueberry on NO inhibition was determined by treating the RAW 264.7 cells with the presence or absence of LPS stimulation. The cells that were stimulated with LPS had been seen to produce significant levels of NO in conditioned medium (Figure 5). The level of NO production induced by LPS in RAW cells decreased in a dose-dependent manner when being treated with different concentrations of 5, 25, 125, 625 µg/mL of the extract samples.
4. Discussion
Antioxidant nutrients such as carotenoids, vitamin C, vitamin E and numerous polyphenolic compounds directly scavenge reactive oxidants. That is why they are hypothesized to constitute an endogenous defense against oxidative cells. Therefore, the plant derived natural antioxidants may contribute to the antioxidant activity and thus they are able to remove towards the total or partial clinical disorders. Phenolic and flavonoids are the polyphenolic compounds which have been found to have free radical scavenging activity[7]. These compounds donate hydrogen molecules to free radicals and thus act as antioxidants. Our results indicate that the water extract has greater phenolic content than the ethanol extract but both extracts have high flavonoid content. Therefore, both the extracts have the potential for antioxidant activity.
DPPH and ABTS cations (ABTS·+) are not biologically relevant but their activities are analyzed to determine the free radical scavenging activity of plant extracts[7]. They are the indicator compounds for testing the hydrogen donating capability of antioxidants. In this assay, results are expressed as the ratio of the inhibition activities with the increase of extract concentrations. Both the extracts exhibited strong free radical scavenging activity comparing with positive control. Water extracts showed slightly higher activity than the ethanol extracts in both the assays. These results indicate that the blueberry extracts have hydrogen donating ability which indicates their role as antioxidants.
Reducing power is widely used to evaluate the antioxidant activity of polyphenols. It is associated with the presence of reductones which shows antioxidant actions by breaking the free radical chain and donating a hydrogen atom[22]. The reduced power of a compound is related with its electron transfer ability and therefore they may serve as a significant indicator of its antioxidant activity[7]. In the reducing power assay, the blueberry extracts displayed a concentrationdependent antioxidant potential. The presence of reducers or antioxidants in the extracts causes the reduction of Fe3+ complex to the Fe2+ form with a significant color change. So the newly formed Fe2+ amount in the solution can be observed by measuring the formation of Perl’s prussian blue at 700 nm. Increased absorbance at 700 nm indicates an increase in reducing power. Both water and ethanol extracts showed reducing ability. Although it was relatively low than both BHT and ascorbic acid. However, the water extract had slightly higher reducing activity than the ethanol extract.
Antioxidants work as an efficient terminator of fatty acid free radical chain reactions[13]. Phenolic compounds donate hydrogen molecules which in terms terminate free radical chain reactions by forming stable compounds. The FTC method is a useful method to quantify the fatty acid chain reactions terminated due to the action of antioxidants[1]. In the linoleic acid oxidation inhibition assay blueberry extracts showed inhibition of linoleic acid oxidation at similar rates as BHT over time. Moreover, both the extracts showed similar capacity in inhibiting the oxidation, although which were significantly lower comparing with ascorbic acid. However, as the extracts showed similar activity with the artificial antioxidant, BHT. So, it is shown in the assay that water and ethanol extracts are strong inhibitors of linoleic acid inhibition.
Nitrite is a toxic substance which is available in proteinrich foods or medicines. Various cell-damaging reactions are induced in the acidic environment of the stomach when it forms ions[1]. Nitrite forms nitrosamine when it reacts with second and third grade amines, whereas nitrosamine can increase the risk of cancer[23]. Consumption of excessamounts of nitrite over time results in the oxidization of hemoglobin, which can lead to methemoglobinemia[23]. Nitric oxide assay determines the ability of extract to inhibit the nitrite production. In our analysis, we found that both the blueberry extracts were very good scavenger of nitrite, specially the ethanol extract which showed around 99% scavenging at the concentration of only 3 mg/mL and IC50only at 0.1 mg/mL. So, blueberry can be used for the remedy of different diseases.
Hydroxyl free radicals (-OH) are one of the main component of oxidants. Additionally, they are the most harmful free radicals formed in biological systems because they cause enormous damage to many types of biomolecules[17]. However, phenolic compounds have the ability to neutralize hydroxyl free radicals[1,24,25]. In the present study, although the ascorbic acid showed very high value of hydroxyl acid scavenging activity comparing with BHT, and ethanol extract of blueberry showed similar activity to BHT. In contrast, the water extract of blueberry had low hydroxyl radical scavenging activity. Therefore, blueberry extracts, particularly the ethanol extract, can protect against biomolecule oxidation by scavenging highly reactive hydroxyl radicals.
All living organisms have a complex antioxidant defense system. The system includes various antioxidant enzymes such as superoxide dismutase and catalase. During aerobic metabolism, superoxide anion is being produced as a byproduct. Superoxide dismutase breaks it up into H2O and H2O2and then H2O2is converted to H2O and O2by catalase. Therefore, they are an important antioxidant defense in nearly all cells exposed to oxygen. On the other hand, the catalase assay is based on the reaction of the enzyme with methanol in the presence of an optimum concentration of H2O2. Formaldehyde is formed at the end of the reaction. It is measured by a colorimetric method using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (purpald) as the chromogen. Afterwards, purpald changes from colorless to purple upon oxidation. In our experiments, both blueberry extracts had similar SOD-like activity although it was not very strong as ascorbic acid. The catalase activity of water extract was slightly higher than the ethanol extract. However, both extracts had catalase activity that was significantly lower compared to the positive control. As evidenced by the SOD-like and catalase activities, the blueberry extracts promote breakdown of hydrogen peroxide that can lead to oxidative damage of cellular biomolecules.
Antioxidant effects of blueberry for the free radicalinduced pBR322 DNA damage were investigated using a free radical-inducedin vitroplasmid DNA breaks system. Both the extracts showed similar prevention of-OH induced DNA breaks. So it can be assumed from our analysis that the blueberry extracts have the capacity to prevent free radicalinduced DNA damage.
Moreover, blueberry also have a role in cell viability and capacity to inhibit the production of nitrogen. It indicates their anti-inflammatory activity as well. After the application of blueberry on LPS stimulated host cell, both the extracts showed an increase number of viable cells and reduction of NO production.
In this study, thein vitroantioxidant activities of water and ethanol extracts from blueberry locally grown in South Korea were investigated. Both the extracts showed significant amount of phenolic and flavonoid content. Likely, the extracts were found to have significant scavenging capacities of different free radicals including DPPH, hydroxyl, superoxide, nitrite,etc. They inhibited lipid peroxidation and showed potent SOD-like and catalase enzymatic activities. Blueberry also showed activity against free radical-induced DNA strand breakage. Furthermore, the blueberry extracts showed good activity to show cell viability and nitrite accumulation inhibitionin vitro. Considering all the findings, it can be concluded that both water and ethanol extracts of Korean blueberry can be used as a potential source of natural antioxidant and anti-inflammatory agent. Furthermore, these results will help to use Korean blueberry for the remedy of different health problems, especially for those involving the complex inflammatory process in which free radicals and ROS play a determinant role. The results are very much significant for the food industries in Korea to find the possible alternative for synthetic antioxidants. Additionally, it will be considered as a new kind of functional food, cosmetics ingredient or nutraceutical to the consumers. Further studies can be performed for the isolation and identification of their individual phenolic compounds of the extracts.In vivostudies can also be applied to understand the mechanisms of antioxidation of the extracts.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research was supported by Basic Science Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (Grant No. 2012-A419-0147).
Comments
Background
Free radicals are substances that occur naturally in our bodies but damage many cellular components including proteins, lipids and DNA, which can cause different types of diseases and accelerate the aging process. Antioxidants are found in fruits and vegetables that help neutralize free radicals.
Research frontiers
This study shows that Korean blueberry extract is a potent antioxidant that has significant scavenging activities for different free radicals. The extracts are found to preventinflammation as well by reducing NO production.
Related reports
Other studies have reported that blueberries have potential biological activities. For example, phenolic compounds from blueberries inhibit colon cancer cell proliferation and protect cells damaged by excess H2O2.
Innovations and breakthroughs
The extracts were found to have significant scavenging capacity of different free radicals. They inhibited lipid peroxidation and showed potent SOD-like and catalase enzymatic activities. Furthermore, the extracts efficiently inhibit nitrite accumulation in macrophage.
Applications
The extracts can be used for the development of a new kind of functional food, cosmetics ingredient or nutraceutical.
Peer review
Authors reported that extracts of Korean blueberries significantly reduced free radicals generation and DNA damage. Moreover, the extracts have a potent antiinflammatory activity. Therefore, the extracts can be useful for the amelioration of oxidative stress and inflammation.
[1] Samad NB, Debnath T, Jin HL, Lee BR, Park PJ, Lee SY, et al. Antioxident activity of Benincasa hispida seeds. J Food Biochem 2013; 37: 388-395.
[2] Gülçin I, Bursal E, Sehitoğlu MH, Bilsel M, Gören AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010; 48: 2227-2238.
[3] Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid Med Cell Longev 2009; 2: 99-106.
[4] Huber GM, Rupasinghe HP. Phenolic profiles and antioxidant properties of apple skin extracts. J Food Sci 2009; 74: C693-C700.
[5] Samad NB, Debnath T, Hasnat MA, Pervin M, Kim DH, Jo JE, et al. Phenolic contents, antioxidant and anti-inflammatory activities of Asparagus cochinchinensis (Loureiro) Merrill. J Food Biochem 2014; 38: 83-91.
[6] Yamada K, Hung P, Park TK, Park PJ, Lim BO. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J Ethnopharmacol 2011; 137: 231-235.
[7] Mayakrishnan V, Veluswamy S, Sundaram KS, Kannappan P, Abdullah N. Free radical scavenging potential of Lagenaria siceraria (Molina) Standl fruits extract. Asian Pac J Trop Med 2013; 6: 20-26.
[8] Madhujith T, Shahidi F. Antioxidant activity of blueberry and other Vaccinium species. Nutraceutical Beverages 2003; 871: 149-160.
[9] Yi W, Fischer J, Krewer G, Akoh CC. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J Agric Food Chem 2005; 53: 7320-7329.
[10] Senevirathne M, Kim SH, Jeon YJ. Protective effect of enzymatic hydrolysates from highbush blueberry (Vaccinium corymbosum L.) against hydrogen peroxide-induced oxidative damage in Chinese hamster lung fibroblast cell line. Nutr Res Pract 2010; 4: 183-190.
[11] Sim KH, Sil HY. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int J Food Sci Technol 2008; 43: 1813-1823.
[12] Salem MZM, Ali HM, El-Shanhorey NA, Abdel-Megeed A. Evaluation of extracts and essential oil from Callistemon viminalis leaves: antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac J Trop Med 2013; 6: 785-791.
[13] Debnath T, Bak JP, Samad NB, Jin HL, Lee BR, Lim BO. Antioxidant activity of mume fructus extract. J Food Biochem 2012; 36: 224-232.
[14] Lee SJ, Kim EK, Hwang JW, Oh HJ, Cheong SH, Moon SH, et al. Purification and characterisation of an antioxidative peptide from enzymatic hydrolysates of duck processing by-products. Food Chem 2010; 123: 216-220.
[15] Patel DK, Kumar R, Laloo D, Hemalatha S. Evaluation of phytochemical and antioxidant activities of the different fractions of Hybanthus enneaspermus (Linn.) F. Muell. (Violaceae). Asian Pac J Trop Med 2011; 4: 391-396.
[16] Debnath T, Jin HL, Hasnat MA, Kim Y, Samad NB, Park PJ, et al. Antioxidant potential and oxidative DNA damage preventive activity of Chrysanthemum indicum extracts. J Food Biochem 2013; 37: 440-448.
[17] Debnath T, Park PJ, Nath NCD, Samad NB, Park HW, Lim BO. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem 2011; 128: 697-703.
[18] Tian B, Hua Y. Concentration-dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem 2005; 91: 413-418.
[19] Li C, Wang MH. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nutr Res Pract 2011; 5: 101-106.
[20] Noh KS, Yang MO, Cho EJ. Nitrite scavenging effect of Umbelligeraeceae. Korean J Food Cookery Sci 2002; 18: 8-12.
[21] Kang YH, Park YK, Lee GD. The nitrite scavenging and electron donating ability of phenolic compounds. Korean J Food Sci Technol 1996; 28: 232-239.
[22] Debnath T, Park DK, Lee BR, Jin HL, Lee SY, Samad NB, et al. Antioxidant activity of Inonotus obliquus grown on germinated brown rice extracts. J Food Biochem 2013; 37: 456-464.
[23] Choi DB, Cho KA, Na MS, Choi HS, Kim YO, Lim DH, et al. Effect of bamboo oil on antioxidative activity and nitrite scavenging activity. J Ind Eng Chem 2008; 14: 765-770.
[24] Siriwardhana N, Lee KW, Jeon YJ, Kim SH, Haw JW. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci Technol Int 2003; 9: 339-346.
[25] Osawa T. Novel natural antioxidants for utilization in food and biological systems. In: Uritani I, Garcia VV, Mendoza EMT, editors. Postharvest biochemistry of plant foodmaterials in the tropics. Japan: Japan Scientific Societies Press; 1994, p. 241-251.
10.12980/APJTB.4.2014C1008
*Corresponding author: Beong Ou Lim, Associate Professor, College of Biomedical & Health Science, Department of Applied Biochemistry, Konkuk University, Chungju-si, Chungbuk-do 380-701, Korea.
Tel: +82-43-840-3570
Fax: +82-43-840-3570
E-mail: beongou@kku.ac.kr
Article history:
Received 12 Dec 2013
Received in revised form 12 Feb, 2nd revised form 16 Feb, 3rd revised form 20 Feb 2014
Accepted 3 Jun 2014
Available online 12 Sep 2014
Methods:Total phenolic and flavonoid contents of the Korean blueberry water and ethanol extracts were determined before determining the potential of the extracts as antioxidant. Antioxidant activity of the extracts was determined by following some well established methods for free radical scavenging such as 2,2-diphenyl-picrylhydrazyl hydrate, 1,2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonicacid), free radical induced DNA damage, superoxide dismutaselike and catalase assay etc. Furthermore, 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan and nitric oxide assay were performed to determine the anti-inflammatory activity of the extracts.
Results:Total phenolic contents were found (115.0±3.0) and (4.2±3.0) mg GAE/100 g fresh mass for both extracts, respectively and flavonoid contents were (1 942.8±7.0) and (1 292.1±6.0) mg CE/100g fresh mass for water and ethonal extracts, respectively. Both the extracts displayed significant scavenging activity of some radicals such as 2,2-diphenyl-picrylhydrazyl hydrate (IC50at 1.8 mg/ mL and 2.05 mg/mL, respectively), 1,2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonicacid) (IC50at 1.5 mg/mL and 1.6 mg/mL, respectively) and nitrite (IC50at 1.7 mg/mL and 1.5 mg/mL, respectively) etc. The extracts were found to prevent inflammation as well by reducing nitric oxide production and cytotoxicity in cell.
Conclusions:The findings suggest that the fresh Korean blueberry could be used as a source of natural antioxidants and anti-inflammatory agents.
 Asian Pacific Journal of Tropical Biomedicine2014年10期
Asian Pacific Journal of Tropical Biomedicine2014年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Disseminated toxocariasis in an immunocompetent host
- Human ophthalmomyiasis externa caused by the sheep botfly Oestrus ovis: a case report from Karachi, Pakistan
- Calcinosis circumscripta in a captive African cheetah (Acinonyx jubatus)
- Production and purification of a bioactive substance against multi-drug resistant human pathogens from the marine-sponge-derived Salinispora sp.
- In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus
- Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae)
