In situ modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in its formulation
Gehad G. Mohamed, F.A. Nour El-Dien, Eman Y.Z. Frag,Marwa El-Badry Mohamed
Chemistry Department, Faculty of Science, Cairo University, Gamaa Str., 12613 Giza, Egypt
1. Introduction
Naphazoline hydrochloride (NPZ-HCl; Fig.1) is used for the temporary relief of redness of the eye associated with minor irritations such as those caused by colds, pollen related allergies,smog, dust, wind, wearing contact lenses, or swimming. It acts
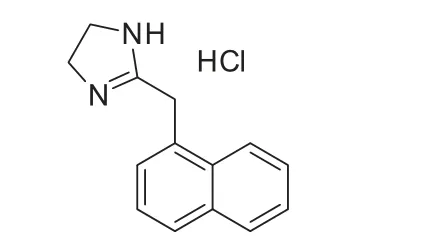
Fig.1 The structure of naphazoline hydrochloride.
Neozoline (Eye/Nasal drops) was produced by Amoun Pharmaceutical Company,El-Obour City,Cairo,Egypt.Every 100 mL contains 50 mg NPZ-HCl.on alpha adrenergic receptors in the arterioles of the conjunctiva to cause vasoconstriction, resulting in decreased conjunctival congestion [1]. Some analytical procedures have been reported for the determination of NPZ in both pure and pharmaceutical preparations including high-performance liquid chromatographic (HPLC) [2],micellar electrokinetic chromatographic [3,4], spectrophotometric[5—9] and potentiometric methods [10,11].
Potentiometry with ion selective electrodes (ISE) is one of the most important analytical tools capable of determining both organic and inorganic substances in medico-biological practice[12]. There is a constant progress in the number of electrodes capable of selectively identifying various drugs such as carbon paste electrodes (CPEs) [13—18]. Although the CPEs play an important role in the electrochemical analysis, the prepared pastes are soft and non-compactable and have to be packed into a special piston shaped holder with definite shape and size. However,shapes and designs of such electrodes are not suitable for every purpose as in the case of measurements in flowing streams or field monitoring with portable analyzers where the respective detection units need electrodes of special constructions [19].
Screen printing technology has increasingly been used for the mass production of inexpensive, reproducible and sensitive disposable electrochemical sensors for the determination of trace levels of compounds in pharmaceutical, biomedical, and environmental samples [20].
In this work, in situ modified screen printed electrodes (SPEs)and CPEs were characterized and optimized with regard to the main experimental parameters affecting the electrode responses,including the nature and content of modifier, pH, response time,temperature and interferences, which were investigated and discussed in detail, and were then applied for the determination of NPZ-HCl in pure form and pharmaceutical preparation (eye drop).
2. Experimental
2.1. Materials and reagents
Analytical grade chemicals and reagents were used.They included NPZ-HCl provided by Misr Company for Pharmaceutical Industry,Egypt. Relative high molecular weight PVC and graphite powder(synthetic 1—2 μm) were supplied by Aldrich. Tricresylphosphate(TCP) was purchased from Alfa-Aesar. Ion pairing agents such as sodium tetraphenylborate (NaTPB) and ammonium reineckate(RN; [NH4(Cr(NH3)2(SCN)4)H2O]) were supplied by Fluka.Phosphotungstic acid (PTA; H3[PW12O40]) and phosphomolybdic acid (PMA; H3[PMo12O40]), were purchased from BDH. Silicotungstic acid (STA, H4SiW12O40) was purchased from Sigma.
Nitric acid was supplied by Merck. Acetonitrile (AR) was supplied by Aldrich. Acetone, cyclohexanone and tetrahydrofuran were supplied by El-Nasr Company, Egypt.
2.2. Solutions
Stock drug solution(1.0×10—2M)was prepared by dissolving the appropriate amount of NPZ-HCl in bidistilled water. Other solutions (1.0×10-3—7.0×10—7M) were prepared by serial dilution from stock solution. NaTPB solution (1.0×10—2M) was prepared by dissolving an accurately weighed amount of the substance in warm water, adjusted to pH 9 by adding sodium hydroxide and made up to the desired volume with water. The resulting solution was standardized potentiometrically against standard thallium acetate solution (1.0×10—2M) [21]. Aqueous solutions of phosphotungstic acid, phosphomolybdic acid, silicotungstic acid and ammonium reineckate were prepared using the analytical grade chemicals and the exact concentrations of these solutions were determined by the appropriate recommended methods [22—24] and lower concentrated solutions were prepared by the appropriate dilutions.
All solutions must be protected from light by keeping them in dark colored quickfit bottles during the whole work.The bidistilled water was used throughout the experiments.
2.3. Equipment
Laboratory potential measurements were performed using a HANNA 211 pH meter. Silver—silver chloride double-junction reference electrode (Metrohm 6.0222.100) in conjugation with different drug ion selective electrodes was used.pH measurements were done using a Jenway 3505 pH meter. A digital multimeter connected to a portable PC and Brand digital buret was used for the measurement of the drug under investigation.
2.4. Procedures
2.4.1. Preparation of the working electrodes
2.4.1.1. Preparation of the SPEs. A manual screen printer was used to produce disposable SPEs. The used substrate which was not affected by the curing temperature or the ink solvent and was easily cut by scissors is a polyvinyl chloride flexible sheet(0.2 mm). The working electrodes were prepared using 6—30 mg ion pairing agent, PVC (8%, w/w), carbon powder (500 mg) and TCP as plasticizer.They were printed using homemade carbon ink and cured at 50°C for 30 min.The prepared electrodes were stored in the refrigerator at 4°C and can be used for measurements directly.
2.4.1.2. Preparation of the CPEs. The CPEs were prepared by thoroughly mixing various amounts (3—20 mg) of ion pairing agents with carbon powder (250 mg) and TCP (100 μL) in the mortar until homogenization of this mixture was achieved. The resulting paste was then packed firmly into the hole of the electrode body. The surface of the resulting CPE was polished using a filter paper to obtain new working surface.
2.4.2. Calibration of the sensors
The calibration of the ISPE and ICPE sensors under investigation was established by immersing the ISPE and ICPE working electrodes in conjunction with Ag—AgCl reference electrode in 50 mL beakers containing known aliquots of 1.0×10-7—1.0×10—2M NPZ-HCl standard solution.The potential reading was plotted against the negative logarithmic value of NPZ-HCl concentrations.The established calibration graph was used for subsequent determination of unknown naphazoline concentrations.
2.4.3. Effect of foreign compounds on the electrode selectivity
The potentiometric selectivity coefficients (KP°tA,B) were determined according to IUPAC guidelines using the separate solutions(SSM) and matched potential (MPM) methods [25,26].
2.4.4. Analytical applications
The proposed ISPE and ICPE sensors were found to be useful in the potentiometric determination of NPZ-HCl in pure solution and in pharmaceutical preparation by using the calibration, standard addition and potentiometric titration methods. Before the application of this potentiometric method,the neozoline eye drop solution was evaporated gently on water bath till dryness. The residue was then dissolved in methylene chloride depending on the fact that NPZ-HCl is insoluble in methylene chloride while chlorophenramine maleate is soluble in methylene chloride, so the residue was washed twice with methylene chloride to get rid of chlorophenramine maleate and the white residue containing NPZ-HCl was dissolved in a definite volume of distilled water and transferred quantitatively to a 50 mL beaker.
In the calibration method the electromotive force (EMF) resulting from immersing the prepared electrodes in conjunction with Ag/AgCl as the reference electrode in the prepared solutions was determined,and then the concentration of NPZ-HCl was calculated from the calibration graph of the corresponding electrode.
In the standard addition method, known increments of NPZHCl standard solution were added to constant volume of samples of different concentrations. The voltage was first measured in the pure sample, then the standard was added and the solutions were mixed well; a second reading was taken. From the change in potential readings for each increment the concentration of the unknown sample was calculated. In the potentiometric titration aliquots of the drug solution containing 0.5—6.0 mg were transferred to a 25 mL beaker.A standard solution of NaTPB was used as a titrant and the titration is monitored using ISPE and ICPE as indicator electrodes conjugated with Ag/AgCl as the reference electrode. The potential values were plotted against the volume of the titrant added and the end points were determined from the Sshaped curves using the first derivative plots. The obtained results were compared with that of British Pharmacopoeia.
3. Results and discussion
3.1. Electrode composition
The electroanalytical performance and electrode potential of an ISE is dependent upon the selective extraction of the target ion which creates the electrochemical phase boundary potential due to thermodynamic equilibria at the sample/electrode interface. Using of a suitable ion pairing agent in the electrode matrix followed by soaking in the drug solution may lead to the formation of an ion exchanger at the electrode surface by adsorption of a counter ion(analyte) from the solution during the measurement itself and can be extracted by the plasticizer into the electrode bulk. Using of a suitable ion pairing agent in the electrode matrix has the advantage of reducing the time required for the electrode preparation where there is no need for ion pair (IP) preparation as well as expansion of the application of ion selective electrodes (ISEs) for the determination of drugs that cannot be precipitated as suitable IPs. Also the same prepared electrodes can be used in preparation of ISEs for many drugs by soaking these electrodes in the solution of the drug under investigation.
In order to determine the suitable type and content of ion pairing agents (sodium tetraphenylborate, phosphotungstic acid,phosphomolybdic acid, silicotungstic acid and ammonium reineckate), several ISPEs and ICPEs were prepared containing 6—30 and 3—20 mg of ion pairing agents. CPE was soaked in 10—2M of NPZ-HCl solution. It was found that the most suitable ion pairing agent was TPB ion and its most suitable amount was 6 mg that gave the highest slope values of 59.7±0.6 and 59.2±0.2 mV decade-1for ISPE and ICPE, respectively, as shown in Table 1 and Fig.2. On the other hand, the lower concentration gave electrodes with lower slope while the higher content caused oversaturation and unsatisfactory performance due to steric hindrance effects at the interface.
3.2. Electroanalytical performance characteristics of the sensors
3.2.1. Calibration plots
The results presented in Table 2 and Fig.2 showed that the prepared sensors can be successfully applied for the potentiometric determination of the drug under investigation with linear response in the concentration range of 7.0×10-7to 1.0×10—2M with slope values of 59.7±0.6 and 59.2±0.2 mV decade-1for ISPE and ICPE, respectively.
3.2.2. Effect of pH
To investigate pH effect on the electrode potential, the potential readings were recorded for two different concentrations (10—4or 10—2M) of NPZ-HCl at different pH values (pH 2—10). The potential changes as a function of pH are shown in Fig.3.This figure indicates that the electrodes response was independent of the pH within the range from 3.1 to 7.9 for ISPE and ICPE with NaTPB ion pairing agent, respectively. At lower pH values the decrease in mV readings may be attributed to the interference from hydronium ion, while at higher pH values the decrease in the mV readings may be due to base precipitates in the test solution and consequently, the concentration of unprotonated species gradually increased. As a result, lower EMF readings were recorded.
3.2.3. Effect of temperature
In order to determine the isothermal coefficient of the electrodes(dE°/dt), the standard electrode potentials (E°) were determined from the calibration graphs (the intercepts at p[NPZ]=0)of ISPE and ICPE carried out at different temperatures (10, 20,30, 40, 50 and 60°C) and plotted versus (t-25), where (t) is the temperature of the experiment in degree centigrade and according to the following equation [27]:
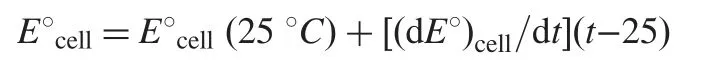
A straight line is obtained and the slope of this line represents the isothermal coefficients of the electrodes which were found to be 0.0022 and 0.0008 V/°C for ISPE and ICPE modified with TPB, respectively. The obtained values of the isothermalcoefficient reveal that the electrodes under investigation had high thermal stability within the used range of temperature. The investigated electrode can be used up to 60°C without noticeable deviation from the Nernstian behavior.

Table 1 Effect of the nature and content of ion pairing agents on the performance of ISPE and ICPE sensors.
3.2.4. Interference studies
As shown in Table 3 the results obtained show no significant interference and this reflects a very high selectivity of the electrodes under investigation toward NPZ-HCl. The results also indicate that there was no serious interference from glucose,sucrose, maltose, fructose, starch and lactose. The inorganic cations did not interfere due to the differences in their ionic size and hence their mobilities, polarities, and permeabilities as compared to those of NPZ+cation.
3.2.5. Response time
The response time is an important factor which characterizes ISEs.It can be defined as the time taken by the electrodes to reach steady potential values of 90% of the final equilibrium values, after successive immersions in a series of solutions, each having a 10-fold concentration difference [31—35]. The dynamic response time of the sensors under study was determined for the concentration range from 1.0×10—6to 1.0×10—3M. Fig.4 illustrates a representative plot of the potential changes versus time for electrodes under study. The proposed sensors have very short response time of 4—7 s and 5—8 s for ISPE and ICPE,respectively.
3.2.6. Effect of soaking time
As shown in Fig.5, it was found that the optimum soaking time for the ICPE electrode was 15 min and longer soaking time affects negatively on the response of the electrode.This may be due to the leaching of the active ingredients (ion-pairing agent and plasticizer) to the bathing solution [36—38].
3.2.7. Lifetime
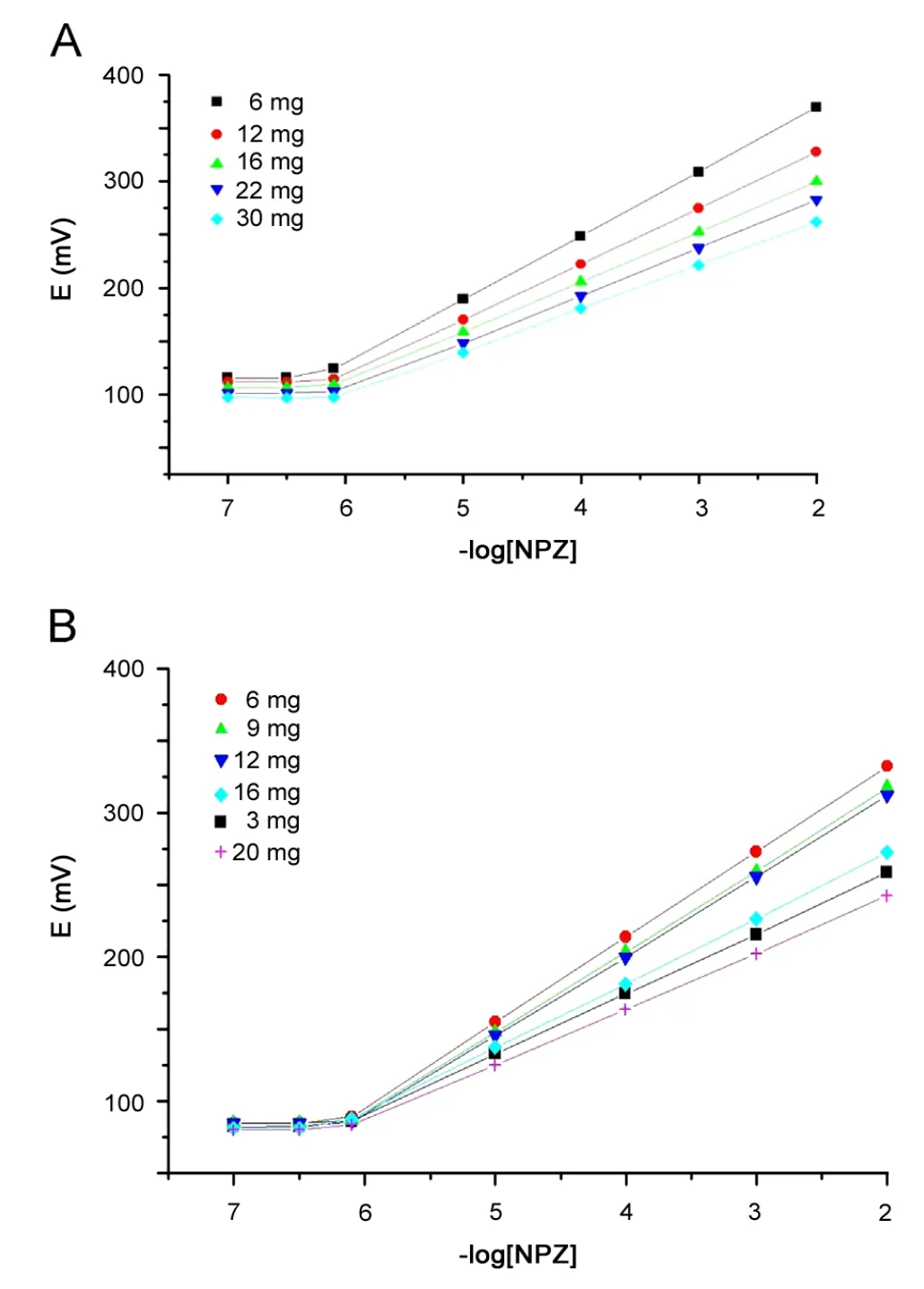
Fig.2 Effect of NaTPB on the performance of(A)ISPE and(B)ICPE sensors.
The lifetime of the investigated sensors was studied by periodically constructing the calibration graphs under optimum conditions on different days.For sensors under investigation it was found that ISPE has a life time of 28 days while ICPE has 30 days. A shiny new surface is obtained every time for measurement using ICPE by squeezing out a small portion of the paste and polishing it on a filter paper.After preparation of ISPEs,they were kept at 4°C and directly used for potentiometric measurements.
3.2.8. Analytical application
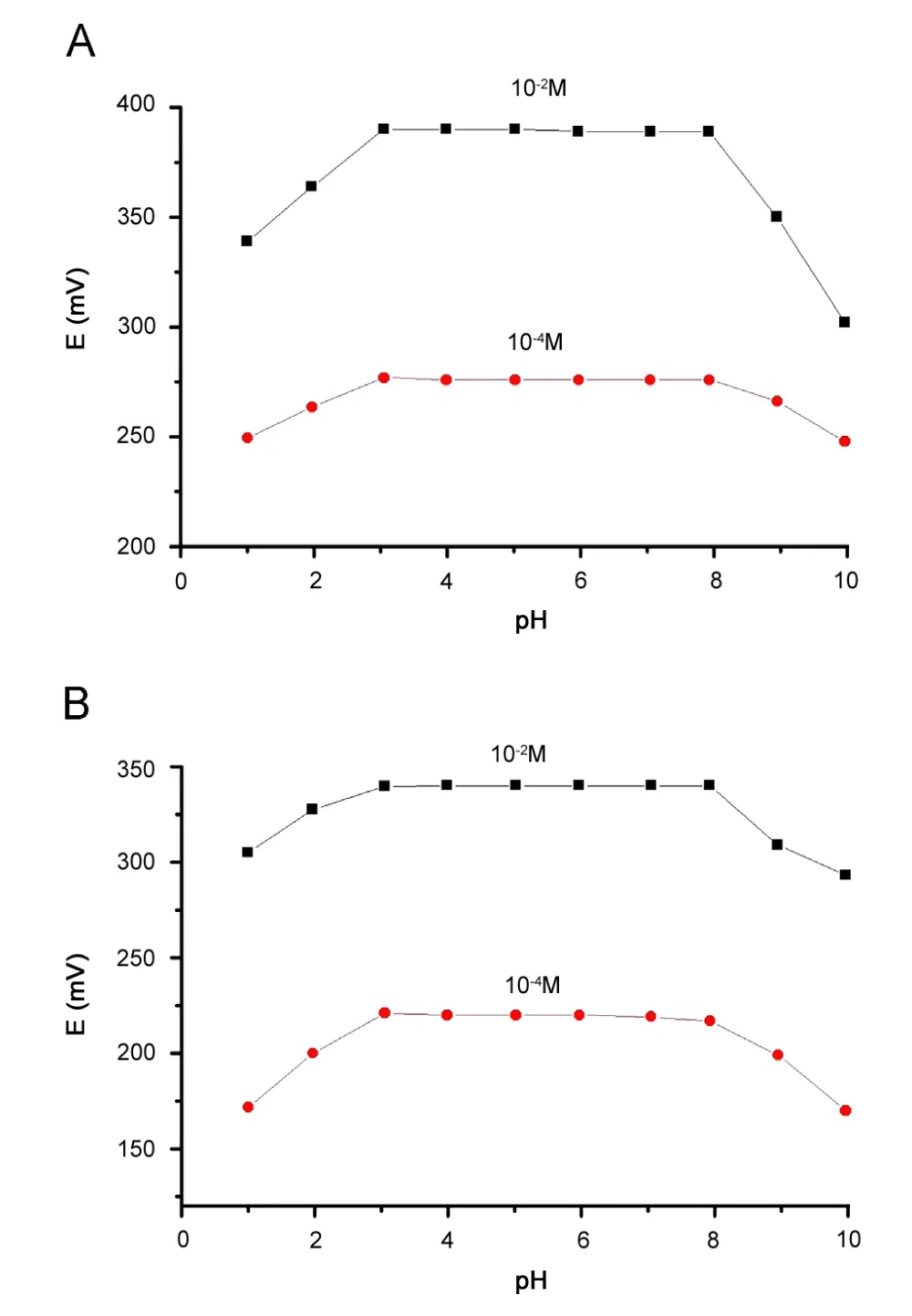
Fig.3 Effect of pH on(A)ISPE and(B)ICPE modified with NaTPB ion pairing agent.
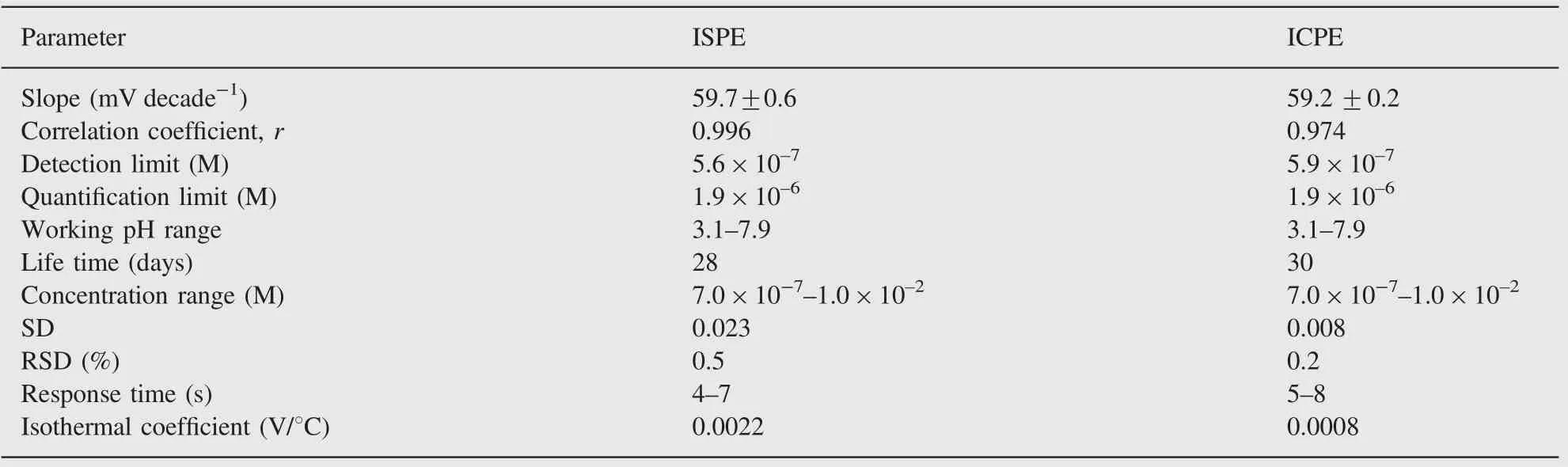
Table 2 Response characteristics of ISPE and ICPE sensors.
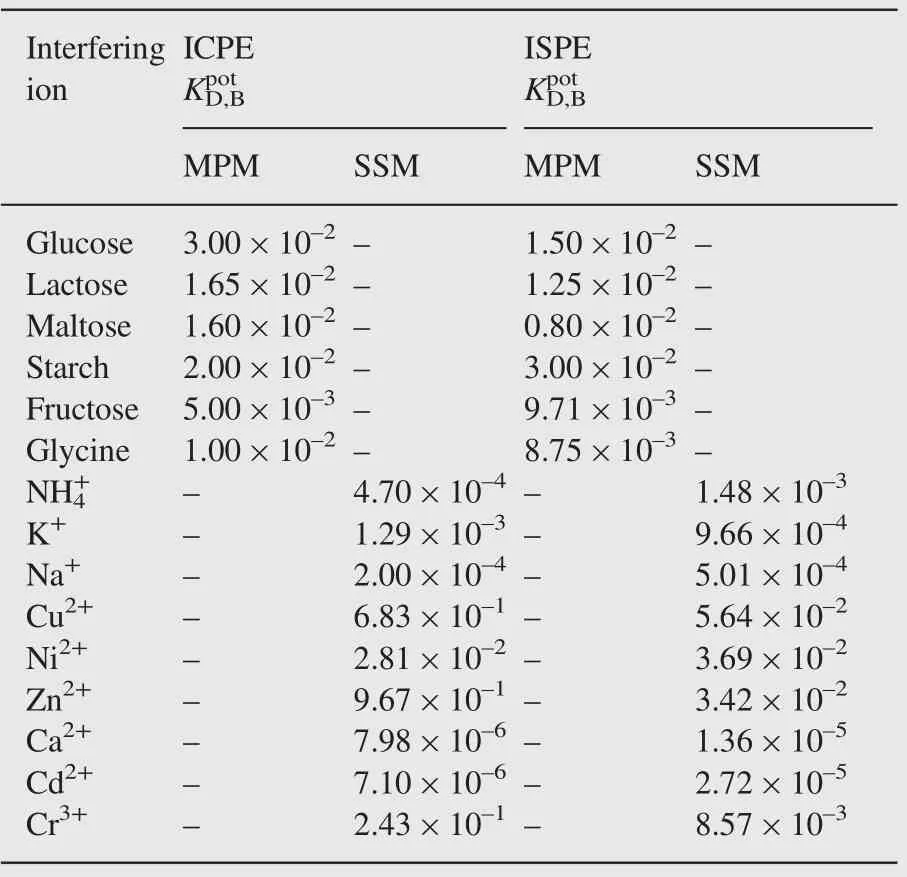
Table 3 Selectivity coefficients values for ISPE and ICPE sensors.
The optimized sensors under investigation have been successfully used for the potentiometric determination of NPZ-HCl by using the standard addition, calibration and potentiometric titration methods, and the results are summarized in Table 4. In order to estimate the quality of the results, recovery values were also determined and are presented in the same table. The obtained results in Table 4 using the proposed sensors were in good agreement with those obtained using the official method [39].
3.3. Method validation
Electroanalytical method validation is the process used to confirm that the determination procedure employed for a specific test is suitable for its intended use like other analytical methods [40].Accuracy,precision,linearity,specificity, limits of detection(LOD)and quantification(LOQ)were achieved using a standard NPZ-HCl stock solution.
3.3.1. Accuracy
Accuracy is an important requirement of electroanalytical methods.It can be defined as the closeness between the true or accepted reference value and the obtained value [40]. The accuracy of the proposed method using in situ SPE and CPE was investigated by the determination of NPZ-HCl in spiked samples prepared from serial concentrations of NPZ-HCl reference standards. The results summarized in Table 5 show high accuracy of the proposed method, as indicated by the percentage recovery values. The statistical analysis of the results using student's t-test and F-test showed no significant differences between them regarding accuracy and precision (Table 4).
3.3.2. Precision
Precision is a measure of how close results are to one another.Precision is also expressed as the closeness of agreement between independent test results obtained under stipulated conditions.Precision is usually expressed as standard or relative standard deviations of the replicate analysis[40].Hence the precision of the proposed potentiometric method using the sensors under investigation was measured as percentage relative standard deviation (RSD %). Intra-day and inter-day precisions were assessed using three concentrations and five replicates of each concentration. The relative standard deviations were found to be very small indicating reasonable repeatability and reproducibility of the proposed method as shown in Table 5.
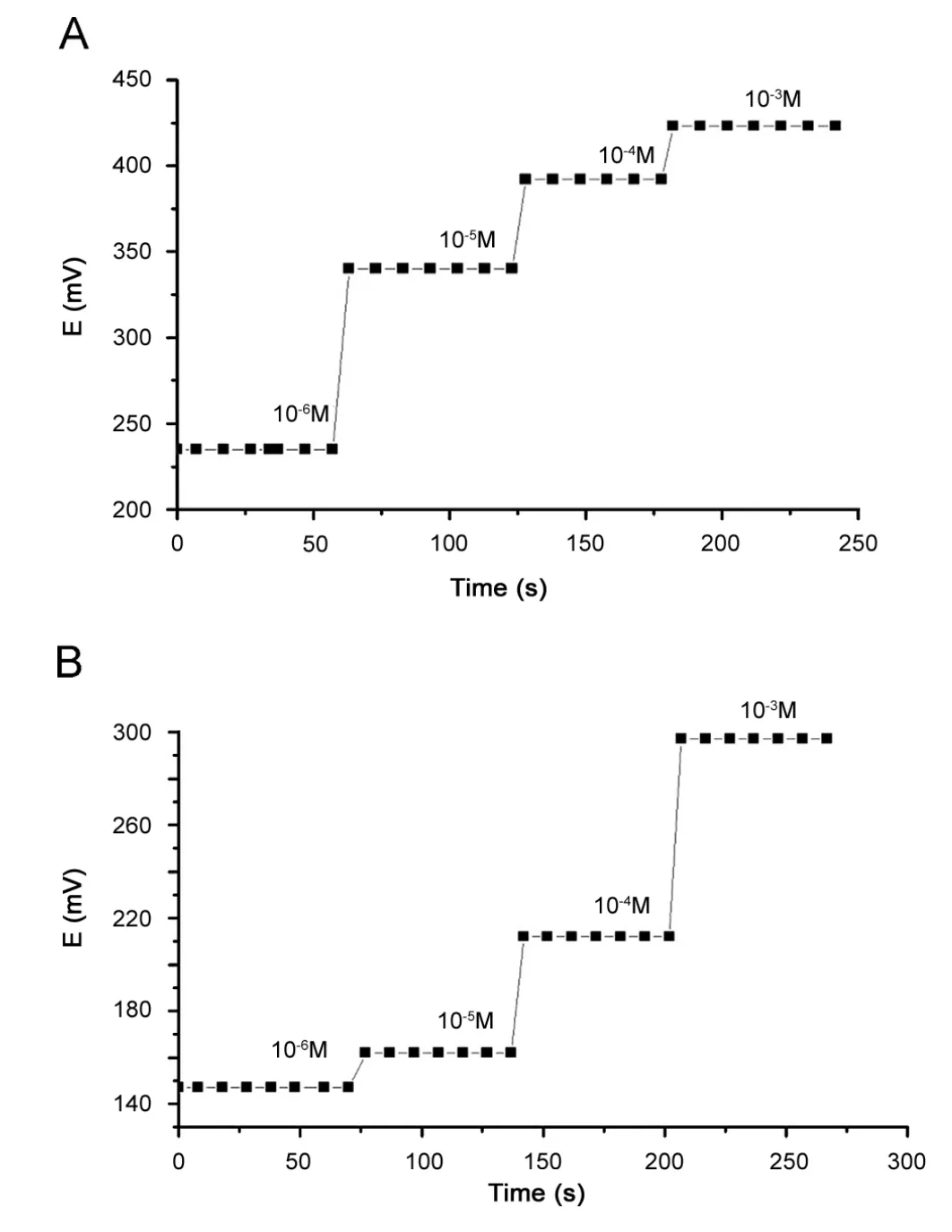
Fig.4 The dynamic response time of (A) ISPE and (B) ICPE potentiometric sensors modified with NaTPB ion pairing agent.
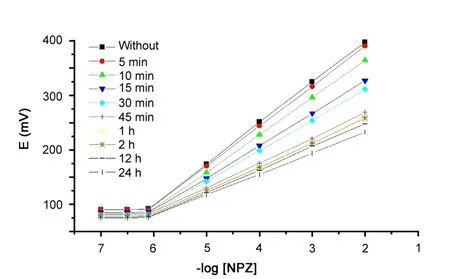
Fig.5 Effect of soaking time on the ICPE.
3.3.3. Linearity
Linearity of an electroanalytical technique can be defined as a measure of how well the calibration plot of electroanalytical response versus concentration approximates a straight line [40].The standard calibration graph was established using five concentrations of standard NPZ-HCl. It was found that a linear relationship is present between the electrode potential (mV) and the log[NPZ-HCl]. The regression data, correlation coefficients(r)and other statistical parameter are presented in Table 2.
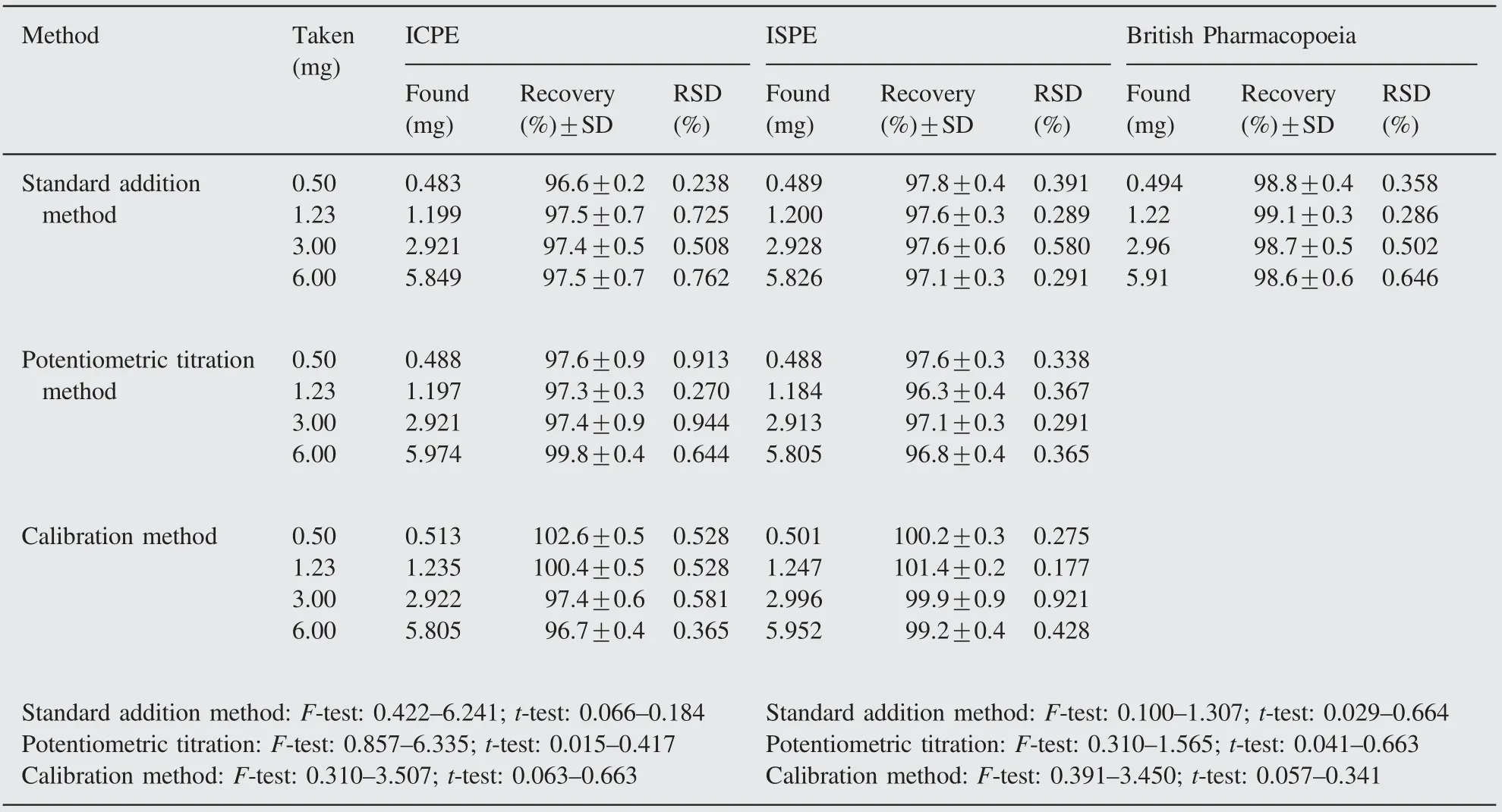
Table 4 Determination of NPZ-HCl in pharmaceutical formulation (Neozoline) using ISPE and ICPE.
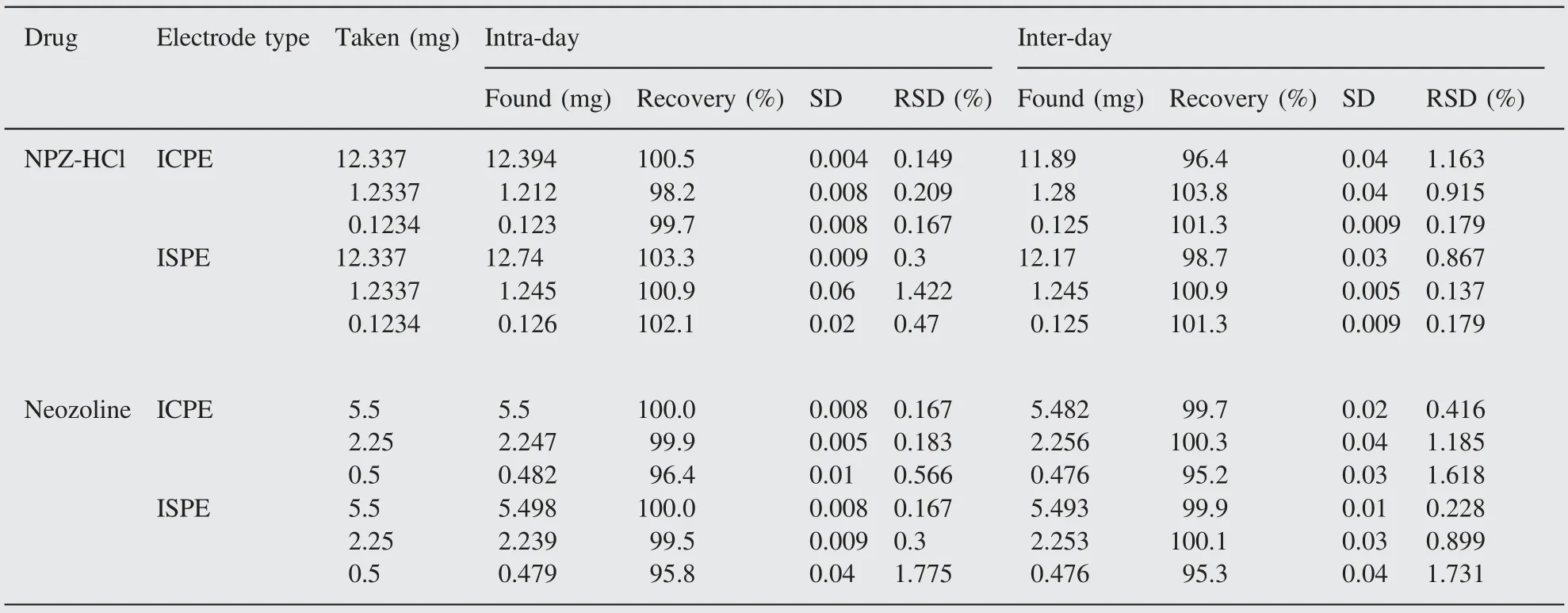
Table 5 Evaluation of accuracy and precision (intra- and inter-day) of the ISPE and ICPE sensors.
3.3.4. Specificity
The specificity of the method was examined by observing any interference caused by the common excipients of the pharmaceutical formulation. It was found that these compounds did not interfere with the results of the proposed method as shown in Table 3.
3.3.5. LOD and LOQ
LOD is the lowest quantity of the investigated compound in a sample that can be detected,but not necessarily quantified with an acceptable uncertainly. LOD of an electroanalytical method is an important factor if quantitative measurements are to be made at concentrations close to it. Especially, LOD is necessary for the trace analysis of drug active components in pharmaceuticals and/or biological samples [40]. The values of LOD that are presented in Table 2 indicate that the sensors under investigation are highly sensitive, selective and can be applied in determination of small amounts of NPZ-HCl.
LOQ is the lowest amount of compound that can be measured in the sample matrix at an acceptable level of accuracy and precision. For many pharmaceutical and biological applications,the LOQ is generally a more useful factor than LOD. The LOQ was determined by establishing the least concentration that can be measured according to ICH Q2(R1)recommendations [41], below which the calibration range is non-linear, and was found to be 1.9×10—6and 1.9×10—6M for ISPE and ICPE, respectively.
4. Conclusion
The present work involves the preparation of screen printed and carbon paste electrodes with in situ mode of modification for the determination of NPZ-HCl drug. The screen printed electrodes were characterized and optimized with respect to the main experimental parameters affecting the electrode performance,including composition, pH, temperature, response time and interferences in comparison to carbon paste electrodes. The electrodes showed good selectivity for NPZ-HCl with respect to some inorganic cations, sugars and glycine. The developed electrodes have been successfully used for the determination of NPZ-HCl in pharmaceutical preparation using the standard addition, potentiometric titration and calibration methods.The obtained results were in good agreement with those obtained using the official method.According to the results obtained, the potentiometric sensors can be successfully applied for the routine analysis of this drug.
[1] 〈http://www.drugs.com/mmx/naphazoline-hydrochloride.html〉,2000—2013.
[2] P. Chocholouš, D. Šatínský, P. Solich, Fast simultaneous spectrophotometric determination of naphazoline nitrate and methylparaben by sequential injection chromatography, Talanta 70 (2) (2006)408—413.
[3] J.M.L. Gallego, J.P. Arroyo, Determination of prednisolone and the most important associated compounds in ocular and cutaneous pharmaceutical preparations by micellar electrokinetic capillary chromatography, J. Chromatogr, B: Anal. Technol. Biomed. Life Sci.784 (1) (2003) 39—47.
[4] J.M.L.Gallego,J.P.Arroyo,Determination of prednisolone,naphazoline, and phenylephrine in local pharmaceutical preparations by micellar electrokinetic chromatography, J. Sep. Sci. 26 (9—10)(2003) 947—952.
[5] E. Souri, M. Amanlou, H. Farsam, et al., A rapid derivative spectrophotometric method for simultaneous determination of naphazoline and antazoline in eye drops, Chem. Pharm. Bull. 54 (1) (2006) 119—122.
[6] H.C. Goicoechea, M.S. Collado, M.S. Satuf, et al., Complementary use of partial least-squares and artificial neural networks for the nonlinear spectrophotometric analysis of pharmaceutical samples, Anal.Bioanal. Chem. 376 (3) (2003) 460—465.
[7] J.J. Charles, M. Bertucat, Simultaneous determination of naphazoline nitrate and tetramethylthionine base in eye drops by first-derivative UV spectrophotometry, Anal. Lett. 32 (2) (1999) 373—382.
[8] S.O. Hman, Direct determination of antazoline and naphazoline in mixtures, Drug Dev. Ind. Pharm. 13 (7) (1987) 1257—1265.
[9] S.Belal,M.A.Elsayed,M.E.Abdel-Hamid,et al.,Utility of chloranil in assay of naphazoline, clemizole, penicillin G sodium, and piperazine, J. Pharm. Sci. 70 (1) (2006) 127—130.
[10] S.M.Ghoreishi,M.Behpour,M.Nabi,A novel naphazoline-selective membrane sensor and its pharmaceutical applications,Sens.Actuators B 113 (2) (2006) 963—969.
[11] E.Y.Z. Frag, G.G.Mohamed,F.A.Nour El-Dien,et al., Construction and performance characterization of screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in pharmaceutical preparations, Analyst 136 (2) (2011) 332—339.
[12] G. Baiulescu, V. Cosofret, Ion-Selective Membrane Electrodes.Applications of Organic Analysis, Wiley, New York, 1977.
[13] E. Khaled, H.N.A Hassan, G.G. Mohamed, et al., Carbon paste and PVC electrodes for the flow injection potentiometric determination of dextromethorphan, Talanta 81 (2010) 510—515.
[14] E. Khaled, H.N.A Hassan, G.G. Mohamed, et al., β-Cyclodextrin-based potentiometric sensors for flow-injection determination of acetylcholines,Int. J. Electrochem. Sci. 5 (2010) 448—458.
[15] E.Y.Z. Frag, G.G. Mohamed, H.M.S. Alelaiwi, Electroanalytical determination of sildenafil in Viagra tablets using screen-printed and conventional carbon paste electrodes, J. Electroanal. Chem. 659(2011) 121—127.
[16] E.Y.Z. Frag, A.M.K. Mohamed, G.G. Mohamed, et al., Construction and performance characterization of ion selective electrodes for potentiometric determination of ranitidine hydrochloride in pharmaceutical preparations and biological fluids, Int. J. Electrochem. Sci. 6(2011) 3508—3524.
[17] E.Y.Z. Frag, G.G. Mohamed, M.M. Khalil, et al., Potentiometric determination of ketotifen fumarate in pharmaceutical preparations and urine using carbon paste and PVC membrane selective electrodes,Int. J. Anal. Chem. 2011 (2011) 1—7.
[18] F.A. Nour El-Dien, G.G. Mohamed, E.Y.Z. Frag, et al., Modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in pure and pharmaceutical preparations, Int. J. Electrochem. Sci. 7 (2012)10266—10281.
[19] I Svancara, P. Kotzian, M. Bartos, et al., Groove electrodes: a new alternative of using carbon pastes in electroanalysis, Electrochem.Commun. 7 (2005) 657—662.
[20] P.F. Boladoa, D.H. Santosa, P.J.L. Ardisanaa, et al., Electrochemical characterization of screen-printed and conventional carbon paste electrodes, Electrochim. Acta 53 (10) (2008) 3635—3642.
[21] K. Vytras, Potentiometric titrations based on ion-pair formation, Ion-Sel. Electrodes Rev. 7 (1985) 77—164.
[22] H. Hayashi, J.B. Moffat, Determination of phosphorus and tungsten in heteropoly acids by EDTA-titration, Talanta 29 (11) (1982) 943—945.
[23] T.G. Towns, Determination of aqueous phosphate by ascorbic acid reduction of phosphomolybdic acid, Anal. Chem. 58 (1) (1986)223—229.
[24] W. Selig, Potentiometric titration of thallium(I) with sodium tetraphenylborate, using ion-selective electrodes, Talanta 27 (11) (1980)914—916.
[25] Y. Umezawa, P. Buhlmann, K. Umezawa, et al., Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (Technical Report), Pure Appl. Chem. 72 (10) (2000) 1851—2082.
[26] IUPAC, Pure Appl. Chem. 72 (2000) 1852.
[27] L.I. Antropov,Theoretical Electrochemistry, Mir Publisher,Moscow,1977.
[28] K. Tohda, D. Dragoe, M. Shibata, et al., Studies on the matched potential method for determining the selectivity coefficients of ionselective electrodes based on neutral ionophore, Anal. Sci. 17 (2001)733—743.
[29] R.P. Buck, E. Lindner, Recommendations for nomenclature of ionselective electrodes. (IUPAC Recommendation 1994), Pure Appl.Chem. 66 (1994) 2527—2536.
[30] Y. Umezawa, K. Umezawa, H. Sato, Selectivity coefficients for ionselective electrodes (Technical Report), Pure Appl. Chem. 67 (3)(1995) 507—510.
[31] M.R. Ganjali, Z. Memari, F. Faridbod,et al., Samarium microsensor:an asymmetric potentiometric membrane sensor, Int. J. Electrochem.Sci. 3 (2008) 1169—1179.
[32] V.K.Gupta,S.Chandra,R.Mangla,Magnesium-selective electrodes,Sens. Actuators B 86 (2—3) (2002) 235—241.
[33] V.K. Gupta, R. Prasad, A. Kumar, Magnesium—tetrazaporphyrin incorporated PVC matrix as a new material for fabrication of Mg2+selective potentiometric sensor, Talanta 63 (4) (2004) 1027—1033.
[34] H.A. Zamani,G. Rajabzadeh, M.R. Ganjali, Highly selective and sensitive chromium(III) membrane sensors based on 4-amino-3-hydrazino-6-methyl-1,2,4-triazin-5-one as a new neutral ionophore.Sens. Actuators B 119(1) (2006) 41—46.
[35] A.K. Jain, V.K. Gupta, S. Radi, et al., A comparative study of Pb2+selective sensors based on derivatized tetrapyrazole and calix[4]arene receptors, Electrochim. Acta 51 (12) (2006) 2547—2553.
[36] E. Linder, K. Toth, E. Pungor, Dynamic Characteristics of Ion-Selective Electrodes, CRC Press, Boca Raton, FL, 1988.
[37] E.Y.Z. Frag, M.A. Zayed, M.M. Omar, et al., Potentiometric determination of chlorpromazine HCl using carbon paste electrode in pure and pharmaceutical preparations, Int. J. Electrochem. Sci. 7(2012) 650—662.
[38] E.Y.Z.Frag,T.A Ali,G.G.Mohamed,et al.,Construction of different types of ion-selective electrodes. Characteristic performances and validation for direct potentiometric determination of orphenadrine citrate, Int. J. Electrochem. Sci. 7 (2012) 4443—4464.
[39] British Pharmacopoeia, Stationary Office London, vol. 1517, 2008.
[40] M. Gumustas, A.S. Ozkan, The role of and the place of method validation in drug analysis using electroanalytical techniques, Open Anal. Chem. J. 5 (2011) 1—21.
[41] ICH Harmonized Tripartite Guideline: Validation of Analytical Procedures. Text and Methodology, Q2(R1), 2005.
 Journal of Pharmaceutical Analysis2013年5期
Journal of Pharmaceutical Analysis2013年5期
- Journal of Pharmaceutical Analysis的其它文章
- A novel and rapid microbiological assay for ciprofloxacin hydrochloride
- Determination of sodium hyaluronate in pharmaceutical formulations by HPLC-UV
- Copper interactions with DNA of chromatin and its role in neurodegenerative disorders
- A critical quality parameter in quantitative fused-core chromatography: The injection volume
- Application of LC-MS/MS for quantitative analysis of glucocorticoids and stimulants in biological fluids
- Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7 μm and 5 μm: Intrinsic evaluation and application to a pharmaceutical test compound
