In vitro germination and propagation of a threatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed
Shreeti Pradhan, Babulal Tiruwa, Bijay Raj Subedee, Bijaya Pant
Plant Biotechnology and Biochemistry Laboratory, Central Department of Botany, Tribhuvan University, Kirtipur, Kathmandu, Nepal
In vitro germination and propagation of a threatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed
Shreeti Pradhan, Babulal Tiruwa, Bijay Raj Subedee, Bijaya Pant*
Plant Biotechnology and Biochemistry Laboratory, Central Department of Botany, Tribhuvan University, Kirtipur, Kathmandu, Nepal
ARTICLE INFO
Article history:
Received 17 Jul 2014
Received in revised form 9 Sep 2014
Accepted 10 Oct 2014
Available online 24 Oct 2014
Media
Propagation
Protocorms
Encapsulation
Artificial seed
Objective:To study thein vitrogermination and plantlet regeneration from artificial seeds ofCymbidium aloifolium(C. aloifolium), a highly threatened medicinal orchid of Nepal.
1. Introduction
Orchids represent the most evolved and one of the largest groups among the angiosperm. They are of immense horticultural as well as medicinal importance which fetches a very high price in the international market. They also play a very useful role to balance the forest ecosystems. Demand for high quality of orchids has been increasing day by day due to their popularity in horticulture industry[1]. A single orchid capsule contains millions of seeds, whichlack any metabolic machinery and do not have any endosperm. In spite of a very large number of seeds produced, only few seeds germinate in nature as they require specific fungus[2]. Currently, wild orchid population decreases rapidly due to biodiversity loss, illegal trade and consumption by local people. Therefore, the development of an artificial means of propagation is needed to reduce collection pressures on wild population. Nowadays, encapsulation technique for producing artificial seeds has become an important asset in micropropagation[3].
Cymbidium aloifoliumL. Sw (C. aloifolium) is one of the highly valuable and threatened medicinal orchids of Nepal. The leaves of this species are extensively used for styptic properties in the treatments of boils and fevers. The roots are used to cureparalysis and chronic illness. The whole plant can also be used as tonic and in the treatment of vertigo, weakness of eyes, burns and sores[4-7]. Besides its medical importance, this orchid also attracts the floriculture market because of its long-lasting highly attractive beautiful flowers. Due to its various uses, people uprooted this plant from wild and hence reach to the threatened category[8]. Thus, an attempt was made to produce artificial seeds ofC. aloifolium, their short term storage and mass propagation within a short span of time by using tissue culture technique. Asymbiotic germination of artificial seed through protocorms provides a useful way to reestablish plants in the wild for germplasm preservation as well as for commercial propagation.
2. Materials and methods
2.1. Source of explants
Protocorms derived fromin vitroculture of seeds ofC. aloifoliumwere used as explants in present study. The undehisced capsules ofC. aloifoliumwere harvested from nature at an elevation of 500 m from tropical region of Central Nepal[8]. Capsule contains a large number of orchid seeds. The freshly collected capsules were first thoroughly washed under running tap water for at least 30 min to remove the external particles attached to it. They were then washed with detergent Tween 20 (0.1%) for 15 min and again washed with tap water until all detergent washed clearly. After that capsules were surface sterilized by dipping it in 70% alcohol for 2 min followed by 1% sodium hypochlorite solution for 15 min and were subsequently rinsed in sterile water for at least three times. The sterilized capsules were dried on Whatman filter paper and dissected longitudinally with the help of sterilized surgical blade to expose the powdery seeds. Seeds were scooped out and spread thinly over the surface of Murashige and Skoog (MS) basal medium under aseptic condition. Protocorms were started to develop after 10 weeks of culture of seeds and 21-days old protocorms were selected as primary explants for present study.
2.2. Germination medium
In vitrogermination and subsequent development of artificial seed was assessed on different strength of liquid MS medium[9],i.e.full (1.0), half (0.5) and quarter (0.25) and full-strength MS liquid media supplemented with different concentration of plant growth regulatorsviz.0.5 mg/L benzyl amino purine (BAP) and 0.5 mg/L naphthalene acetic acid (NAA) (Table 1). MS medium was fortified with 3% sucrose as carbon source without solidified with agar. The pH of MS media was adjusted to 5.8 before autoclaving. About 16-20 mL of media was dispensed into each culture tube (150×25 mm, Borosil) and autoclaved at pressure 15 psi and temperature of 121 °C for 20 min. All cultures were maintained at (25±2) °C under 500-1 000 lux illuminance for 16/8 h. (light/dark) photoperiod using cool white light (Philips, India).
2.3. Gel matrix and encapsulation
Protocorms were used as primary explants to produce artificial seeds. For encapsulation of protocorms, sodium alginate was used with calcium chloride dihydrate for complexation. Sodium alginate of 4% and 0.2 mol/L of calcium chloride dihydrate (CaCl2.2H2O) solution were prepared separately by dissolved in sterile water. Individual protocorms were separated and mixed in 4% sodium alginate matrix. Alginate matrix containing single protocorm were taken up with the help of sterile micropipette and then gently dropped into 0.2 mol/L CaCl2.2H2O solution. The drops each containing single protocorm were left in CaCl2.2H2O solution for at least 30 min to harden the alginate beads. After that, the alginate beads were washed with sterile water for three times and blot dried. The alginate beads were then called as artificial seeds or synthetic seeds or encapsulated seeds. Artificial seeds [(3±1) mm diameters] were placed on sterile filter paper containing sterile glass petriplates (30 beads per plate), sealed with parafilm and stored at 4 °C or used immediately. Freshly prepared artificial seeds were inoculated on different strength of MS liquid mediumi.e.0.25 MS, 0.5 MS, 1.0 MS, and 1.0 MS liquid medium supplemented with 0.5 mg/L BAP and 0.5 mg/L NAA for their germination and regeneration (Table 1). The cultures were kept at (25±2) °C under 16/8 h photoperiod from cool-white-light in culture room.
2.4. Sterilization and viability test of storage artificial seed
Artificial seeds stored at 4 °C were regularly taken out at regular intervals of 7 d till 28th d and were inoculated on culture tubescontaining hormone free MS liquid medium in order to test their germination percentage. All the inoculation process was done in laminar air flow cabinet. Before inoculation, storage artificial seeds were surface sterilized in different ways to reduce the chance of contamination. Different sterilization treatments were named as T1, T2, T3, T4and T5. In each treatment, total 10 artificial seeds were used (Table 2).
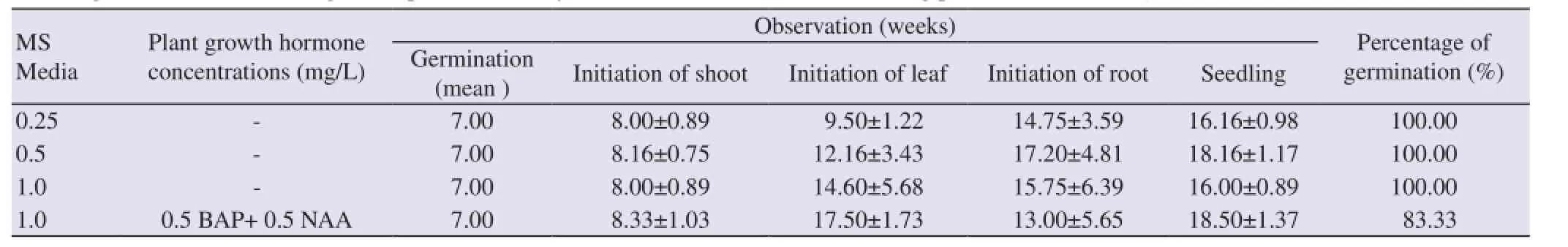
Table 1In-vitrogermination and seedling development of freshly inoculated artificial seeds containing protocorms ofC. aloifolium(L.) Sw.
Storage artificial seeds were directly inoculated on medium and this treatment was designated as T1(Control).
Some artificial seeds were first washed with sterile water, dried on blotting paper and inoculated on medium. This treatment was designated as T2.
Some artificial seeds were washed with fungicide bavistine (0.1%) for 5 min and then rinsed with sterile water. After that, artificial seeds were dried on sterile blotting paper and finally inoculated on medium. This treatment was designated as T3.
Some artificial seeds were first dipped into fungicide bavistine (0.1%) for 5 min, then surface sterilized with sodium hypochlorite (1%) solution for 5 min followed by 70% ethyl alcohol for 2 min. They were then rinsed with sterile water and soaked on sterile blotting paper. Finally, artificial seeds were ready for inoculation on medium and this treatment was designated as T4.
Some artificial seeds were first surface sterilized with sodium hypochlorite (1%) solution for 5 min followed by 70% ethyl alcohol for 2 min. Then, artificial seeds were rinsed with sterile water and soaked on sterile blotting paper. Finally, artificial seeds were inoculated on medium and this treatment was designated as T5.
After surface sterilization treatments, storage artificial seeds were inoculated on culture tubes containing hormone free MS liquid medium. Then, they were transferred in culture room where temperature was maintained at (25±2) °C and photoperiod of 16/8 h. The cultures were observed regularly till complete germination and development of shoot, leaves and roots from storage artificial seeds.
2.5. Hardening
Plantlets regenerated fromin vitroculture of artificial seeds ofC. aloifoliumwere used for hardening process. Plantlets with well developed shoots and roots in culture vessels were opened up and kept in culture room for one week before transferring to earthen pots. Plantlets were then taken out from culture vessels and washed thoroughly with running tap water to remove the traces of medium without causing harm to roots. These plantlets were treated with 0.1% fungicide (bavistin) solution for 5 min and again washed with sterile water. After washing, they were blot dried and finally transferred to an earthen pot containing potting mixture of cocopeat, litter and sphagnum moss in a ratio of 2:1:1. The potted plants were watered once a day and fertilized at weekly intervals with a foliar spray of a mixture of nitrogen, phosphorous and potassium (20:10:10). The potted plants were covered with a perforated plastic bag to maintain the humidity and kept under greenhouse until the seedlings were established. They were observed regularly.
2.6. Stastical analysis
Each treatment consisted of at least 6 explants and each experiment was repeated twice. Statistical analysis was done using One-way ANOVA classification system. The data obtained were analyzed using application software-Microsoft excel. The significant difference between the MS medium and MS medium supplemented with different growth hormones were analysed atP≤0.05 using SPSS version 16.0 (SPSS Inc. USA).
3. Results
Germination of orchid seeds were favoured by different factorsviz.explants, media, pH, growth hormones and culture condition.C. aloifoliumis one of the high value medicinal orchids of Nepal. In the present study, protocorms were used as explants for production and germination of artificial seed. Sodium alginate of 4% and 0.2 mol/L of CaCl2.2H2O solution were actively participated on production of artificial seed (Figure 1A).
Each freshly prepared artificial seed containing protocorm was cultured on different strength of MS liquid mediaviz.0.25, 0.5, 1.0 and 1.0 MS liquid medium supplemented with 0.5 mg/L BAP and 0.5 mg/L NAA (Table 1). Each test consisted six replicates. Germination of freshly inoculated artificial seed was started after 7 weeks of culture in all the condition. Artificial seed containing protocorm first undergoes swelling, become more greening and directly gave rise to shoot bud without undergoing multiplication of protocorms (Figure 1B). First shoot primordia was developed after 8 weeks of culture in all the conditions while first leaf primordia were developed after 9 weeks of culture on 0.25 MS and first root primordia was observed after 13 weeks of culture on 1.0 MS+0.5 mg/L BAP+0.5 mg/L NAA.
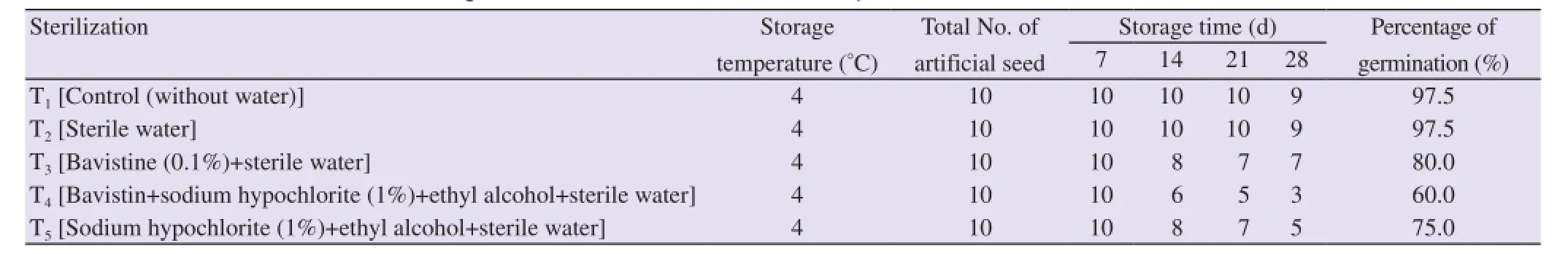
Table 2 Effect of different sterilization treatments on germination of artificial seeds ofC. aloifolium.
In present study, it was found that 0.25 MS medium was effective for earlier shoot, leaf and root initiation which took 8, 9 and 14 weeks of culture respectively (Figure 1C). This condition was followed by 0.5 MS, 1.0 MS and 1.0 MS supplemented with 0.5 mg/L BAP and 0.5 mg/L NAA. While complete seedling was first observed on 1.0 MSi.e.after 16 weeks of culture, rather than 0.25 MS (Table 1). Large shoot multiplication was also found on hormone free 1.0 MS medium (Figure 1D). After 24 weeks of culture, plantlets of 5-6 cm height with well developed root system were selected for hardening. These plantlets were potted in earthen pots containing cocopeat, litter and sphagnum moss in 2:1:1 ratio and covered with perforated plastic bags to maintain require humidity (Figure 1E). About 85% plantlets were survived in this condition.

Figure 1.Production, germination and regeneration of plantlet from artificial seed ofC. aloifolium.
Also, some freshly prepared artificial seeds were stored at 4 °C for 28 d to check their viability percentage. All the storage artificial seeds showed viability on hormone free MS liquid medium. The highest viability percentage was observed on storage artificial seeds treated with T1and T2i.e.97.5% of germination whereas T3, T4and T5showed 80.0%, 60.0% and 75.0% of germination respectively (Table 2). Out of five different treatments, T1and T2were found to be the best ways for plant conversion from storage artificial seeds followed by T3. Viability percentage was observed on the basis of time taken and survivality of artificial seeds during germination and seedling development. In present study, it was found that artificial seeds stored for 7 d showed 100% germination in all the sterilization treatments.
4. Discussion
All the tested conditions responded positively on germination and plantlet development of freshly inoculated artificial seed containing protocorms ofC. aloifolium. All the strength of MS liquid mediumviz.0.25 MS, 0.5 MS and 1.0 MS showed 100% germination of artificial seeds while 1.0 MS liquid medium supplemented with 0.5 mg/L BAP and 0.5 mg/L NAA showed only 83.33% of germination.
In nature, only 2%-5% of orchid seeds were germinated due to their symbiotic requirement of fungus Rhizectonia[10]. Among tested conditions, hormone free 1.0 MS liquid medium was found to be the most effective condition for complete conversion of artificial seed into seedling after 16 weeks of culture. Zhanget al. (2011) found that protocorms were the best propagator for artificial seed production inDendrobium candidium[11]. Pradhanet al. (2013) reported that non- encapsulated protocorms took 30 weeks of culture for complete conversion into seedling on 1.0 MS medium which is longer time than in the present study[12]. This suggested that artificial seeds containing protocorms gives earlier response than nonencapsulated protocorms. It may be due to the presence of alginate complex that enhance the growth of artificial seed.
The requirement of exogenous auxins and/or cytokinins for regeneration of protocorm like bodies (PLBs) or shoot buds and plantlet development has been reported for many orchid species[13]. However, the combinations, concentrations, and the ratio between them are usually critically important. The ratio of auxin to cytokinin for shoot buds or PLBs formation varies from species to species. In present investigation, the synergistic effect of BAP and NAA along with MS supplemented media delayed the development of seedling from artificial seed ofC. aloifolium.
The shoots emergence from artificial seed grown continuously by breaking through the capsule of artificial seed. In present study, complete seedling was achieved after 16-19 weeks of culture in all tested conditions. One-hundred percent of conversion of artificial seed into whole plantlet was found in all conditions except hormone treated medium. This finding was supported by Corrie and Tandon (1993) who reported 100% conversion of encapsulated PLBs into plantlet underin vitrocondition inCymbidium giganteum[14]. Similarly, Sarmahet al. (2010) producedVanda coeruleasynseeds by encapsulating PLBs regenerated from the leaf base with a 94.9 % conversion frequency[15]. Naganandaet al. (2011) encapsulated the PLBs ofFlickingeria nodosaand achieved 95% conversion after 3 months’ storage at 4 °C[16]. Gantaitet al. (2012) obtained 96.4% conversion in alginate- encapsulatedAranda×VandaPLBs with 3% sodium alginate and 75 mmol/L calcium chloride[17]. The present result was in contrast with the findings obtained by Saiprasad and Polisetty (2003) who obtained 100% conversion of encapsulated PLBs when cultured on MS medium supplemented with BA and NAA (Dendrobium) or NAA alone (OncidiumandCattleya)[18].
Artificial seeds also present an excellent way to store orchid material at room temperature, under cold storage, or even cryopreservation for weeks to months, or even years while maintaining the clonal stability of the material[19]. The germinationpercentage of storage artificial seed was affected by composition of encapsulation matrix and duration of pre-germination storage[20]. Artificial seeds stored at 4 °C in present study, were tested their viability rate after every 7 d upto 28th d. Total 10 stored artificial seeds were inoculated on hormone free full strength of MS liquid media in each interval of 7 d under five different treatments. It was found that as the storage time increases, chances of contamination also increases which ultimately lowers the survival rate of storage artificial seeds. Use of fungicide like bavistine can enhance the growth of artificial seed by reducing the chances of contamination. Therefore, in present investigation, low concentration of bavistinei.e.0.1% was used which comparatively gave satisfactory responsed on germination and plantlet development from storage artificial seeds. This result was supported by Butset al. (2013) who reported that below 0.5% concentration of bavistine was effective for growth and yield ofVigna radiate[21]. Similarly, Ramsehet al.(2009) observed the maximum shoot length [(8.20±0.37) cm] from encapsulated node cuttings incorporated with 3.0 mg/L bavistin on MS basal medium[22]. Aggarwalet al. (2005) also found that high doses of fungicide like bavistine (above 0.5%) caused remarkable adverse effect on growth of sun flower plant[23]. However, combine effect of bavistine, sodium hypochlorite (1%) and ethylalcohal (70%) decline the germination rate by earlier rupturing of calcium alginate beads.
One-hundred percent of germination was achieved when artificial seeds were stored for 7 d. Later, germination percentage was gradually decreased upto 66% when stored for 28 d at 4 °C in all five treatments. But, when germination pattern was compared between different treatments, T1and T2were found to be the most effective sterilization techniques for regeneration of contamination free plant from alginated encapsulated artificial seeds stored at 4°C. They showed 97.5% of germination which was the highest percentage in present investigation as compare to the other treatments. It may be due to the less ion exchange occurred between calcium alginate beads and sterile water which not only lowers the risk of contamination but also protects the protocorm in gel for longer period.
According to Zhanget al., 2011, protocorms stored large nutrients hence they are capable to undergo successive phages of division and give rise to plant even when no nutrients was added to artificial seed[11]. Similar results were also obtained in present study. Several workers have tried various medium and various concentrations of growth hormones to promote artificial seed germination and seedling growth of different orchids[15,18,24-26].
Higher germination percentage (100%) in case of artificial seeds without storage could be due to the matrix which not only facilitates regular nutrient supply but also protects the dispersed, delicate tissue from any mechanical injury during handling and from desiccation[27,28]. Artificial seeds inoculated on MS liquid medium free of growth regulators eventually developed into multiple plantlets within 24 weeks of culture. Complete seedlings were started to develop after 16 weeks of culture on this medium. Later, thein vitroraised seedlings developed from artificial seeds were successfully established in the potting medium. The survival rate of plantlets ofC. aloifoliumwas reached about 85% in the greenhouse.
Artificial seeds were successfully produced by encapsulating the individual protocorms in calcium alginate beads for mass propagation, storage and conservation. Hormone free full strengthi.e.1.0 MS liquid medium in the present study was found to be more effective for germination and plantlet regeneration from artificial seed ofC. aloifolium. Artificial seeds containing protocorms gave 97.5% germination upto 28th d of pre-germination storage period. A significant outcome of the present study was the observation that the synthetic seeds retained their viability even after 28th d at 4 °C and the plants regenerated also maintained their morphological identity. Hence, the present paper is expected to be a novel research which open new vista in germplasm preservation, short term storage and large volume propagation of a threatened medicinal orchid,C. aloifoliumin the form of artificial seed.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
Authors are grateful to Central Department of Botany, Tribhuvan University, Kirtipur, Nepal for providing all laboratory facilities for this investigation. We are also highly acknowledged to Nepal Academy of Science and Technology, Khumaltar, Lalitpur, Nepal for providing the partial financial support (Grant No. 443/66/67) to carry out the present research work.
[1] Chugh S, Guha S, Rao, IU. Micropropagation of orchids: a review on the potential of different explants.Sci Hortic2009;122(4): 507-520.
[2] Kalimuthu K, Senthilkumar R, Vijayakumar S.In vitromicropropagation of orchid,Oncidiumsp. (Dancing Dolls).Afr J Biotechnol2007;6(10): 1171-1174.
[3] Taha RM, Saleh A, Mahmad N, Hasbullah NA, Mohajer S. Germination and plantlet regeneration of encapsulated microshoots of aromatic rice (Oryza sativaL. Cv. MRQ 74).Scientific World Journal2012; doi: 10.1100/2012/578020.
[4] Das PK, Sahoo S, Bal S. Ethnobotanical studies on orchids of Niyamgiri Hill Ranges, Orissa, India.Ethnobot Leaflets2008;12: 70-78.
[5] Hossain MM, Sharma M, Pathak P. Cost effective protocol forin vitromass propagation ofCymbidium aloifolium(L.) Sw.-a medicinally important orchid.Eng Life Sci2009;9(6): 444-453.
[6] Nongdam P, Chongtham N.In vitrorapid propagation ofCymbidium aloifolium(L.) Sw.: a medicinally important orchid via seed culture.J Biol Sci2011;11(3): 254-260.
[7] Pant B, Raskoti BB.Medicinal orchids of Nepal. Nepal: Himalayan Map House Pvt. Ltd.; 2013.
[8] Raskoti BB.The orchids of Nepal. Nepal: Bhakta Bahadur Raskoti and Rita Ale; 2009.
[9] Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures.Physiol Plant1962;15(3): 473-497.
[10] Rao AN. Tissue culture in orchid industry. In: Reinert J, Bajaj YPS, editors.Applied and fundamental aspects of plant cell, tissue and organ culture.Berlin: Springer-Verlag; 1977, p. 46-49.
[11] Zhang YF, Yan SQ, Zhang Y. Factors affecting germination and propagators of artificial seeds ofDendrobiumcandidum.Adv Biomed Eng2011;1-2: 404-410.
[12] Pradhan S, Regmi T, Parmar G, Pant B. Effect of different media onin vitroseed germination and seedling development ofCymbidium aloifolium(L.) Sw.Nepal J Sci Technol2013;14(1): 51-56.
[13] Arditti J, Ernst R.Micropropagation of orchids. NewYork: John Wiley and Sons; 1993.
[14] Corrie S, Tandon P. Propagation ofCymbidium giganteumWall, through high frequency conversion of encapsulated protocorms underin vivoandin vitroconditions.Indian J Exp Biol1993;31: 61-64.
[15] Sarmah DK, Borthakur M, Borua PK. Artificial seed production from encapsulated PLBs regenerated from leaf base ofVanda coeruleaGrifft. ex. Lindl.--an endangered orchid.Curr Sci2010;98(5): 686-690.
[16] Nagananda GS, Satishchandra N, Rajath S. Regeneration of encapsulated protocorm like bodies of medicinally important vulnerable orchidFlickingeria nodosa(Dalz.) Seidenf.Int J Bot2011;7: 310-313.
[17] Gantait S, Bustam S, Sinniah UR. Alginate-encapsulation, shortterm storage and plant regeneration from protocorm-like bodies ofArandaWan Chark Kuan ‘blue’×Vanda coeruleaGrifft. ex. Lindl. (Orchidaceae).Plant Growth Regul2012;68(2): 303-311.
[18] Saiprasad GVS, Polisetty R. Propagation of three orchid genera using encapsulated protocorm-like bodies.In Vitro Cell Dev Biol Plant2003;39(1): 42-48.
[19] da Silva JAT. Production of synseed for hybridCymbidiumusing protocorm-like bodies.J Fruit Ornam Plant Res2012;20(2): 135-146.
[20] Siong PK, Mohajer S, Taha RM. Production of artificial seeds derived from encapsulatedin vitromicro shoots of cauliflower,Brassica oleraceavar.botrytis.Rom Biotechnol Lett2012;17(4): 7549-7556.
[21] Buts AK, Singh D, Chaudhary VL, Singh M. Effect of bavistin on seed germination, morphological features and yield ofVigna radiate.Indian J Life Sci2013;3(1): 15-20.
[22] Ramseh M, Marx R, Mathan G, Pandian SK. Effect of bavistin onin vitroplant conversion from encapsulated uninodal microcuttings of micropropagated Bacopa monnieri (L.)-an Ayurvedic herb.J Environ Biol2009;30(3): 441-444.
[23] Aggarwal A, Sharma D, Prakash V, Sharma S, Gupta A. Effect of bavistin and dithane M-45 on theMycorrhizaeandRhizospheremicrobes of sunflower.Helia2005;28(42): 75-88.
[24] Pinker I, Abdel-Rahman SSA. Artificial seeds for propagation ofDendranthema X grandiflora(Ramat.).Propag Ornam Plants2005;5(4): 186-191.
[25] Qin Z, Zhao T, Qiu J, Lin Y, Cai Y. [Germination and propagators of artificial seeds ofDendrobium huoshanense].Sheng Wu Gong Cheng Xue Bao2008;24(5): 803-809.
[26] Mohanty P, Das J. Synthetic seed technology for short term conservation of medicinal orchidDendrobium densiflorumLindl. Ex Wall and assessment of genetic fidelity of regenerants.Plant Growth Regul2013;70: 297-303.
[27] Sharma A, Tandon P, Kumar A. Regeneration ofDendrobium wardianumWarner. (Orchidaceae) from synthetic seeds.Indian J Exp Biol1992;30: 747-748.
[28] Reddy MC, Murthy KSR, Pullaiah T. Synthetic seeds: a review in agriculture and forestry.Afr J Biotechnol2012;11(78): 14254-14275.
10.12980/APJTB.4.2014APJTB-2014-0369
*Corresponding author: Dr. Bijaya Pant, Associate Professor, Central Department of Botany, Tribhuvan University, Kirtipur, Kathmandu, Nepal.
Tel: +977 1 4331322, + 977 9841359626
E-mail: pant_bijaya@yahoo.com
Foundation Project: Partially supported by Nepal Academy of Science and Technology (NAST), Khumaltar, Lalitpur, Nepal (Grant No. 443/66/67).
Methods:Artificial seeds were producedin vitroby encapsulation of protocorms with 4% sodium alginate and 0.2 mol/L calcium chloride solution.In vitrogermination and plantlet regeneration of the artificial seeds were tested by culturing them on different strength of Murashige and Skoog (MS) liquid media (0.25, 0.5 and 1.0) and MS liquid medium supplemented with 0.5 mg/L benzyl amino purine and 0.5 mg/L naphthalene acetic acid. Freshly produced artificial seeds were stored up to 28 d at 4 ºC. In order to check the viability, storage artificial seeds were treated with five different sterilization techniques (T1, T2, T3, T4, T5) and inoculated on full strength (1.0) of MS liquid medium after each 7 d of interval upto 28th days.
Results:The highest percentage of germination (100%) of artificial seed was obtained on quarter (0.25), half (0.5) and full (1.0) strength of MS liquid medium. Experimentally, full strength of MS liquid medium was more effective for earlier seedling development ofC. aloifolium. Artificial seeds were successfully stored at 4 ºC till 28th days. Treatments T1and T2showed 97.5% viability of storage artificial seeds and hence considered as the most effective sterilization techniques to recover the plant from storage artificial seeds. Plantlets developed from artificial seeds were successfully acclimatized in potting mixture containing cocopeat, litter and sphagnum moss with 85% survival rate.
Conclusions:The present study revealed that artificial seeds are the good alternative explants forin vitromass propagation and short term conservation ofC. aloifolium.
 Asian Pacific Journal of Tropical Biomedicine2014年12期
Asian Pacific Journal of Tropical Biomedicine2014年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Changing trends of cardiovascular risk factors among Indians: a review of emerging risks
- An unusual cause of optic neuritis: rickettsiosis disease
- Seroprevalence of syphilis in patients attending a tertiary care hospital in Southern India
- Histopathological and molecular study of Neospora caninum infection in bovine aborted fetuses
- Genetic polymorphisms of GSTM1, GSTP1 and GSTT1 genes and lung cancer susceptibility in the Bangladeshi population
- Expression of p-PPARγ in the aging thoracic aorta of spontaneously hypertensive rat and inhibitory effect of rosiglitazone
