Cu 纳米团簇发光性能和结构的研究
葛梦然 李丹丹 姚 涛 潘国强
(中国科学技术大学 国家同步辐射实验室 合肥 230029)
Cu 纳米团簇发光性能和结构的研究
葛梦然 李丹丹 姚 涛 潘国强
(中国科学技术大学 国家同步辐射实验室 合肥 230029)
利用光致发光谱(Photoluminescence, PL)、X射线吸收精细结构(X-ray Absorption Fine Structure, XAFS)和MALDI-TOF质谱等技术研究了化学还原法合成的Cu金属纳米团簇的发光性能及其结构。PL谱表明随着反应溶液中十二硫醇(C12SH)和2-巯基-5-正丙烷基嘧啶(C7H10N2S, 2-mercapto-5-n-propylpyrimidine, MPP)配体比例的增加,Cu纳米团簇主发光峰的波长从618 nm逐渐蓝移到571 nm。质谱的结果说明以MPP作为单一配体时的主要产物为Cu5[MPP]3;而当为MPP与C12SH两种混合配体时,Cu纳米团簇中Cu原子数变小,组成变为Cu4[MPP][C12SH];并且随着C12SH比例增加,Cu4[MPP][C12SH]产物的组成保持不变。XAFS结果则进一步表明随着C12SH比例的增加,Cu纳米团簇的Cu-S键长从0.228 nm缩短到0.224 nm,原子构型从双三角锥结构转变为四面体。综合以上结果,认为Cu纳米团簇的原子数的减少导致团簇的光致发光从618nm蓝移至597 nm;而Cu-S键长的缩短引起Cu(I)-S杂化的HOMO-LUMO带隙增大,从而导致团簇的发光波长进一步从597 nm蓝移至571 nm。
Cu纳米团簇,发光可调,X射线吸收精细结构(XAFS),同步辐射
金属纳米团簇(尺寸通常小于2 nm)因其独特的物理和化学性质,以及在发光和催化等领域的广泛应用前景,近年来逐渐成为纳米材料研究领域的热点[1-3]。由于特殊的量子尺寸效应,金属纳米团簇的电子能级将呈现出与大的纳米晶所不同的离散型特征,主要体现在光致发光和光吸收的变化[4-7]。长期以来,该领域的研究主要集中在贵金属(如Au、Ag等)纳米团簇的合成和结构表征上,例如Au25(SR)18、Au102(SR)44(SR=巯基配体)等纳米团簇已被合成,并确定其结构[8-9]。然而,由于固有的不稳定性,关于Cu纳米团簇的合成和性能调控研究却很少[8-11],Cu纳米团簇具有独特的电子[12]、光学[13]和催化性能[14],同时作为过渡族金属,储量丰富,实现对Cu纳米团簇的可控合成并对其性能进行深入调控将具有重要的应用价值。
近年来,一些稳定的不同原子个数的Cu纳米团簇合成已经相继被报道[14-16],例如Salorinne等[17]最近成功制备出苯并三唑包覆的CumBTAn(m≤6)纳米团簇,并计算出Cu6BTA4是正四面体的Cu4金属内核被两个BTA-Cu(I)-BTA保护的结构。陈卫课题组[18]利用2-巯基-5-正丙烷基嘧啶作为表面活性剂,通过单相法合成出具有425 nm和593 nm双发射波长的Cun(n≤8)纳米团簇,并在氧还原反应中表现出良好的电催化性能。但是从发光性能来看,目前报道的Cu纳米团簇发光波长无法调节,对发光机理研究也一直是一个难于解决的问题。最近,对纳米团簇发光机理认识有了一定的进展。例如,Wu等[19]基于[Au25(SR)18]q纳米团簇(q是团簇的电荷值)的发光性研究,发现金属纳米团簇的发光主要来自于团簇表面配体-金属作用和金属内核。
本文通过调节初始配体2-巯基-5-正丙烷基嘧啶(MPP)和十二硫醇(C12SH)的比例,合成出从618nm到571 nm可控发光的Cun(n≤6)纳米团簇。利用紫外可见吸收光谱(Ultraviolet-visible spectroscopy, UV-vis)、基质辅助激光解吸-飞行时间质谱仪(Matrix Assisted Laser Desorption Ionization Time of Flight Spectrometer, MALDI-TOF)质谱和X射线吸收精细结构(X-ray Absorption Fine Structure, XAFS)技术对不同初始比例生成的Cu纳米团簇的结构进行研究,利用最小二乘法对XAFS函数FT(k3χ(k))进行模拟计算,明确初始配体中C12SH量对Cu纳米团簇的结构影响,并了解其发光机理。
1 实验
硝酸铜、硼氢化钠购于上海国药化学;四正辛基溴化铵购于阿拉丁;MPP购于Alfa Aesar。可控发光的Cu纳米团簇的合成方法基于对已有的方法进行改进得到[18]。硝酸铜(0.025 g,0.103 mmol)和四正辛基溴化铵(0.136 g,0.249 mmol)共溶在乙醇中(25 mL),再将其在80 °C环境下搅拌30 min。然后把该溶液在冰水中冷却后加入MPP或MPP和C12SH的初始混合配体。溶液在氩气流下剧烈搅拌反应6 h后加入硼氢化钠(0.047 g,1.241 mmol)和乙醇(5 mL)继续反应7 h。经离心分离10 min、转速16 000 r·min-1和乙醇洗涤多次,最后将沉淀的产物再分散在乙醇中以供进一步使用。
Cu纳米团簇的MALDI-TOF质谱在Bruker Autoflex III质谱仪上测得,基质采用反式-2-[3-(4-叔丁基苯基)-2-甲基-2-丙烯]丙二腈(DCTB),与样品摩尔比例是1:1000,测量范围:500-1100 Da(避免基质在465 Da的峰)。Cu纳米团簇的Cu边X射线吸收谱在北京同步辐射装置(Beijing Synchrotron Radiation Facility, BSRF)1W1B线站测得,储存环电子能量为2.5 GeV,最大电流强度为250 mA。XAFS实验数据通过IFEFFIT的Athena分析后,利用IFEFFIT的Artermis进行拟合。
2 结果与讨论
图1(a)展示不同初始配体比例的Cu纳米团簇在365 nm激发光下的光致发光谱(Photoluminescence, PL)及其白光下照片(最大发射波长和吸收峰位置归纳见表1)。由图1(a)可知,样品的最大发光波长位置随着初始配体中C12SH比例的增大从618 nm逐渐蓝移到571nm。由于反应中的其他参数都保持一致,表明Cu纳米团簇发光波长的改变是由于初始配体比例的变化导致的。此外,自然光下的Cu纳米团簇的溶液颜色也随着初始配体中C12SH比例的增大从深黄色变到淡黄色。根据陈卫等[18,20]对Cu纳米团簇的电子结构分析可知,Cu纳米团簇通常会表现出400 nm和600 nm附近的两种发光,其中400 nm附近的发光来源于sp带到d带的跃迁,而600 nm附近的发光来源于sp带内HOMO-LUMO 跃迁。因此,这里的PL谱蓝移可能归因于HOMO-LUMO带隙的变化。
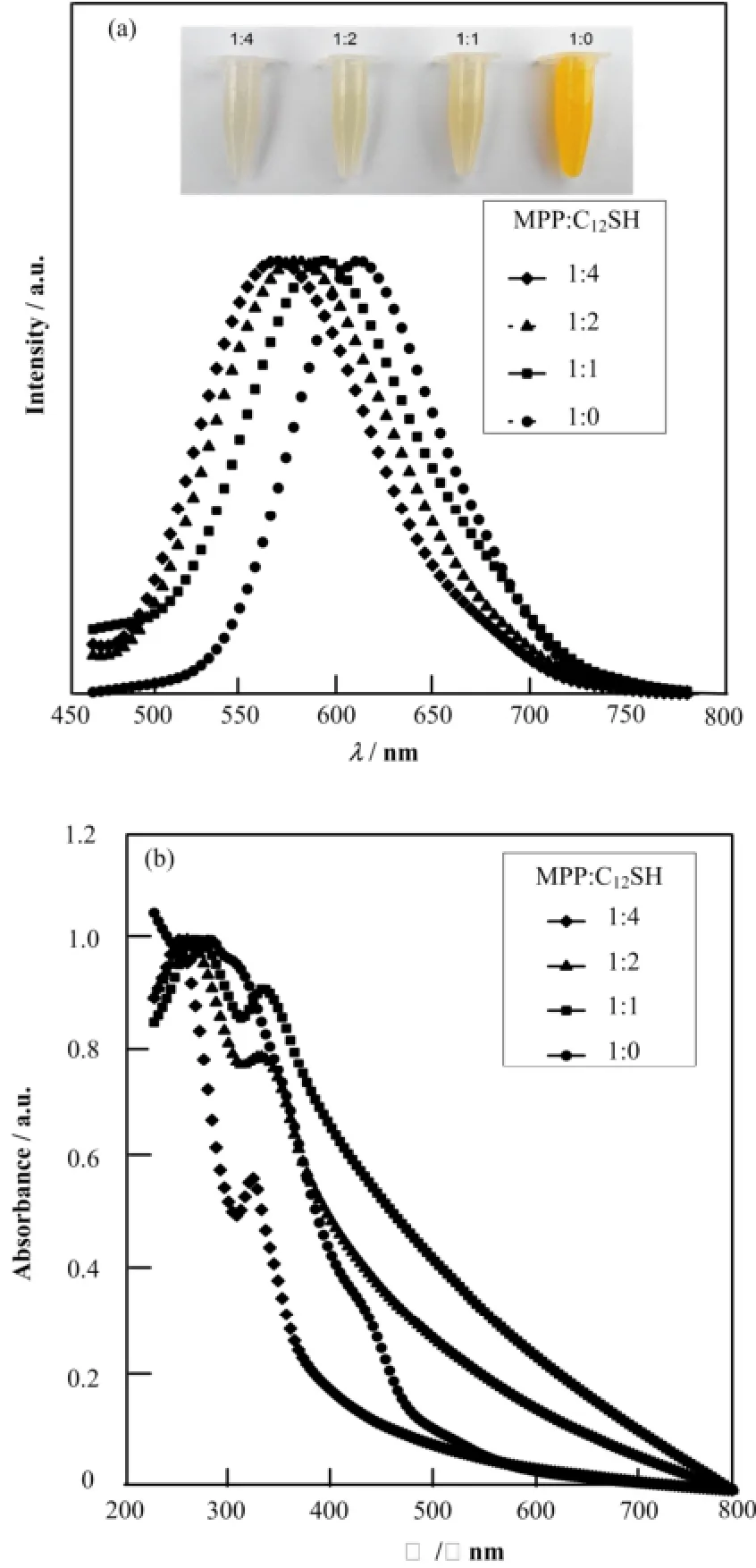
图1 不同初始配体比例样品光致发光谱(激发波长365 nm)和白光下照片(a)以及UV-vis吸收谱(b)Fig.1 Photoluminescence spectra (excited at 365 nm) and optical photograph (a) and UV-vis absorption spectra of the samples (b).

表1 Cu纳米团簇合成参数及相应的吸收峰位置、最大发光峰位置Table 1 Synthesis parameters, absorption peaks and emission peaks of copper clusters.
图1(b)显示了不同初始配体比例的Cu纳米团簇的UV-vis吸收谱,未发现存在560 nm附近的Cu纳米晶体的特征表面等离子共振(Surface Plasmon Resonance, SPR)吸收峰和由Cu2+的d-d跃迁产生的750 nm附近处的特征吸收峰,证实了团簇尺寸较小(小于4 nm),并且样品中的Cu原子至少被还原到+1价[17,21-22]。随着初始配体中C12SH所占比例的增加,所有样品在350 nm和280 nm附近的吸收峰发生蓝移,这两个吸收峰已被确定来自于Cu纳米团簇的d带到sp带的带间吸收跃迁[18,20],这说明初始配体比例的不同会对Cu纳米团簇的电子结构产生影响。因此,PL谱中的蓝移主要来源于产物的电子结构变化。由于金属纳米团簇的电子结构和团簇的化学组成和尺寸以及结构有关[18-19],为了了解PL谱中蓝移现象的机理,分别采用质谱和具有元素分辨的XAFS技术研究不同初始配体比例的Cu纳米团簇的化学组成和尺寸以及结构信息。
图2给出了样品的MALDI-TOF质谱在550-1150 Da的结果。从图2可知,初始配体MPP和C12SH 比例为1:0制备的Cu纳米团簇展现出995.2 Da、778.5 Da和561.7 Da三个峰,分别对应于Cu6M4、Cu5M3和Cu4M2纳米团簇(M代表MPP配体)。根据Salorinne等[17]对铜纳米团簇的质谱分析,由于三个质谱峰各相差一个CuM(216.8 Da)碎片单元,因MALDI-TOF质谱工作中存在激光对样品电离造成的解离,可认为无C12SH制备的Cu纳米团簇是Cu5M3占主导的纳米团簇。当C12SH加入之后,三个样品都含有较强的608.8 Da和较弱的561.7 Da峰,对应于Cu4ML(L代表C12SH配体)和Cu4M2纳米团簇,并且以Cu4ML占主导。MALDI-TOF质谱表明初始配体中引入C12SH会减少Cu纳米团簇的Cu原子数,但进一步增加C12SH在初始配体中比例则不会对Cu纳米团簇的Cu原子个数产生影响。根据文献报道[23-25],金属纳米团簇的发光能量会随着团簇中金属原子数目的增加而降低。由于无C12SH制备的Cu纳米团簇,且Cu原子数数目最多,所以发光能量最低,波长最大。当配体中引入C12SH时,由于Cu纳米团簇中Cu原子数减少到4,因此纳米团簇的发光波长相应的从618 nm蓝移到597 nm。然而对于其他三个初始配体中引入C12SH的样品,它们拥有相同的化学组成,却表现出不同发光。
图3(a)和(b)显示了Cu纳米团簇Cu原子的k空间EXAFS函数kχ(k)曲线和R空间的FT(k3χ(k))函数曲线及相应的拟合曲线。图3(a)表明,不同初始配体比例的Cu纳米团簇的kχ(k)曲线与参照物体相Cu表现出很大差别,但与参照物CuS类似。这表明Cu纳米团簇的尺寸很小,并且纳米团簇的Cu原子与S原子有较强成键。从图3(b)可以进一步看出,在所有样品的RSF曲线中,都存在一个0.18 nm附近的主振幅峰。与参比样品CuS的RSF曲线对比可知,0.18 nm处的主振幅峰对应着Cu-S配位,并且主振幅随着C12SH所占比例的增大向低R方向有微弱移动。对于初始配体无C12SH得到的Cu纳米团簇,Cu-S配位峰强度较低并在0.22 nm附近表现出明显肩膀峰,对比体相Cu的RSF可知,该肩膀峰对应于Cu-Cu配位;当C12SH引入时,Cu-Cu配位峰减弱并消失,并且Cu-S向低R方向微弱移动,表明C12SH的引入导致纳米团簇的原子结构发生较大变化,相对于无C12SH引入的Cu纳米团簇,Cu-Cu配位数的减少。
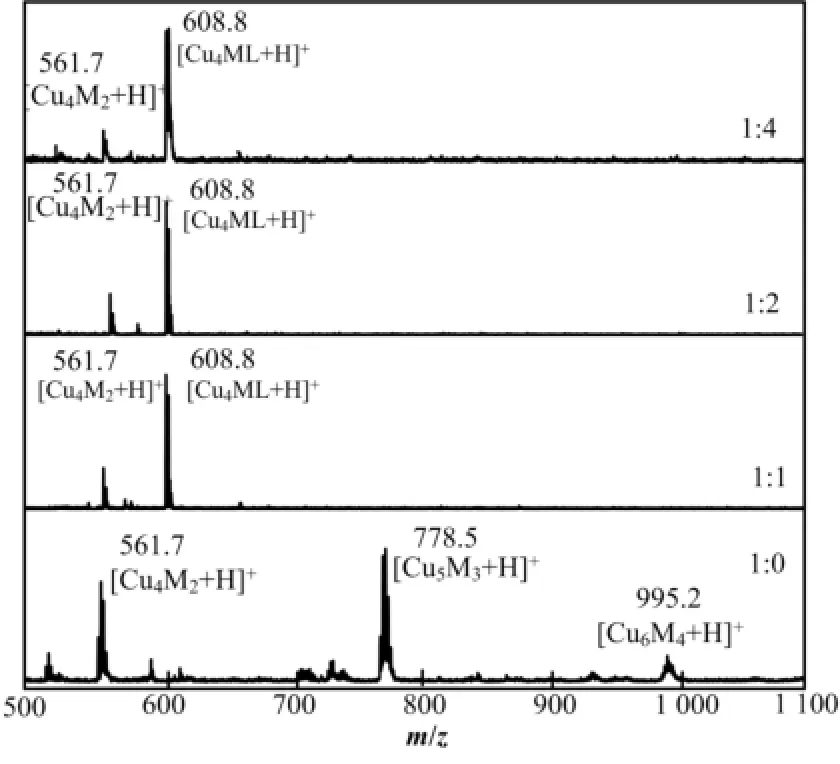
图2 不同初始配体比例样品的MALDI-TOF质谱Fig.2 MALDI-TOF mass spectra of the samples showing the main clusters and fragments.
为获得Cu纳米团簇结构的局域结构信息,通过IFEFFIT中的ARTEMIS对FT(k3χ(k))函数曲线进行最小二乘法的拟合(拟合区域0.14-0.30 nm)。拟合结果如图3(b)(空心圈)所示,结构参数归纳于表2。由表2可知,当初始配体中引入C12SH时,Cu纳米团簇中的Cu-Cu的键长从0.254 nm 缩短到0.24 nm,Cu-Cu配位数从3.6降到3.0,Cu-S的配位数从1.2降到1.0。随着C12SH在初始配体中的比例升高,除了Cu-S键长从0.228 nm 逐渐缩短到0.224 nm,Cu纳米团簇的整体结构参数保持不变。综合MALDI-TOF质谱和XAFS拟合结果,认为配体仅为MPP的Cu5M3纳米团簇,它是一种类双三角锥结构(图4(a));当初始配体中引入C12SH,Cu纳米团簇变为四面体结构(图4(b)),虽然C12SH和MPP中S原子与Cu原子结合的Cu-S键长可能不同,但是图3(b)中样品的RSF曲线仅有一个配位峰,因此我们认为这两种Cu-S键长十分接近;此外,随着C12SH所占比例的继续增大,Cu纳米团簇除Cu-S键长外,四面体的结构保持不变。
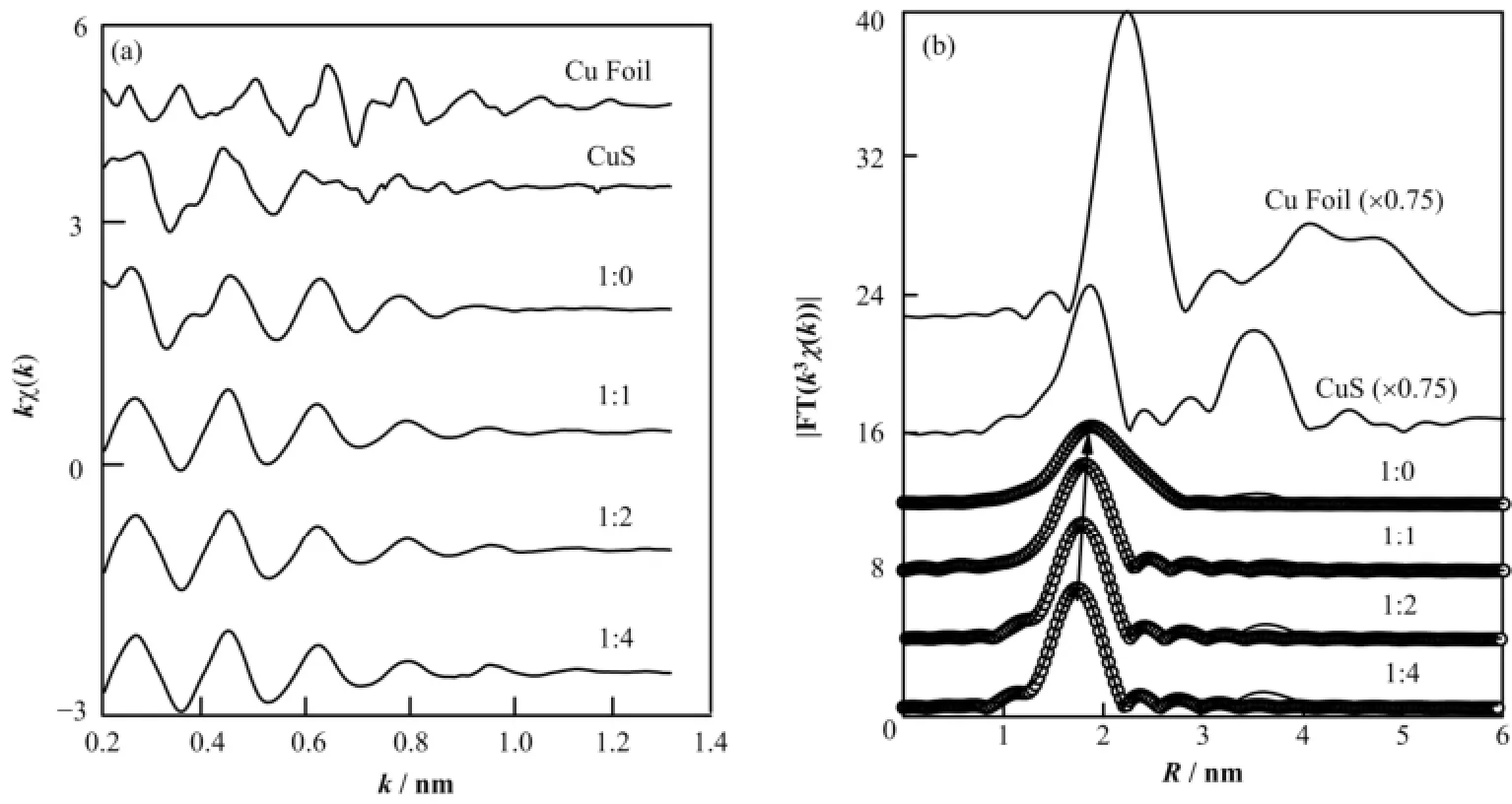
图3 不同初始配比Cu纳米团簇EXAFS谱(a) k空间kχ(k)谱,(b) 径向结构函数k3χ(k)谱(实线)及其拟合曲线(空心圈)Fig.3 Structure determination EXAFS k-space spectra kχ(k) (a), RSF curves (solid lines) and simulated fit (open circles) (b).
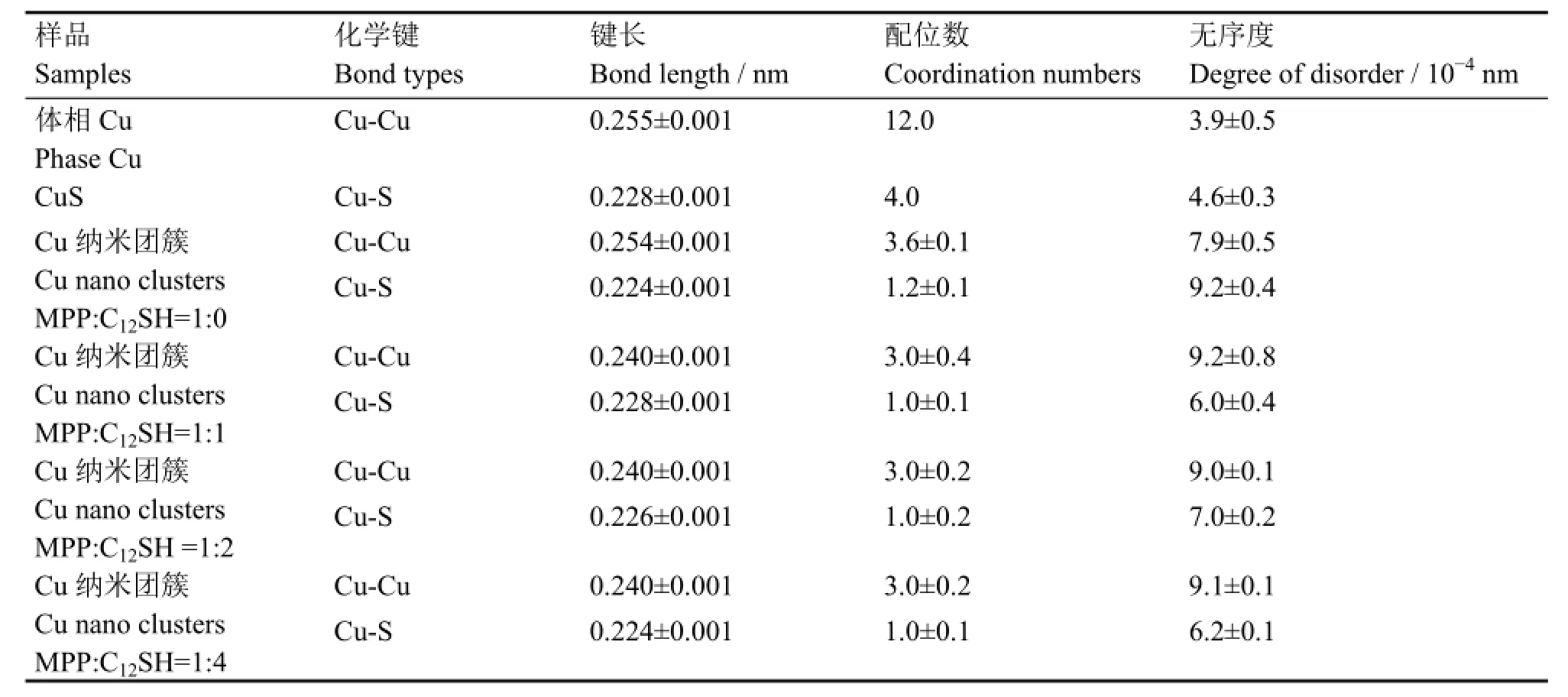
表2 Cu纳米团簇对Cu边的EXAFS进行拟合所得的结构参数Table 2 Structural parameters of Cu clusters fitting for Cu K-edge EXAFS spectra.

图4 Cu5M3 (a)和Cu4ML (b)纳米团簇的晶体结构Fig.4 Computational Cu5M3 cluster model (a) and Cu4ML cluster model (b).
根据以往对Au纳米团簇电子结构分析[26-28],我们认为Cu纳米团簇的Cu原子的3d10轨道组成d带;表面Cu原子的4sp轨道和S原子的3p轨道形成的Cu(I)-S混合态组成sp带内的HOMO能级和LUMO能级的低能量部分;内部Cu原子的sp轨道组成了LUMO能级的高能量部分。因此,我们认为样品中的600 nm发光是由LUMO能级中高能量部分的激发态电子向sp带内的HOMO能级跃迁产生,即发光来源于内部Cu原子向表面Cu(I)-S电荷转移。反应中,C12SH在初始配体中的比例增大导致生成的Cu纳米团簇的Cu-S键长发生缩短,从而导致了sp带内的HOMO能级的降低,使HOMO-LUMO带隙增大,进而导致Cu纳米团簇的发光波长的蓝移。
3 结语
通过调节初始配体MPP和C12SH的比例,合成出618-571 nm可控发光的Cun(n≤6)纳米团簇。根据UV-vis吸收谱、MALDI-TOF质谱以及XAFS谱的结果分析,认为仅有MPP作为配体时的产物主要为双三角锥结构的Cu5MPP3;当初始配体中引入C12SH后则变为四面体结构的Cu4[MPP][C12SH],纳米团簇中的Cu原子数的减少导致Cu纳米团簇的发光从618 nm蓝移至597 nm。随着C12SH在初始配体中所占比例的继续增加,Cu纳米团簇除Cu-S键长从0.228 nm缩短到0.224 nm外,四面体结构保持不变。但是Cu-S键长的缩短导致了由Cu(I)-S所构成的HOMO能级的降低,HOMO-LUMO带隙增大导致Cu纳米团簇的发光波长从597 nm逐渐蓝移至571 nm。
1 Brust M, Walker M, Bethell D, et al. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system[J]. Journal of the Chemical Society, Chemical Communications, 1994, 7: 801-802
2 Dahl J A, Maddux B L S, Hutchison J E. Toward greener nanosynthesis[J]. Chemical Reviews, 2007, 107(6): 2228-2269
3 Daniel M C, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology[J]. Chemical Reviews, 2004, 104(1): 293-346
4 Deheer W A. The physics of simple metal-clusters experimental aspects and simple-models[J]. Reviews of Modern Physics, 1993, 65(3): 611-676
5 Gao Y, Shao N, Pei Y, et al. Catalytic activities of subnanometer gold clusters (Au-16-Au-18, Au-20, and Au-27-Au-35) for CO oxidation[J]. ACS Nano, 2011, 5(10): 7818-7829
6 Kawasaki H, Yamamoto H, Fujimori H, et al. Surfactant-free solution synthesis of fluorescent platinum subnanoclusters[J]. Chemical Communications, 2010, 46(21): 3759-3761
7 Rastogi S K, Denn B D, Branen A L. Synthesis of highly fluorescent and thio-linkers stabilize gold quantum dots and nano clusters in DMF for bio-labeling[J]. Journal of Nanoparticle Research, 2012, 14(1): 1-12
8 Wu Z W, Gayathri C, Gil R R, et al. Probing the structure and charge state of glutathione-capped Au-25(SG)(18) clusters by NMR and mass spectrometry[J]. Journal of the American Chemical Society, 2009, 131(18): 6535-6542
9 MacDonald M A, Zhang P, Chen N, et al. Solution-phase structure and bonding of Au-38(SR)(24) nanoclusters from X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry C, 2011, 115(1): 65-69
10 Zhu M, Lanni E, Garg N, et al. Kinetically controlled, high-yield synthesis of Au-25 clusters[J]. Journal of the American Chemical Society, 2008, 130(4): 1138-1139
11 Pei Y, Pal R, Liu C Y, et al. Interlocked catenane-like structure predicted in Au-24(SR)(20): implication to structural evolution of thiolated gold clusters from homoleptic gold(I) thiolates to core-stacked nanoparticles[J]. Journal of the American Chemical Society, 2012, 134(6): 3015-3024
12 Chen W, Ghosh D, Sun J, et al. Dithiocarbamate-protected ruthenium nanoparticles: synthesis, spectroscopy, electrochemistry and STM studies[J]. Electrochimica Acta, 2007, 53(3): 1150-1156
13 Diez I, Ras R H A. Fluorescent silver nanoclusters[J]. Nanoscale, 2011, 3(5): 1963-1970
14 Vilar-Vidal N, Blanco M C, Lopez-Quintela M A, et al. Electrochemical synthesis of very stable photoluminescent copper clusters[J]. The Journal of Physical Chemistry C, 2010, 114(38): 15924-15930
15 Yuan X, Luo Z T, Zhang Q B, et al. Synthesis of highly fluorescent metal (Ag, Au, Pt, and Cu) nanoclusters by electrostatically induced reversible phase transfer[J]. ACS Nano, 2011, 5(11): 8800-8808
16 Vazquez-Vazquez C, Banobre-Lopez M, Mitra A, et al. Synthesis of small atomic copper clusters in microemulsions[J]. Langmuir, 2009, 25(14): 8208-8216
17 Salorinne K, Chen X, Troff R W, et al. One-pot synthesis and characterization of subnanometre-size benzotriazolate protected copper clusters[J]. Nanoscale, 2012, 4(14): 4095-4098
18 Wei W T, Lu Y Z, Chen W, et al. One-pot synthesis, photoluminescence, and electrocatalytic properties of subnanometer-sized copper clusters[J]. Journal of the American Chemical Society, 2011, 133(7): 2060-2063
19 Wu Z K, Jin R C. On the ligand's role in the fluorescence of gold nanoclusters[J]. Nano Letters, 2010, 10(7): 2568-2573
20 Lu Y Z, Wei W T, Chen W. Copper nanoclusters: synthesis, characterization and properties[J]. Chinese Science Bulletin, 2012, 57(1): 41-47
21 Lisiecki I, Billoudet F, Pileni M P. Control of the shape and the size of copper metallic particles[J]. The Journal of Physical Chemistry, 1996, 100(10): 4160-4166
22 Murrie M, Collison D, Garner C D, et al. Synthesis, structure and magnetic properties of [Cu-5(bta)(6)L-4] (bta=benzotriazolate; L=beta-diketonate) clusters[J]. Polyhedron, 1998, 17(17): 3031-3043
23 Duchesne P N, Zhang P. Local structure of fluorescent platinum nanoclusters[J]. Nanoscale, 2012, 4(14): 4199-4205
24 Forward J M, Bohmann D, Fackler J P, et al. Luminescence studies of gold (I) thiolate complexes[J]. Inorganic Chemistry, 1995, 34(25): 6330-6336
25 Zheng J, Nicovich P R, Dickson R M. Highly fluorescent noble-metal quantum dots[J]. Annual Review of Physical Chemistry, 2007, 58: 409-431
26 Zhu M, Aikens C M, Hollander F J, et al. Correlating the crystal structure of a thiol-protected Au-25 cluster and optical properties[J]. Journal of the American Chemical Society, 2008, 130(18): 5883-5885
27 Lee H M, Ge M F, Sahu B R, et al. Geometrical and electronic structures of gold, silver, and gold-silver binary clusters: origins of ductility of gold and gold-silver alloy formation[J]. The Journal of Physical Chemistry B, 2003, 107(37): 9994-10005
28 Zheng J, Zhou C, Yu M X, et al. Different sized luminescent gold nanoparticles[J]. Nanoscale, 2012, 4(14): 4073-4083
CLCTL99
Study on photoluminescence and structure of copper nanoclusters
GE Mengran LI Dandan YAO Tao PAN Guoqiang
(National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, China)
Background:During the past few decades, metal nanoclusters have
considerable research interests for their distinct optical, catalytic and electronic properties. However, till today, most studies are focused on the synthesis, structure and properties of Au and Ag clusters, while the studies on Cu nanoclusters are rather rare because of its inherent instability.Purpose:We aim to synthesize the Cu nanocluster that has the tunable photoluminescence.Methods:Through varying the initial legend molar ratio of 2-mercapto-5-n-propylpyrimidine and dodecanethiol, a series of Cu nanoclusters with different emission wavelength were prepared. The photoluminescence property and structure of these nanoclusters were studied by the photoluminescence spectrometry (PL), X-ray absorption fine structure (XAFS) and Matrix Assisted Laser Desorption Ionization Time of Flight Spectrometer (MALDI-TOF) mass spectrometry.Results:(1) PL spectra showed that with the increase of the proportion of dodecanethiol, the maximum emission wavelength of Cu nanoclusters shifted from 618 nm to 571 nm. (2) Mass spectra results indicated that the main products were changed from Cu5MPP3(MPP: 2-mercapto-5-n-propylpyrimidine) in the presence of MPP alone to Cu4[MPP][C12SH] when the dodecanethiol was introduced, and remains unchanged with increasing the dodecanethiol. (3) XAFS results showed that with the increase of dodecanethiol, the Cu-S bond length was shortened from 0.228 nm to 0.224 nm.Conclusion:The reduction of the amount of Cu atomic number would lead to the shift of emission wavelength from 618 nm to 597 nm, and the shortening of Cu-S bond length pushed down the HOMO level which was formed by the Cu(I)-S. With the increase of the HOMO-LUMO band gap, the emission wavelength of the products shifted from 597 nm to 571 nm.
Copper nanoclusters, Tunable photoluminescence, X-ray absorption fine structure (XAFS), Synchrotron radiation
TL99
10.11889/j.0253-3219.2014.hjs.37.090104
国家自然科学基金课题(No.11079032、No.U1232132)资助
葛梦然,男,1990年出生,现就读于中国科学技术大学国家同步辐射实验室
潘国强,E-mail: gqpan@ustc.edu.cn
2014-04-15,
2014-05-08

