Fused-core particle technology in high-performance liquid chromatography: An overview
Joseph J. Kirklnd, Stephnie A. Schuster,*, Willim L. Johnson,Brry E. Boyes,b
aAdvanced Materials Technology, Inc., 3521 Silverside Road, Suite 1-K, Quillen Building, Wilmington, DE 19810, USA
bComplex Carbohydrate Research Center, 315 Riverbend Road, Athens, GA 30602, USA Available online 27 February 2013
1. Introduction
The introduction of particles with Fused-Core®technology by Advanced Materials Technology, Inc., in 2006, has resulted in a strong change in the manner in which high-performance liquid chromatography (HPLC) separations are conducted. These sub-3 μm diameter high-purity silica particles with a solid core and a thin, porous outer shell created sudden interest because of the very high efficiency of columns operating at modest backpressures [1,2]. The success of these fused-core particles quickly resulted in closely-similar superficially porous particles (SPPs)(often called porous-shell, core-shell) being introduced by competitors. The superior performance of these materials may be a function of the very narrow particle size distribution of the SPPs, and perhaps their higher particle density, which results in very homogeneous, efficient packed beds. Columns of these materials rival the efficiency of columns with 1.7 μm totally porous particles, but with only about one-half the operating pressure[3,4].As a result,practitioners can use columns of these particles with the existing conventional HPLC equipment so that expensive very high pressure instruments are not required. Also,laboratory manipulations are simplified since the special techniques required for very high pressure operation are not necessary.In this report, ‘‘fused-core particles'' are those particles specifically produced by Advanced Materials Technology, Inc. having a solid core and a thin porous outer shell.The term‘‘superficially porous particles(SPPs)''is used when referring to other particles that are available in a similar generic configuration.
A family of fused-core particles has been designed for applications with specific compound classifications. The original fused-core Halo®particles with 90 ˚A pores were directed toward separating small molecules [1,4]. Since their initial introduction, fused-core particles now are available with larger pores that are designed for separating peptides and small proteins [5], and particles with even larger pores have been produced for separating proteins and large biomacromolecules[6,7]. In addition, larger particle-size fused-core particles have been made available for special applications[8].Table 1 lists the fused-core particles that have been described to date,with their physical characteristics and properties. This report describes these particles in more detail,providing reasons for their design,and showing typical applications for which these particles were intended.
2. Experimental
Sample mixture components, mobile phase modifiers, peptides and proteins were obtained from Sigma-Aldrich(St.Louis,MO).Myosin was purchased from Cytoskeleton, Inc. (Denver, CO).Trifluoroacetic acid was from Pierce Chemical(Rockford,IL)and acetonitrile from EMD (Gibbstown, NJ). Particle sizes were determined with a Coulter Multisizer 3 instrument(Fullerton,CA)and surface area BET measurements using nitrogen were conducted with a Micromeritics TriStar II (Norcross, GA). Shell thicknesses were determined by the difference in Coulter measurements for the starting solid cores and the final particles.All of the fused-core particles of this study showed a particle size distribution of about 5% standard deviation from the average, which may contribute to the very high efficiency of these materials [1,4].
Columns of fused-core particles were obtained from Advanced Materials Technology, Inc. (Wilmington, DE).Columns of conventional totally porous particles were from Mac-Mod Analytical (Chadds Ford, PA) or Supelco (Bellefonte, PA). The HPLC data were collected with an Agilent Model 1100, Agilent Model 1200 SL liquid chromatographs,Shimadzu Prominence UFLC XR and Shimadzu Nexera liquid chromatographs. Peak widths (full width half max)were used for measuring plate numbers. To minimize extracolumn band broadening effects,a 5 μL flow cell with the heat exchanger bypassed was used for the Agilent 1100, while a 2 μL flow cell was used for the Agilent 1200. A 2.4 μL semimicro flow cell was used for both the Shimadzu Prominence and Nexera. Additionally, all of the capillary tubing connections and needle seats were of 0.12 mm ID for both Agilent instruments. No corrections for instrumental extra-column band broadening effects were applied to any of the chromatographic data in this study.
2.1. MS conditions
Samples of synthetic peptide standards[9]or tryptic digests of various proteins were resolved on three series coupled columns of 150 mm×2.1 mm (ID) Halo Peptide ES-C18, for a total column length of 450 mm. MS analysis used the Shimadzu Nexera LC upstream from the Shimadzu LCMS-2020 single quadrupole MS, operated in the electrospray ionization mode(ESI). The ESI source was maintained at 4.2 kV with 15 L/min N2drying gas and pneumatic assisted nebulization at 1.4 L/min,a block temperature of 400°C and desolvation capillary at 250°C. Scans were collected at 2.5 points per second in the range of 350-1800 m/z, with or without selected ion monitoring (SIM), which used the default band width of 1 amu.Separations of peptides or digests were monitored using an SPD-20 A absorbance detector at 220 nm, a 50 ms time constant, and fitted with a low dispersion UHPLC flow cell,in series with the MS. In some cases, the absorbance detector was removed, to qualify the minimal contribution of this cell to peak band-broadening. Peak capacity calculations were conducted as previously described [9], using the particular peptides and conditions specified below.
3. Results and discussion
3.1. Halo
As noted in Table 1, 2.7 μm fused-core particles with 0.5 μmthick porous shell were designed to separate small molecules with high efficiency.Early studies in which these particles were synthesized with 60 ˚A pores and a C18 stationary phase showed some restricted diffusion and reduced efficiency for some molecules above about 600 molecular weight [10].Subsequent particles synthesized with 90 ˚A pores showed no such effects for solutes up to about 2000 molecular weight,so this pore size was set for the commercial particles. This90 ˚A pore size resulted in a surface area of about 135 m2/g for these high-purity Type B silica particles, which allows for adequate retention and sample loading properties.

Table 1 Characteristics of fused-core particles.
Why was the overall diameter of these particles set at 2.7 μm? This diameter was not an arbitrary decision but a result of calculations based on known HPLC theory. Particle size can be related to the available pressure using the approximate equation [11]
This relationship can be expressed as
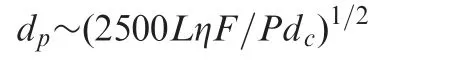
where P is the column pressure(psi),L is the column length(cm),η is the mobile phase viscosity(cP),F is the flow rate(mL/min),dpthe particle diameter(μm),and dcthe column ID(cm). If the maximum pressure of the pumping system is known, and the column dimensions and flow rate are described for a typical mobile phase with a known viscosity, the optimum particle size for these conditions can be estimated. For example, for a 5 cm×0.46 cm column and a typical mobile phase viscosity of 1.6 cP,at a flow rate of 1.0 mL/min with a maximum pressure of 400 bar (5880 psi, typical of instruments at the time of this calculation), the calculated optimum particle size for these conditions is 2.7 μm. The 2.7 particle diameter was selected for the first fused-core particles also so that 2 μm porosity frits could be conveniently used to capture the particles within the column.This frit porosity is widely used in columns of 5 μm particles,allowing consistent routine operation without problems of fouling and plugging with typical samples.
Why was the shell thickness of 0.5 μm selected for this Halo particle? This judgment was based on a compromise of physical properties. The thicker the porous shell, the higher the surface area for higher sample loading potential and greater solute retention.A thinner porous shell reduces sample loading qualities and retention characteristics, but could have higher efficiency,particularly at higher mobile phase velocities.The 0.5 μm porous shell on a 2.7 μm particle which results in a silica fused-core particle with a surface area of 135 m2/g has been found to be a good compromise for chromatographic utility. A thinner shell results in only a slight improvement in mass transfer characteristics and efficiency at higher flow rates for small molecules(favorable diffusion properties),but at the expense of reduced retention and sample loading.
To provide optimum performance, the high efficiency and sharp, low-volume peaks produced by the Halo fused-core particles do require instruments with good design[11].However,most current well-designed HPLC instruments meet this qualification and expensive, ultra-high-pressure instruments are not required. Data collection is optimized by using low volume detector cells (e.g., 1.5 μL), small diameter (75-125 μm ID)connecting tubing between the column, injector and detector,and 80 Hz data sampling. The experimental handling of instrumental extra-column band broadening effects for maximum column efficiency has been thoroughly defined in published studies [12,13].

Fig.1 Fast steroid separation with fused-core column.Column:50 mm×2.1 mm Halo C18; mobile phase: 40% water/60% methanol;flow rate: 1.0 mL/min; temperature: 40°C; pressure: 480 bar; detector, UV: 254 nm; 1 μL injection; and solutes: (1) uracil, (2) prednisone, (3) hydrocortisone, and (4) dexamethasone.
The properties of the Halo fused-core particle are exemplified by the chromatographic separation in Fig.1.This mixture of three steroids was well resolved in less than 30 s as a result of the particle efficiency at an elevated mobile phase velocity.This property is characteristic of fused-core particles, showing only moderate loss in efficiency when operated at mobile phase velocities well above the velocity of highest efficiency.The thin porous shell of the particles allows rapid mass transfer (kinetics) of solutes even when high mobile phase velocities are used.Fig.2 compares high-efficiency separations of a mixture of seven anticoagulants with fused-core columns containing different stationary phases to obtain variations in separation selectivity. In this case, the mobile phase velocity was set for optimum efficiency of these columns.
Researchers looking to improve their separations for bioanalysis have benefitted from the advantages of fused-core particle columns.To increase throughput,fast LC/MS/MS methods were developed using fused-core particle columns for the analysis of rimonabant in mouse plasma [14] and for the quantification of sinafloxacin in rat plasma[15].Badman et.al.have reported LC/MS/MS methods of 1 min or less for discovery pharmacokinetic samples [16]. The enhanced robustness of fused-core particle columns has been utilized for several bioanalytical applications[17,18]. Finally, higher sensitivity was observed when fused-core particle columns were used for the analysis of imipramine and desipramine in rat plasma [19].
3.2. Halo Peptide
This fused-core particle was designed specifically for the highperformance separation of peptides and small proteins [5].Solutes above about 2000 molecular weight show broader peaks with Halo fused-core C18 particles with 90 ˚A pores apparently as a result of restricted diffusion. In this case, the pores are too small to allow adequate access of larger molecules;particles with larger pores are required. Studies showed that particles with 160 ˚A pores allow full access of peptides and small proteins up to about 15 kDa without restricted diffusion, depending on solute configuration. Fig.3 shows that some components in a small protein mixture demonstrate restricted diffusion for 90 ˚A fusedcore particles,but no such problem is seen for a column of fusedcore particles with 160 ˚A pores.

Fig.2 Separation of anticoagulants with fused-core columns of different selectivities.Columns:50 mm×4.6 mm Halo C18,50 mm×4.6 mm Halo RP-amide, 50 mm×4.6 mm Halo phenyl-hexyl; mobile phase: A/B=40/60; A=0.1% formic acid, pH 2.6; B=methanol/acetonitrile,50/50; flow rate: 2.0 mL/min; temperature: 45°C; pressure: 210 bar; detector, UV: 254 nm; 1 μL injection; and solutes: (1) uracil,(2) 4-hydroxycoumarin, (3) coumarin, (4) 6-chloro-4-hydroxycoumarin, (5) warfarin, (6) coumatetralyl, and (7) coumachlor, i=impurity.

Fig.3 Effect of pore size on separation of small proteins. Columns: 100 mm×4.6 mm Halo C18 (90 ˚A pores) and 100 mm×4.6 mm Halo Peptide ES-C18 (160 ˚A pores); mobile phase: A=water/0.1% trifluoroacetic acid; B=acetonitrile/0.1% trifluoroacetic acid;gradient: 25-42% B in 10 min; flow rate: 1.5 mL/min; temperature: 30°C; detection: 215 nm; and solutes in order of elution:(1) ribonuclease A, (2) bovine insulin, (3) human insulin, (4) cytochrome c, and (5) lysozyme. Peak widths in minutes above each peak.

Fig.4 High resolution separation of a complex peptide mixture. Columns: 3@ 150 mm×2.1 mm Halo Peptide ES-C18 (160 ˚A pores);mobile phase: A=water/0.1% formic acid/20 mM ammonium formate; B=A with 80% acetonitrile; gradient 5-55% B in 150 min; flow rate: 0.5 mL/min; temperature: 70°C; detection: 220 nm; and injection volume: 50 μL [25 μg each] of α-1-glycoprotein tryptic digest and apotransferrin tryptic digest. Inset shows an expanded scale to demonstrate excellent peak symmetry and resolution.
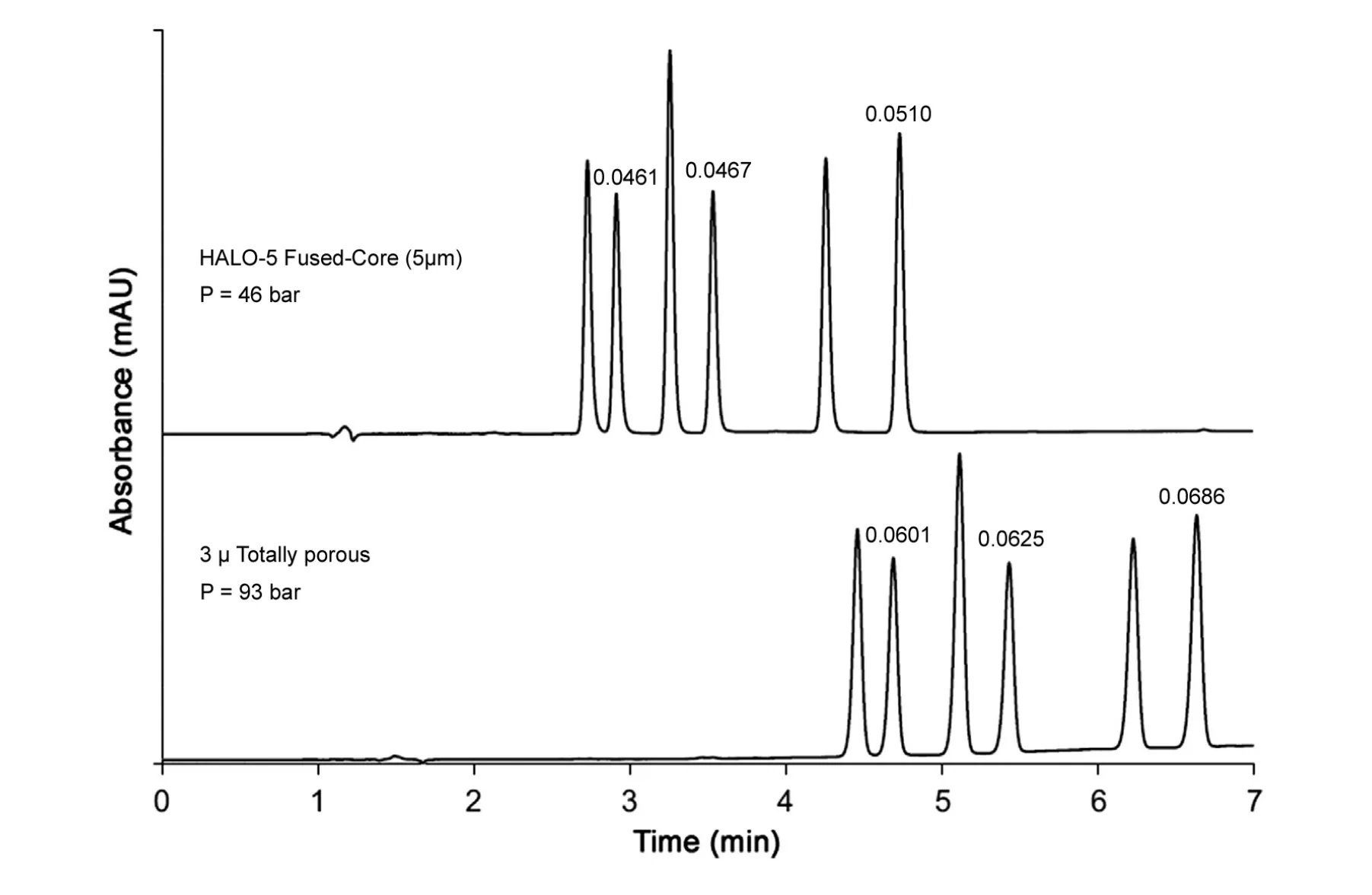
Fig.5 Comparative separations with 5 μm fused-core and 3 μm totally porous particles. Columns: 100 mm×4.6 mm Halo 5 PFP(pentafluorophenylpropyl), 100 mm×4.6 mm 3 μm totally porous PFP; mobile phase: A=25 mM ammonium acetate, pH 5.5,B=acetonitrile; gradient: 36-65% B in 7 min; flow rate: 0.75 mL/min; temperature: 35°C; pressure: Halo 5=46 bar, 3 μm=93 bar;and detection: 254 nm. Solutes in order of elution: (1) oxazepam, (2) lorazepam, (3) nitrazepam, (4) clonazepam, (5) flunitrazepam,(6) diazepam. Peak widths in minutes above selected peaks.
Note that retention for components in Fig.3 is lower for the particles with 90 ˚A pores. This effect is in spite of the higher surface area (Table 1) and the higher carbon content of this C18 stationary phase compared to the sterically-protected(ES) C18 phase for the particles with 160 ˚A pores. A potential complicating factor is that the C18 stationary phase for the 90 ˚A particles is endcapped while the ES-C18 phase for the 160 ˚A particles is not. Nevertheless, we speculate that the reduced retention in the separation of particles with 90 ˚A pores is influenced by the restricted diffusion for these proteins. Additional studies are needed to elucidate this apparent anomaly.

Fig.6 ‘‘Ballistic chromatography''separation.Column:20 mm×2.1 mm Halo-5 PFP,5 μm with 0.6 μm shell;mobile phase:A=25 mM ammonium acetate, pH 5.5, B=acetonitrile; gradient: 17-25% B in 30 s; flow rate: 4.0 mL/min; temperature: 60°C; and solutes in order of elution: (1) oxazepam, (2) lorazepam, (3) clonazepam, (4) temazepam, (5) flunitrazepam, and (6) diazepam.

Fig.7 Comparative van Deemter plots for 400 ˚A fused core and 300 ˚A totally porous particles.Columns:100 mm×4.6 mm fused-core ES-C18 (400 ˚A pores), 100 mm×4.6 mm totally porous C18 (300 ˚A pores); mobile phase: fused-core: 41% acetonitrile/59% of 0.1%trifluoroacetic acid, totally porous: 42.5% acetonitrile/57.5% of 0.1% trifluoroacetic acid; mobile phase velocity as shown; temperature:60°C; and solute: carbonic anhydrase, MW=29 kDa.

Fig.8 Rapid separation of protein mixture with wide-pore fused-core particles. Column: 100 mm×2.1 mm Halo wide-pore, 3.4 μm,0.2 μm porous shell,ES-C8; mobile phase gradient:25-50% acetonitrile/water/0.1% trifluoroacetic acid in 5 min; flow rate:0.5 mL/min;temperature:60°C;UV detection:215 nm;and peak identities:(1)cytochrome c(12.4 kDa);(2)lysozyme(14.3 kDa);(3)α-chymotrypsin(25 kDa); (4) catalase (250 kDa); (5) carbonic anhydrase (29 kDa); (6) enolase (46.7 kDa); and (7) β-amylase (200 kDa).
Very rapid separations for simple mixtures of peptides can be obtained with the Halo Peptide columns, or alternatively, highresolution gradient separations can be performed with longer columns, as shown in Fig.4. In this example, three columns of 150 mm are operated in series,yielding 450 mm of column length.At the moderate flow rate for these 2.1 mm ID columns of 0.5 mL/min,and column temperature of 70°C,the back-pressure observed was less than 800 bar over the course of the gradient separation of peptides, which is well within the range of typical UHPLC instruments. The excellent resolution of the complex protein digest mixture shown in Fig.4 is possible due to the high peak capacities that result from both the high efficiency fused-core particles, as well as the extended column length that can be employed. This high efficiency is due to the high permeability of the columns, relative to those that are observed for sub-2-μm particle columns. Thus, for a representative 11 peptide mixture,ranging from the dipeptide Asp-Phe to the 26 amino acid long melittin peptide, we measured a peak capacity of 574 for the conditions shown in Fig.4.It should be noted that this high peak capacity is obtained in a reasonable separation time (c. 150 min),using instruments that are not specially modified to reduce band dispersion,and represents conditions using an MS-friendly mobile phase (formic acid/ammonium formate). Therefore, higher peak capacities can be obtained,but at the expense of significant elution run time. In all cases, peak capacities were calculated to include only the gradient range for acetonitrile compositions that encompasses the useful range for elution of peptides and tryptic fragments that are observed for digests of simple protein mixtures,(c. 5-55% acetonitrile, as used in Fig.4). This range is also observed for much more complex proteomic samples.Including a gradient range of acetonitrile modifier that is outside this elution window artificially improves peak capacities.

Fig.9 Separation of large protein with wide-pore fused-core particles. Column: 100 mm×2.1 mm, 3.4 μm fused-core, ES-C8,400 ˚A; mobile phase: A=water/0.1% trifluoroacetic acid,B=acetonitrile/0.1% trifluoroacetic acid; gradient=35-50% B in 7 min; flow rate: 0.45 mL/min; temperature: 80°C; detection:215 nm; and solute: myosin (440 kDa).
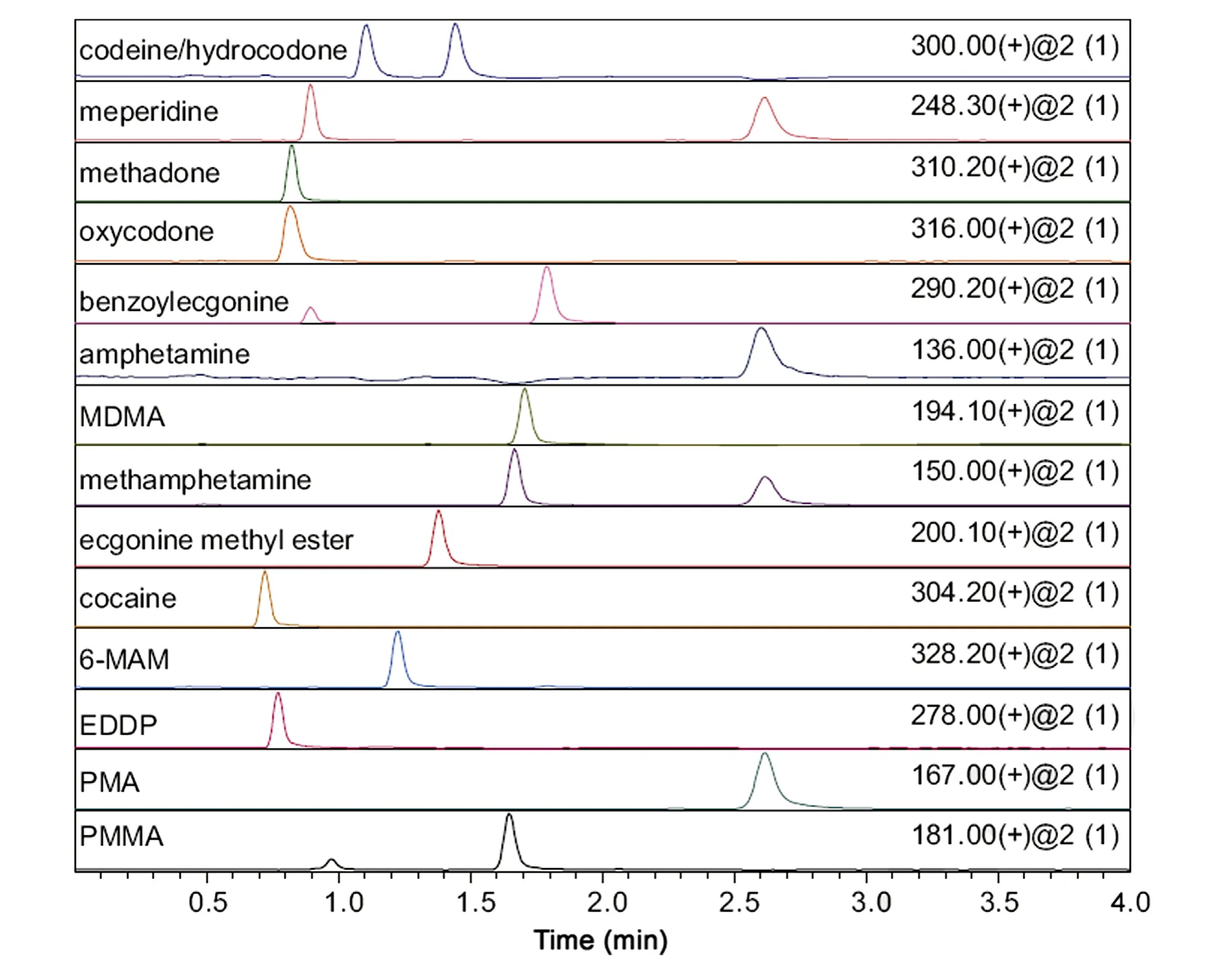
Fig.10 HILIC separation of drugs of abuse and selected metabolites.Column:100 mm×2.1 mm Halo Penta-HILIC;mobile phase:95/5 acetonitrile/water with 5 mM ammonium formate, pH 3.0; flow rate: 0.5 mL/min; temperature 60°C; 1 μL [500 pg each except amphetamine and PMA 100 ng each] injected; SIM, unit resolution, positive ion mode, 2 kV, 400°C heat block, 225°C capillary;Shimadzu Nexera and LCMS 2020; and solutes as indicated.
3.3. Halo 5
Studies interestingly showed that large particle size fused-core particles produce columns that have smaller reduced plate height h than smaller fused-core particles(h=H/dp,where H is the plate height, and dpis the particle size) [8]. Columns of 5 μm fused-core particles routinely demonstrate h values of about 1.2 for small molecules, without corrections for instrumental extra-column band broadening effects.Because of high efficiency, these 5 μm fused-core particles produce separations superior to those of 3 μm totally porous particles, but, also importantly, with lower back pressures, as shown in Fig.5.Therefore,columns of these 5 μm fused-core particles have the same operating characteristics as columns of traditional 5 μm totally porous particles (e.g., low pressure, resistance to plugging, high stability), but with much higher efficiency for faster, better component resolution.
Because of superior mass transfer characteristics, columns of the Halo 5 fused-core particles have demonstrated interesting performance when used in the ballistic chromatography mode[20].Here, short, low-volume columns (e.g., 2.1 mm×20 mm) are used with gradients at very high mobile phase velocities to produce extremely fast separations (30-60 s) of simple mixtures typical for monitoring reactions, or to quickly analyze a large number of samples. An example of such a ballistic chromatography separation is shown in Fig.6 for the separation of a mixture of anti-anxiety drugs in about 27 s. This very fast chromatographic approach also is useful for the fast analysis of the fractions sampled in initial 2-D chromatographic separations to provide rapid and efficient resolution of very complex component mixtures.
3.4. Halo Wide-pore

Fig.11 Separation of nucleosides and bases with fused-core Halo Penta-HILIC. Column: 100 mm×4.6 mm; mobile phase: A/B gradient, A=8/92 10 mM ammonium formate, pH 6/acetonitrile, B=15/85 ammonium formate, pH 6/acetonitrile; gradient: 0 min, 0%B; 3.6 min, 0% B; 3.61 min, 30% B; 4.0 min, 50% B; 6.0 min, 70% B; temperature: 35°C; detector response: 0.02 s; and solutes:(1) thymine, (2) uracil, (3) thymidine, (4) 2′-deoxyadenosine, (5) adenine, (6) uridine; (7) adenosine, (8) hypoxanthine, (9) xanthine,(10) cytosine, (11) 2′-deoxycytidine, (12) guanine, (13) 2-deoxyguanosine, (14) cytidine, and (15) guanosine.
Fused-core particles with even wider pores have been synthesized for separating larger proteins and other larger biomacromolecules.To accommodate these large solutes without restricted diffusion,efficient particles with 400 ˚A pores have been developed [7].Columns containing these fused-core particles show superior characteristics over columns of comparable totally porous particles,as shown in the van Deemter plot in Fig.7.Here,data for a high-quality 3 μm totally porous particle and a 2.7 μm Halo fusedcore particle are compared for a protein using a van Deemter plot of plate height H (μm) versus linear mobile phase velocity to illustrate differences for the two particles. Although there is a slight difference in particle size, the wide-pore Halo fused-core particle clearly shows higher efficiency(smaller plate height)at the mobile phase velocity plate height minimum,and a much smaller increase in plate height as the mobile phase velocity is increased.The latter factor is a result of the superior mass transfer allowed by the thin porous shell, compared to the totally porous particle structure. Columns of the larger pore fused-core particles can be used for rapidly separating protein mixtures, as shown in Fig.8.
The wide pores of the Halo 400 particles allow the separation of very large proteins as illustrated in Fig.9.The starting myosin protein used for this separation dissociates in the trifluoroacetic acid mobile phase to form smaller sub-units of widely varying size. Tests have shown that proteins at least as large as 400 kDa can be efficiently separated with columns of these fused-core particles without any evidence of restricted diffusion[7].Limited data suggest that C4 and C8 stationary phases on these particles may be preferred over a C18 stationary phase because of higher efficiency and reduced potential for loss in recovery of some proteins during separations.We speculate that this loss is due to aggregation of some highly hydrophobic segments on proteins as a result of reduced protein solubility as the concentration of organic mobile phase increases during the gradient. Under such conditions,longer chain alkyl groups of the C18 phase could trap or precipitate proteins. However, the resulting C18 coated particles can be regenerated by purging the column with the initial mobile phase of the gradient (higher aqueous concentration, less organic), and re-eluting the material with repetitive gradients.
3.5. Special Halo columns
Fused-core particles with 90 ˚A pores have been modified with special stationary phases such as ligands for HILIC to provide separation opportunities for compounds that are difficult to separate by conventional reversed-phase chromatography [21].For example, a Halo Penta-HILIC column with a highly polar poly-hydroxylic stationary phase is especially useful for separating basic, highly polar drugs by the HILIC technique, as shown in Fig.10. Separations on the Halo Penta-HILIC column are shown for a mixture of 15 drugs of abuse and metabolites. The high efficiency and excellent peak shape for these basic compounds on the Halo Penta-HILIC column are a result of the highly-deactivated particle surface by the poly-hydroxylic stationary phase.This modified fused-core particle is also useful for separating highly polar solutes such as sugars, peptides, and nucleic acid components, as shown for the HILIC separation of nucleosides and nucleotides in Fig.11.
4. Conclusions and future
The superficially porous design of particles in the fused-core family clearly has utility for rapidly and efficiently separating a wide range of compounds.Particles with specific diameters and pore sizes have been developed to optimize the separation of compound types, usually based on solute size. The practical utility of the fused-core particles has been demonstrated as a result of their high efficiency, ease of operation, modest operating pressure and column stability. References describing the characteristics and applications of Halo fused-core particles can be found at http://www.advanced-materials-tech.com. The future of fused-core particles appears to be quite bright as there are additional possibilities for different particle types,sizes and stationary phases that will even further expand the utility of this family of particles.
We appreciate the partial support of this study provided by the NIH with SBIR Grants GM099355 and GM093747. We also thank Robert Moran and William Miles for assistance with obtaining chromatographic measurements. We are also grateful to Joseph DeStefano, Timothy Langlois, and Jason Lawhorn for valuable discussions regarding this manuscript.
[1] J. DeStefano, T. Langlois, J. Kirkland, Characteristics of superficially-porous silica particles for fast HPLC: some performance comparisons with sub-2-μm particles, J. Chromatogr. Sci.46 (2008) 254-260.
[2] J. Cunliffe, T. Maloney, Fused core particle technology as an alternative to sub-2 μm particles to achieve high separation efficiency with low backpressure,J.Sep.Sci.30(2007)3104-3109.
[3] J. Salisbury, Fused-core particles: a practical alternative to sub-2 μm particles, J. Chromatogr. Sci. 46 (2008) 883-886.
[4] J. Kirkland, T. Langlois, J. DeStefano, Fused core particles for HPLC columns, Am. Lab. 39 (2007) 18-21.
[5] S.A. Schuster, B.M. Wagner, B.E. Boyes, et al., Wider pore superficially porous particles for peptide separations by HPLC,J.Chromatogr. Sci. 48 (2010) 566-571.
[6] S. Fekete, R. Berky, J. Fekete, et al., Evaluation of a new wide pore core-shell material (AerisTMWIDEPORE) and comparison with other existing stationary phases for the analysis of intact proteins, J. Chromatogr. A 1236 (2012) 177-188.
[7] B.M. Wagner, S.A. Schuster, B.E. Boyes, et al., Superficially porous silica particles with wide pores for biomacromolecular separations, J. Chromatogr. A (2012) 22-30.
[8] J. DeStefano, S.A. Schuster, J.M. Lawhorn, et al., Performance characteristics of new superficially porous particles, J. Chromatogr. A 1258 (2012) 76-83.
[9] S.A. Schuster, B.E. Boyes, B.M. Wagner, et al., Fast high performance liquid chromatography separations for proteomic applications using Fused-core®silica particles,J.Chromatogr.A 1228 (2012) 232-241.
[10] J.J. Kirkland, Advanced Materials Technology, Inc., Wilmington, DE, 2005 unpublished studies.
[11] L.R. Snyder, J.J. Kirkland, J.W. Dolan,Introduction to Modern Liquid Chromatography, John Wiley, Hoboken, NJ, 2010.
[12] J.J. Kirkland, W.W. Yau, H.J. Stocklosa, et al., Sampling and extra-column effects in high-performance liquid chromatography;influence of peak skew on plate count calculations, J. Chromatogr. Sci. 15 (1977) 303-316.
[13] F. Gritti, C.A. Sanchez, T. Farkas, et al., Achieving the full performance of highly efficient columns by optimizing conventional benchmark high-performance liquid chromatography instruments, J. Chromatogr. A 1217 (2010) 3000-3012.
[14] Y. Hsieh, C. Duncan, J. Brisson, Fused-core silica column highperformance liquid chromatography/tandem mass spectrometric determination of rimonabant in mouse plasma, Anal. Chem. 79(2007) 5668-5673.
[15] S. Wang,J. Wen,L. Cui, et al., Optimization and validation of a high performance liquid chromatography method for rapid determination of sinafloxacin, a novel fluoroquinolone in rat plasma using a fused-core C18-silica column, J. Pharm. Biomed.Anal. 51 (2010) 889-893.
[16] E.R. Badman, R.L. Beardsley, Z. Liang, et al., Accelerating high quality bioanalytical LC/MS/MS assays using fused-core columns, J. Chromatogr. B 878 (2010) 2307-2313.
[17] J. Cunliffe, C. Noren, R. Hayes, et al., A high-throughput LCMS/MS method for the quantitation of posaconazole in human plasma:implementing fused core silica liquid chromatography, J.Pharm. Biomed. Anal. 50 (2009) 46-52.
[18] D.N. Mallett, C. Ramirez-Molina, The use of partially porous particle columns for the routine, generic analysis of biological samples for pharmacokinetic studies in drug discovery by reversed-phase ultra-high performance liquid chromatographytandem mass spectrometry, J. Pharm. Biomed. Anal. 49 (2009)100-107.
[19] W.Song,D.Pabbisetty,E.Groeber,et al.,Comparison of fusedcore and conventional particle size columns by LC-MS/MS and UV: application to pharmacokinetic study, J. Pharm. Biomed.Anal. 50 (2009) 491-500.
[20] L.A. Romanyshyn, P.R. Tiller, Ultra-short columns and ballistic gradients: considerations for ultra-fast chromatographic liquid chromatographic-tandem mass spectrometric analysis, J. Chromatogr. A 928 (2001) 41-51.
[21] S.A. Schuster, B.E. Boyes, J.J. DeStefano, R. Orlando, J.J.Kirkland, Novel superficially porous HILIC column packing material for high speed seperations of biological molecules,Poster presented at the 38th International Symposium on High Performance Liquid Seperations and Related Techniques, Anaheim,CA, USA, June (2012) 16-21.
 Journal of Pharmaceutical Analysis2013年5期
Journal of Pharmaceutical Analysis2013年5期
- Journal of Pharmaceutical Analysis的其它文章
- A novel luminol-based chemiluminescence method for the determination of amikacin sulfate in serum by using trivalent copper-periodate complex
- Assay method for quality control and stability studies of a new antimalarial agent (CDRI 99/411)☆
- Simultaneous determination of human plasma protein binding of bioactive flavonoids in Polygonum orientale by equilibrium dialysis combined with UPLC-MS/MS
- Chiral separation of bavachinin in Fructus Psoraleae and rat plasma by liquid chromatography using permethylated-β-CD as a chiral selector
- Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7 μm and 5 μm: Intrinsic evaluation and application to a pharmaceutical test compound
- In situ modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in its formulation
