Expression of p-PPARγ in the aging thoracic aorta of spontaneously hypertensive rat and inhibitory effect of rosiglitazone
Hai-Feng Yuan, Xiao-Lin Niu, Deng-Feng Gao, Guang-Hua Hao, An-Qi Song, Jin Wei
1Department of Encephalopathy, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, China
2Department of Cardiology, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, China
3Key Laboratory of Environment and Genes Related to Diseases of Ministry of Education, Xi’an Jiaotong University, Xi’an 710061, China
Expression of p-PPARγ in the aging thoracic aorta of spontaneously hypertensive rat and inhibitory effect of rosiglitazone
Hai-Feng Yuan1*, Xiao-Lin Niu2,3, Deng-Feng Gao2, Guang-Hua Hao2, An-Qi Song2, Jin Wei2
1Department of Encephalopathy, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, China
2Department of Cardiology, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, China
3Key Laboratory of Environment and Genes Related to Diseases of Ministry of Education, Xi’an Jiaotong University, Xi’an 710061, China
ARTICLE INFO
Article history:
Received 28 Sep 2014
Received in revised form 23 Oct 2014
Accepted 24 Oct 2014
Available online 31 Oct 2014
P-PPARγ
Spontaneously hypertensive rat
Thoracic aorta
Rosiglitazone
Aging
Objective:To investigate the expression of phosphorylated peroxisome proliferators-activated receptor γ (p-PPARγ) in the aging thoracic aorta of spontaneously hypertensive rat (SHR) and the inhibitory effect of rosiglitazone on the phosphorylation of PPARγ.
1. Introduction
Peroxisome proliferators-activated receptors γ (PPARγ) is a ligands-activated nuclear transcription factor. Activated PPARγ binds to the peroxisome proliferator responsive element in thepromoter of target gene to regulate gene expression[1,2]. For the past few years, PPARγ has been considered as a well-known antiaging molecule[3,4]. Activated PPARγ plays a role in regulating adipocyte differentiation and lipid metabolism, improving insulin resistance, inhibiting inflammatory reaction[5] and upregulating longevity gene expression such as Klotho[6]. Previous studies reported that PPARγ inactivation by phosphorylation was observed in many kinds of tissues, such as kidney[7], cerebral cortex[8] and fat[9], suggesting that PPARγ phosphorylation may play an important role in the initiation and development of aging and leads to functional disorder and aberrant expressionof downstream target gene. Vascular aging can change the threshold and severity of cardiovascular diseases including hypertension[10]. It is an independent risk factor for the increasing incidence of hypertension with older age[10,11]. However, whether PPARγ inactivation and the degree of PPARγ phosphorylation are presented during hypertension related vascular aging remains undetermined.
Recently, Choiet al.reported that cyclin-dependent kinase 5 (Cdk5) could lead to PPARγ phosphorylation in adipocytes and subsequently result in functional disorder and aberrant expression of obesity related gene. PPARγ phosphorylated by Cdk5 is inhibited by rosiglitazone, a PPARγ agonist,in vitroandin vivo[12]. Furthermore, the inhibitory effect is independent of canonical receptor transcriptional activation pathway. These results indicate that rosiglitazone may inhibit PPARγ phosphorylation in vascular aging of hypertension patients. In this study, 16, 32 and 64 weekold spontaneously hypertension rats (SHR) were treated with rosiglitazone. We aimed to investigate PPARγ phosphorylation in the thoracic aorta of SHR during vascular aging and explore the inhibitory effect of rosiglitazone on PPARγ phosphorylation.
2. Materials and methods
2.1. In vivo experiments
The 16, 32 and 64 week-old SHR (n=54) and Wistar-Kyoto (WKY) rats (n=27) were purchased from Shanghai Slack Laboratory Animal Co., LTD (Permit number: SCXK: 2007-0005, Shanghai, China) and cultured in the Centre of Laboratory Animals, Medical College of Xi’an Jiaotong University. Rats were housed in sterilized cages (2 rats/cage) at a constant temperature (23 °C) and humidity and fed a regular autoclaved diet with waterad libitum[13]. Rats were respectively divided into WKY (n=9), SHR (n=9) and SHR+rosiglitazone groups (n=9). Rats in the SHR+rosiglitazone group were treated with rosiglitazone (5 mg/ kg) daily for 8 weeks through intragastric administration. While, rats in the WKY and SHR groups were treated with the same volume of saline. Blood pressure measurement was performed after treatment. After treatment, systolic blood pressure (SBP) was measured using tail cuff method as previous described[14]. All rats were sacrificed by cervical vertebra dislocation under anesthesia with ether and the thoracic aorta tissues were excised and prepared for routine pathological examination with Hematoxylin-Eosin (HE) staining[13]. Some thoracic aorta tissues (n=3) were subjected to immunohistochemical analysis, and some (n=6) were homogenized and the proteins extracted for immunoblotting. All animal protocols were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
2.2. Immunohistochemical staining
Immunohistochemistry was performed on paraformaldehydefixed paraffin sections with streptavidin peroxidase conjugated method to detect the positive expression of phosphorylated PPARγ (p-PPARγ) in the aortas of rats according to the manufacturer’s instructions (Zhongshan Golden Bridge Biotechnology Ltd. Co., Beijing, China). The sections of thoracic aorta were routinely dewaxed and antigen retrieval in citrate buffer (0.01 mol/L, pH 6.0). The sections were subsequently blocked using 10% goat plasma and incubated using p-PPARγ (Ser 273; Beijing Bioss Biological Technology Ltd. Co., Beijing, China) (1:600) primary antibody at 4°C overnight. Biotinylated secondary antibodies were used to detect the primary antibody. The sections were visualized with diaminobenzidine (Wuhan Boster Biological Engineering Ltd. Co., Wuhan, China) and counterstained with hematoxylin, then dehydrated in alcohol and xylene and mounted onto glass slides. Phosphate buffer solution was used as primary antibody for negative control.
2.3. Western blot
The total proteins were isolated from thoracic aorta and measured using Protein Quantitative Reagent Kit-BCA Method (Beijing Bioss Biological Technology Ltd. Co.). After protein denaturation at 95 °C, proteins were separated using 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred into the nitrocellulose filter. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (G8140; US Biological, Swampscott, MA, USA) (1:5 000) and p-PPARγ (1:800) primary antibodies were used in the immunoblotting assays. Horseradish peroxidaseconjugated goat anti-mouse or anti-rabbit secondary antibodies (Bio-Rad, Hercules, CA, USA) were used at a 1:1 000-1: 5 000 dilution and detected using a Western Blotting Luminol Reagent (sc-2048; Santa Cruz Biotechnology), as described in previous study[15].
2.4. Statistical analysis
The data were analyzed using the SPSS statistical package for Windows Version 13 (SPSS, Chicago, IL, USA). Results were expressed as mean±SEM. Levene test and Shapiro-Wilk test were performed to determine the homogeneity test for variance and normality test, respectively. Significance was established using One-way ANOVA and LSD-ttest when appropriate. Difference was considered significant whenP<0.05.
3. Results
3.1. The SBP of SHR and WKY rats with different age
As shown in Figure 1, the SBP in 16, 32 and 64 week-old SHR were significantly higher than those in matched WKY rats (P<0.05). However, the SBP in rosiglitazone treated SHR were obviously lower as compared with those in matched SHR(P<0.05). In SHR, 64 week-old rats showed the highest SBP as compared with either 32 week-old or 16 week-old rats (P<0.05, respectively). But 16 week-old SHR exhibited the lowest SBP as compared with 32 week-old rats (P<0.05). Comparison of SBP in 16, 32 and 64 week-old WKY rats, the highest SBP was observed in 64 week-old rats (P<0.05, respectively, Figure 2), while 16 week-old rats showed the lowest SBP (P<0.05, respectively, Figure 2).
3.2. The structure of thoracic aorta in SHR and WKY rats with different age
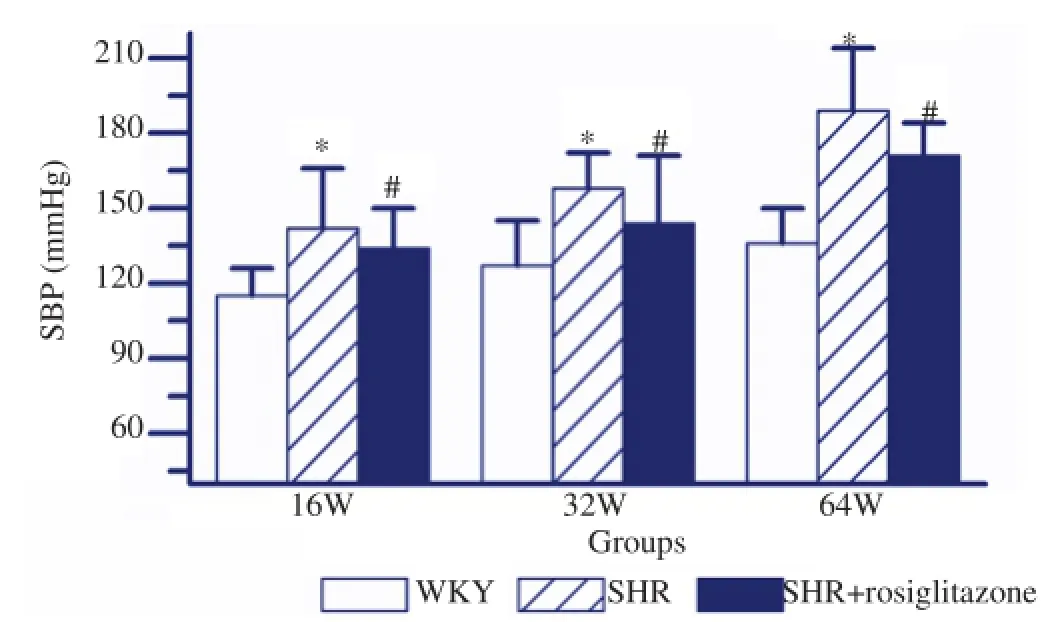
Figure 1.SBP in different groups with different age.The SBP in SHR group was significantly higher than that in WKY group. However, The SBP in SHR+rosiglitazone group was obviously lower than that in SHR group. Values are depicted as mean±SEM,n=9.*P<0.05vsWKY group;#P<0.05vsSHR group.
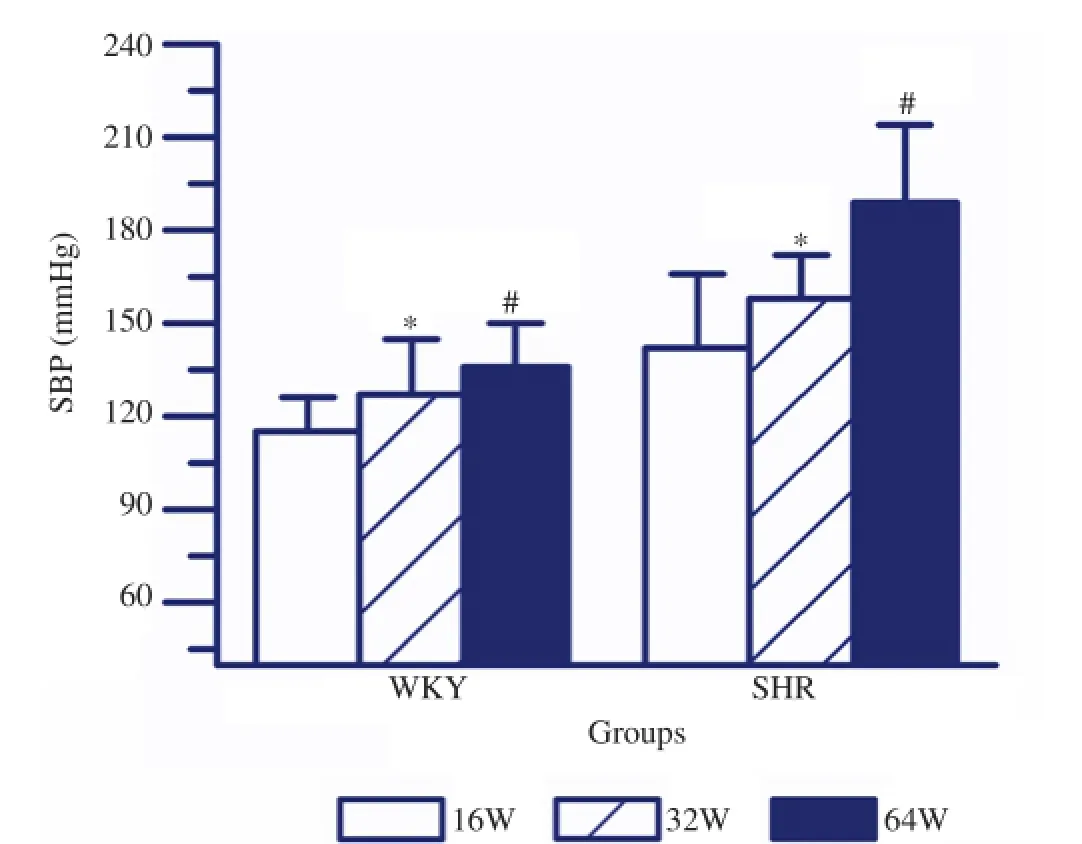
Figure 2.SBP in SHR and WKY rats with different age.The SBP in 64 week-old SHR was prominently higher than that in either 32 or 16 week-old rats. While 16 week-old SHR showed the lowest SBP. Similarly, The SBP in 64 week-old WKY rats was significantly higher than that in either 32 or 16 week-old rats. While 16 week-old WKY rats showed the lowest SBP. Values are depicted as mean±SEM,n=9.*P<0.05vs16 week;#P<0.05vs32 week.
As shown in Figure 3, HE staining showed the layer of elastic fibers was no big change, but exhibited increased content of smooth muscle cell, wrinkled lining endothelium and increased thickness of internal elastic lamina in the thoracic aorta of SHR. These histopathological changes were worsened by the increase of age. The histopathological changes in SHR+rosiglitazone group were less than those in SHR group. WKY group only showed increased layer of elastic fibers with the increase of age. But other histopathological changes were not observed in WKY group.
3.3. The expression of p-PPARγin the thoracic aorta of rats
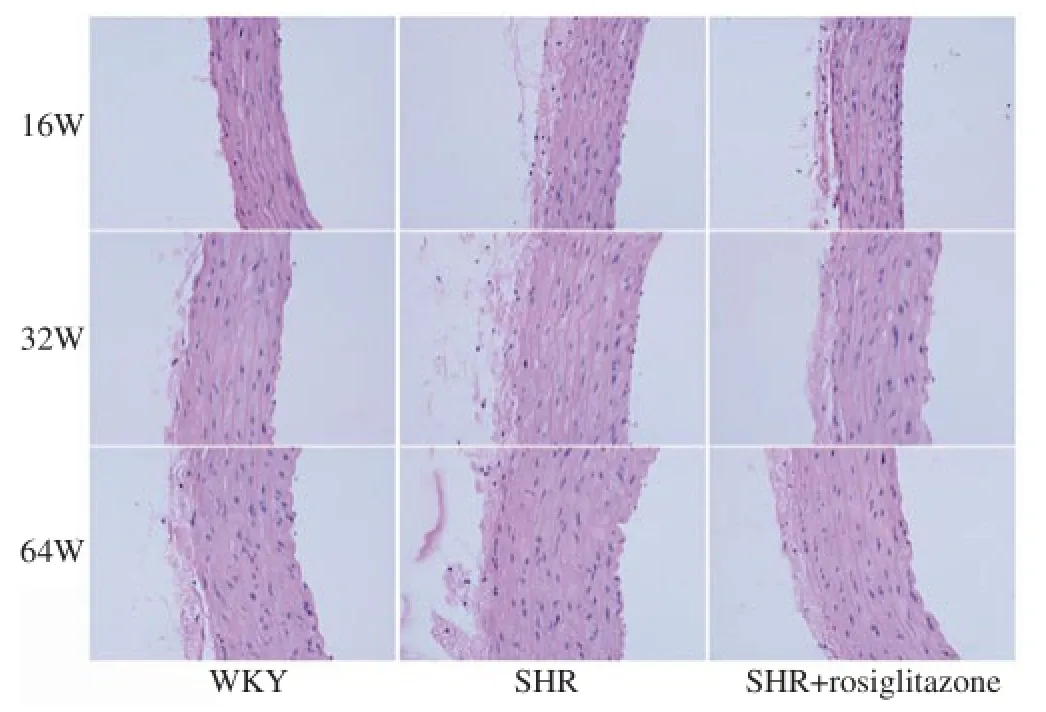
Figure 3.HE staining of the thoracic aorta of rats in different groups.The histopathological changes were worsened by the increase of age in SHR group. The histopathological changes in SHR+rosiglitazone group were less than those in SHR group. However, WKY group showed slight histopathological changes. Original magnification ×100.
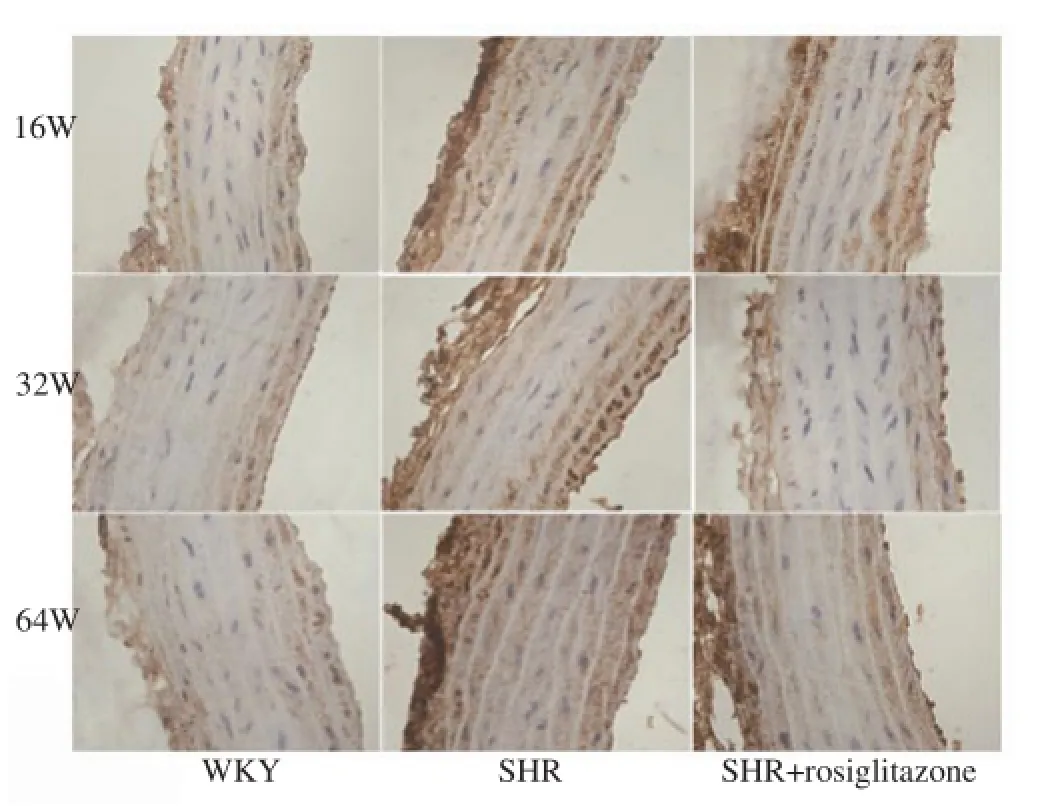
Figure 4.Immunohistochemical analyses of p-PPARγ in the thoracic aorta of rats in different groups.The p-PPARγ signal was up-regulated by the increase of age in both SHR and WKY groups. The p-PPARγ signal in SHR group was higher than that in WKY group. However, SHR+rosiglitazone group showed a weak p-PPARγ signal as compared with SHR group. Original magnification ×400.
The thoracic aortas arising from rats in different groups were subjected to immunostaining for p-PPARγ. As shown in Figure 4, our results found that p-PPARγ signal was increased by the increase of age in both SHR and WKY rats. P-PPARγ signal in SHR group was obviously higher than that in matched WKY group. However, SHR+rosiglitazone group showed less p-PPARγ signalas compared with SHR group. As shown in Figures 5A and 5B, western blot analysis indicated that the levels of p-PPARγ in the thoracic aorta isolated from 16, 32 and 64 week-old SHR were significantly higher than those in matched WKY rats (P<0.05, respectively). Whereas, the levels of p-PPARγ in the thoracic aorta arising from rosiglitazone treated SHR were obviously lower than those in matched SHR (P<0.05, respectively). In SHR, 64 weekold rats showed the highest p-PPARγ expression as compared with either 32 week-old or 16 week-old rats (P<0.05, respectively). But 16 week-old SHR exhibited a lower p-PPARγ expression as compared with 32 week-old rats (P<0.05). Comparison of p-PPARγ expression in 16, 32 and 64 week-old WKY rats, the highest p-PPARγ expression was observed in 64 week-old rats (P<0.05, respectively, Figure 5B), while 16 week-old rats showed the lowest p-PPARγ expression (P<0.05, respectively, Figure 5B).
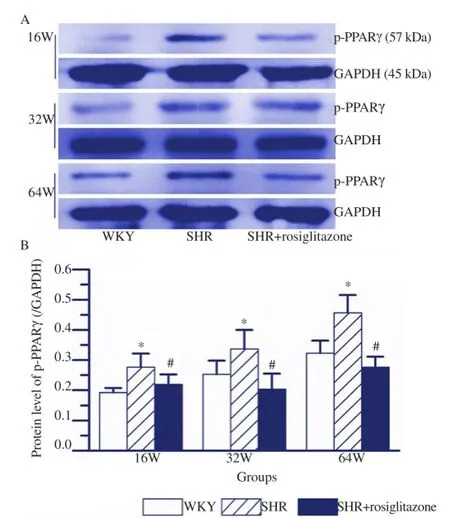
Figure 5.Western blot analysis of p-PPARγ protein in the thoracic aorta of rats in different groups.A: Representative western blot analysis of p-PPARγ expression in the thoracic aorta of rats in different groups; B: Quantification of the data revealed that p-PPARγ expression in SHR group was higher than that in WKY group. However, SHR+rosiglitazone group showed a less p-PPARγ level as compared with SHR group. Values are depicted as mean±SEM,n=6.*P<0.05vsWKY group;#P<0.05vsSHR group.
4. Discussion
Vascular aging is mainly characterized as characteristic remodeling of the structure and function by the increase of age, which is an independent risk factor for promoting the incidence of hypertension[10,11]. In this study, SBP were measured using a tail cuff method. Our data indicated that the SBP in SHR was elevated by the increase of age. HE staining showed that the histopathological changes including increased content of smooth muscle cell, wrinkled lining endothelium and increased thickness of internal elastic lamina in the thoracic aorta of SHR were worsened by the increase of age. These results indicate that vascular aging is observed in the thoracic aorta of SHR. PPARγ acts as a nuclear transcription factor for regulating several senescenceassociated genes[3-6]. PPARγ phosphorylation is an important post-transcriptional modification and it has been found in many aging diseases[7-9]. However, whether PPARγ phosphorylation is presented in vascular aging induced hypertension has not been investigated. We, for the first time, approved that p-PPARγ was observed in the thoracic aorta of 16, 32 and 64 week-old SHR by the increase of age, which was consistent with vascular aging in the thoracic aorta. These results suggest that PPARγ phosphorylation is enhanced during hypertension related vascular aging. But the priority of PPARγ phosphorylation or vascular aging needs to be further confirmed.
Different phosphorylated residues of PPARγ in different cells and under different stimulations exert different biological effects[16]. Mitogen-activated protein kinases induce the phosphorylation of PPARγ on Ser112 and inhibit PPARγ activity[17-19]. However, cyclindependent kinase 7 (Cdk7) and Cdk9 lead to phosphorylation of PPARγ on Ser112 and increase PPARγ activity[20,21]. Otherwise, Cdk5 mediates Ser273 phosphoryaltion, which is located at hinge region between PPARγ DNA binding domain and ligand binding domain, to regulate target gene expression[12]. Our studies just detected the level of p-PPARγ with Ser273 phosphorylation. Thus, the level of p-PPARγ with Ser112 phosphorylation still needs further studies. Previous studies have approved that PPARγ agonist, rosiglitazone, inhibits Cdk5 mediated phosphorylation of PPARγ on Ser273 in adipocytes and is independent of canonical receptor transcriptional activation signaling pathway[12]. Our studies further confirmed that rosiglitazone inhibits the phosphorylation of PPARγ on Ser273 in the thoracic aorta of SHR. But further researches are needed to confirm that the effect is mediated by Cdk5 pathway or not. Otherwise, PPARγ functions as a nuclear transcription factor for regulating many target gene expression. Whether rosiglitazone binds to PPARγ and regulates phosphorylation related target gene expression to inhibit PPARγ phosphorylation still need to be approved.
In conclusion, PPARγ phosphorylation is observed in the thoracic aorta of SHR during vascular aging. Furthermore, the level of p-PPARγ protein is up-regulated by the increase of age. Rosiglitazone inhibits PPARγ phosphorylation and vascular aging. These results contribute to revealing the mechanisms involved in hypertension vascular aging.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No. 81070219).
[1] Houseknecht KL, Cole BM, Steele PJ. Peroxisome proliferator-activated receptor gamma (PPARgamma) and its ligands: a review.Domest Anim Endocrinol2002;22(1): 1-23.
[2] Monsalve FA, Pyarasani RD, Delgado-Lopez F, Moore-Carrasco R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases.Mediators Inflamm2013; doi: 10.1155/2013/549627.
[3] Erol A. The functions of PPARs in aging and longevity.PPAR Res2007; doi: 10.1155/2007/39654.
[4] Speeckaert MM, Vanfraechem C, Speeckaert R, Delanghe JR. Peroxisome proliferator-activated receptor agonists in a battle against the aging kidney.Ageing Res Rev2014;14: 1-18.
[5] Chung JH, Seo AY, Chung SW, Kim MK, Leeuwenburgh C, Yu BP, et al. Molecular mechanism of PPAR in the regulation of age-related inflammation.Ageing Res Rev2008;7(2): 126-136.
[6] Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, et al. Klotho is a target gene of PPAR-gamma.Kidney Int2008;74(6): 732-739.
[7] Ulrich-Lai YM, Ryan KK. PPARγ and stress: implications for aging.Exp Gerontol2013;48(7): 671-676.
[8] Sanguino E, Roglans N, Rodríguez-Calvo R, Alegret M, Sánchez RM, Vázquez-Carrera M, et al. Ageing introduces a complex pattern of changes in several rat brain transcription factors depending on gender and anatomical localization.Exp Gerontol2006;41(4): 372-379.
[9] Zheng F, Zhang S, Lu W, Wu F, Yin X, Yu D, et al. Regulation of insulin resistance and adiponectin signaling in adipose tissue by liver X receptor activation highlights a cross-talk with PPARγ.PLoS One2014;9(6): e101269.
[10] Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease.Circulation2003;107(1): 139-146.
[11] Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension.Biomed Res Int2014; doi: 10.1155/2014/406960.
[12] Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5.Nature2010;466(7305): 451-456.
[13] Tu K, Zheng X, Zhou Z, Li C, Zhang J, Gao J, et al. Recombinant human adenovirus-p53 injection induced apoptosis in hepatocellular carcinoma cell lines mediated by p53-Fbxw7 pathway, which controls c-Myc and cyclin E.PLoS One2013;8(7): e68574.
[14] Fritz M, Rinaldi G. Blood pressure measurement with the tail-cuff method in Wistar and spontaneously hypertensive rats: influence of adrenergic- and nitric oxide-mediated vasomotion.J Pharmacol Toxicol Methods2008;58(3): 215-221.
[15] Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, et al. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma.Mol Cancer2014;13: 110.
[16] Floyd ZE, Stephens JM. Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ.Biochim Biophys Acta2012;1822(7): 1090-1095.
[17] Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma.Science1996;274(5295): 2100-2103.
[18] Katsura S, Okumura T, Ito R, Sugawara A, Yokoyama A. Identification of posttranslational modifications in peroxisome proliferatoractivated receptor γ using mass spectrometry.PPAR Res2014; doi: 10.1155/2014/468925.
[19] Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma.Nature1998;396(6709): 377-380.
[20] Corona JC, de Souza SC, Duchen MR. PPARγ activation rescues mitochondrial function from inhibition of complex I and loss of PINK1.Exp Neurol2014;253: 16-27.
[21] Silva IAL, Cox CJ, Leite RB, Cancela ML, Conceição N. Evolutionary conservation of TFIIH subunits: implications for the use of zebrafish as a model to study TFIIH function and regulation.Comp Biochem Physiol B Biochem Mol Biol2014;172-173: 9-20.
10.12980/APJTB.4.201414B416
*Corresponding author: Hai-Feng Yuan, M.D., Department of Encephalopathy, the Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, China.
Tel: +86-13572234796
E-mail: yuanhaifeng323@163.com
Foundation Project: Supported by a grant from the National Natural Science Foundation of China (Grant No. 81070219).
Methods:16, 32 and 64 week-old Wistar-Kyoto rats (WKY) and SHR were randomly and respectively divided into WKY, SHR and SHR+rosiglitazone group (9 in each group). The rats in SHR+rosiglitazone group were treated with rosiglitazone (5 mg/kg, intragastrically) for 56 d, whereas normal saline was applied in WKY and SHR groups. Systolic blood pressure (SBP) of rats was measured by tail cuff method. Histopathological damage of thoracic aorta was analyzed using Hematoxylin-Eosin (HE) staining. Immunohistochemical staining and western blot were performed to test the level of p-PPARγ protein in the thoracic aorta arising from each group.
Results:The SBP in 16, 32 and 64 week-old SHR were significantly higher as compared with those in matched WKY rats (P<0.05, respectively). HE staining showed increased content of smooth muscle cell, wrinkled lining endothelium and increased thickness of internal elastic lamina in the thoracic aorta of SHR. Immunohistochemical staining and western blot indicated that the levels of p-PPARγ in the thoracic aorta arising from SHR were obviously higher than those in the thoracic aorta arising from WKY rats (P<0.05, respectively). Importantly, the high SBP, histopathological abnormalities of the thoracic aorta and elevated p-PPARγ expression were prominently abrogated by rosiglitazone treatment in SHR (P<0.05, respectively). Furthermore, the SBP, histopathological abnormalities of the thoracic aorta and p-PPARγ expression were positively correlated with age in SHR (P<0.05, respectively).
Conclusions:The PPARγ phosphorylation was observed in the thoracic aorta of SHR and its expression was increased by the increase of age. Furthermore, rosiglitazone inhibited the PPARγ phosphorylation and suppressed vascular aging in SHR.
 Asian Pacific Journal of Tropical Biomedicine2014年12期
Asian Pacific Journal of Tropical Biomedicine2014年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Changing trends of cardiovascular risk factors among Indians: a review of emerging risks
- An unusual cause of optic neuritis: rickettsiosis disease
- Seroprevalence of syphilis in patients attending a tertiary care hospital in Southern India
- Histopathological and molecular study of Neospora caninum infection in bovine aborted fetuses
- Genetic polymorphisms of GSTM1, GSTP1 and GSTT1 genes and lung cancer susceptibility in the Bangladeshi population
- In vitro germination and propagation of a threatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed
