Assay method for quality control and stability studies of a new antimalarial agent (CDRI 99/411)☆
Kirn Khndelwl, Shkti Deep Pchuri, Sofi Zidi, Pnkj Dwivedi,Ashok Kumr Shrm, Chndn Singh, Anil Kumr Dwivedi,*
aDivision of Pharmaceutics, CSIR-Central Drug Research Institute, Chattar Manzil Palace, Lucknow 226001, India
bDivision of Medicinal and Process Chemistry, CSIR-Central Drug Research Institute, Chattar Manzil Palace, Lucknow 226001, India
Available online 6 May 2013
1. Introduction
Malaria is still one of the major infectious diseases caused by a protozoa Plasmodium falciparum, with billions of people at risk for this deadly disease especially in underdeveloped countries[1—4]. The developing world urgently needs new, safe, and effective antimalarial drugs as the failure of the contemporary drugs due to the increasing resistance of the malaria parasite has further complicated the problem of treating malaria [5]. Semisynthetic derivatives of artemisinin are effective against both the chloroquine-sensitive and chloroquine-resistant malaria, hence used for the treatment of malaria caused by multidrug resistant P. falciparum [6]. CDRI 99/411 (Fig.1) is a potent orally active 1,2,4-trioxane antimalarial candidate compound for the treatment of malaria [7—8]. 1,2,4-Trioxane ring system in artemisinin is essential for the antimalarial activity due to the presence of peroxide group [9]. A synthetic analog of this is a better replacement for management of falciparum malaria. Patent for CDRI 99/411 is granted as it has shown a good profile for development as a drug and hence has been taken up for further development at our Institute [10]. Synthesis of CDRI 99/411 is shown in Fig.2. In this reaction 1-(4-cyclohexylphenyl) ethanone(1) is converted to ethyl 3-(4-cyclohexylphenyl)-but-2-enoate (2)and the resulting ester is converted to 3-(4-cyclohexyl- phenyl)-but-2-en-1-ol (3), which finally results into CDRI 99/411 after peroxidation with 2-admantanone. In a multistep synthesis, the starting material and the intermediates may form the process impurities; thus there is a need for an analytical tool for identification and quantification of CDRI 99/411[11]. Liquid chromatography is usually an analytical method of choice for pharmaceutical quality control [12], so the present study was undertaken to develop and validate an HPLC method for CDRI 99/411 having good separation of starting material, intermediates for process quality control and degradation products in stress stability studies.
2. Experimental
2.1. Reagents and standards
Standard CDRI 99/411,starting material(1)and intermediates(2,3)were supplied by the Medicinal and Process Chemistry Division of our Institute. HPLC grade acetonitrile was obtained from Spectrochem Pvt. Ltd. (Mumbai, India) and glacial acetic acid (analytical grade) from E. Merck (India) Ltd. (Mumbai, India). Milli-Q pure water was obtained from a Millipore Elix water purification system purchased from Millipore India Pvt.Ltd.(New Delhi,India). Other reagents used were of analytical grade.
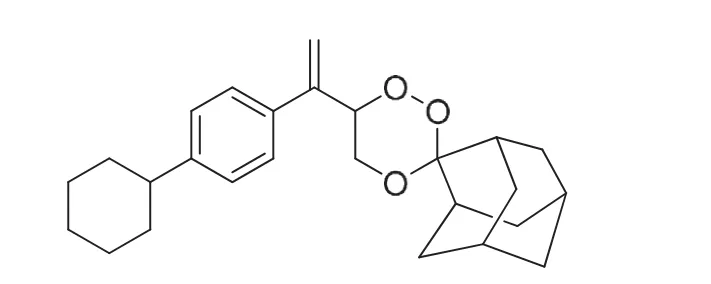
Fig.1 Chemical structures of CDRI 99/411.
2.2. Apparatus and chromatographic conditions
Waters HPLC (Milford, MA, USA) system was equipped with a binary gradient pump (model 515, Waters), an auto sampler injector (Model 2707, Waters) and a diode array detector (Model 2998,Waters).The sample and standard solution were analyzed by HPLC after injecting 20 μL.The following gradient was used with(A) water containing 0.1% glacial acetic acid and (B) acetonitrile:0 min, 30:70 (A:B); 5 min, 10:90; 15 min, 10:90; 17 min, 30:70;and 20 min, 30:70. HPLC separation was achieved on a RP-select B Lichrospher®column (250×4 mm, 5 μm, Merck, Germany).Column effluent was monitored at 245 and 275 nm. The solutions were filtered and degassed before use. Chromatography was performed at 30°C at a flow rate of 1.0 mL/min. Data were acquired and processed using Waters HPLC interface and software, Empower 2.
2.3. Preparation of stock and working standard solutions
Primary stock solutions of compounds 1,2,3 and CDRI 99/411 were prepared by dissolving the respective compounds in acetonitrile to achieve concentrations of 2.06, 1.92, 1.42 and 1.36 mg/mL respectively.These solutions were further diluted by transferring 1 mL each of the above stocks into 10 mL volumetric flask and making up to the volume with acetonitrile.Finally,solutions were prepared by diluting the diluted solution with acetonitrile to achieve different dilutions required for calibration of these compounds.
2.4. Preparation of sample solution
The stock solutions for samples of bulk candidate drug prepared for preclinical studies and samples from the stability chambers were prepared by dissolving these samples in acetonitrile. These samples were analyzed after dilution of stock with acetonitrile.
2.5. Method validation
2.5.1. Specificity and selectivity
The chromatographic interferences were assessed by comparing chromatograms of blank acetonitrile with those of samples spiked with compounds 1, 2, 3 and CDRI 99/411 in acetonitrile.
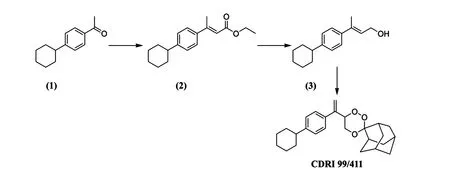
Fig.2 Schematic representation of synthesis of CDRI 99/411.
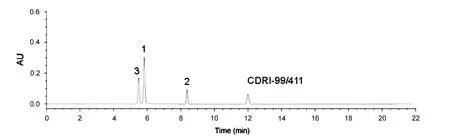
Fig.3 HPLC chromatogram of mixture of compounds 1, 2, 3 and CDRI 99/411.
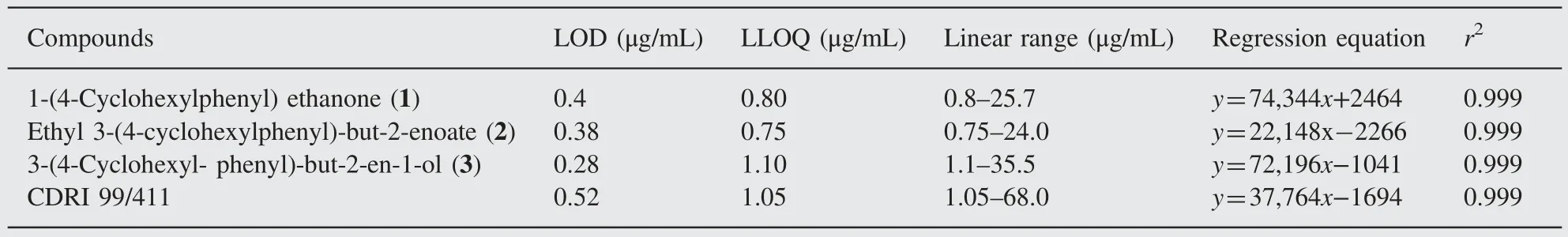
Table 1 Regression data, limit of detection (LOD) and lower limit of quantification (LLOQ) for compounds CDRI 99/411, 1, 2 and 3.
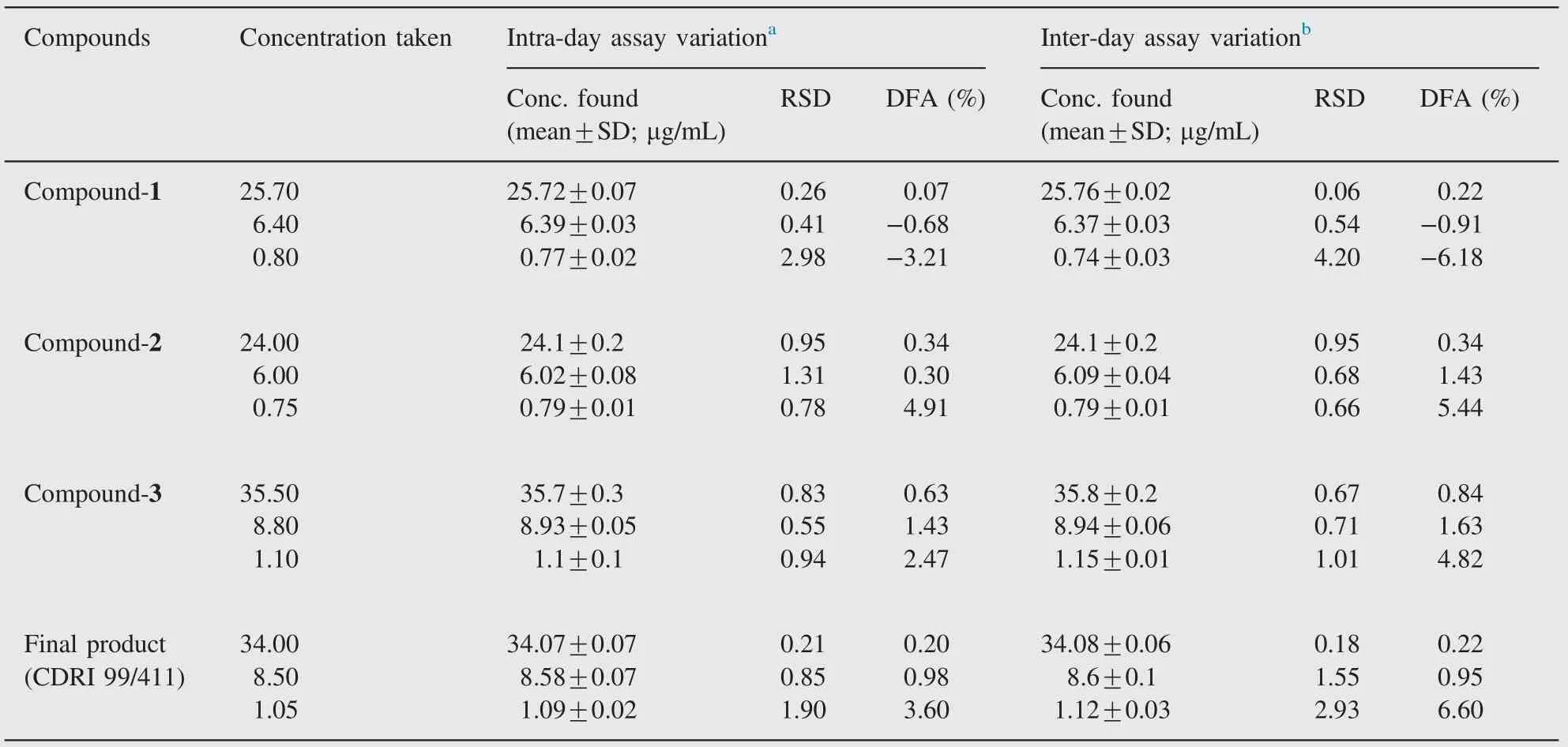
Table 2 Intra- and inter-day assay variation of compounds 1, 2, 3 and CDRI 99/411.
2.5.2. Linearity of calibration curves
The linearity of the method was established by constructing calibration curves over a concentration range of 0.8—25.7, 0.75—24.0,1.1—35.5 and 1.05—68.0 μg/mL for compounds 1, 2, 3 and CDRI 99/411, respectively. Plots of peak area (response) against analyte concentration were used. The slope, intercept and the correlation coefficient of each calibration curve were determined using linear regression analysis.
2.5.3. Accuracy and precision
In order to assess the intra- and inter-day precision and accuracy of the assay, quality control (QC) samples at low, medium and high concentrations were prepared as described above. The intra-day precision of the assay was assessed by calculating the relative standard deviation(RSD)for the analysis of QC samples in three replicates and inter-day precision was determined by the analysis of three replicates QC samples on three consecutive days. The accuracy was calculated on the basis of the difference in the mean calculated concentration and concentration taken (% deviation from actual concentration, % DFA).
2.6. Stability studies
Forced degradation studies were performed to evaluate the stability indicating properties and specificity of the method [13]. All solutions used for stress studies were prepared with acetonitrile at an initial concentration of 1 mg/mL of CDRI 99/411 and heated at 80°C and then diluted with acetonitrile to give a final concentration of 50 μg/mL.
The acidic degradation of CDRI 99/411 was studied in hydrochloric acid(0.1 M)at 80°C for 8 h and alkaline degradation was studied in sodium hydroxide (0.1 M) at 80°C for 1 h and the stressed samples were instantly cooled and diluted with acetonitrile.Oxidation was conducted by refluxing with 3%H2O2for 1 h at 80°C and then diluted with acetonitrile.
3. Results and discussion
3.1. Chromatography
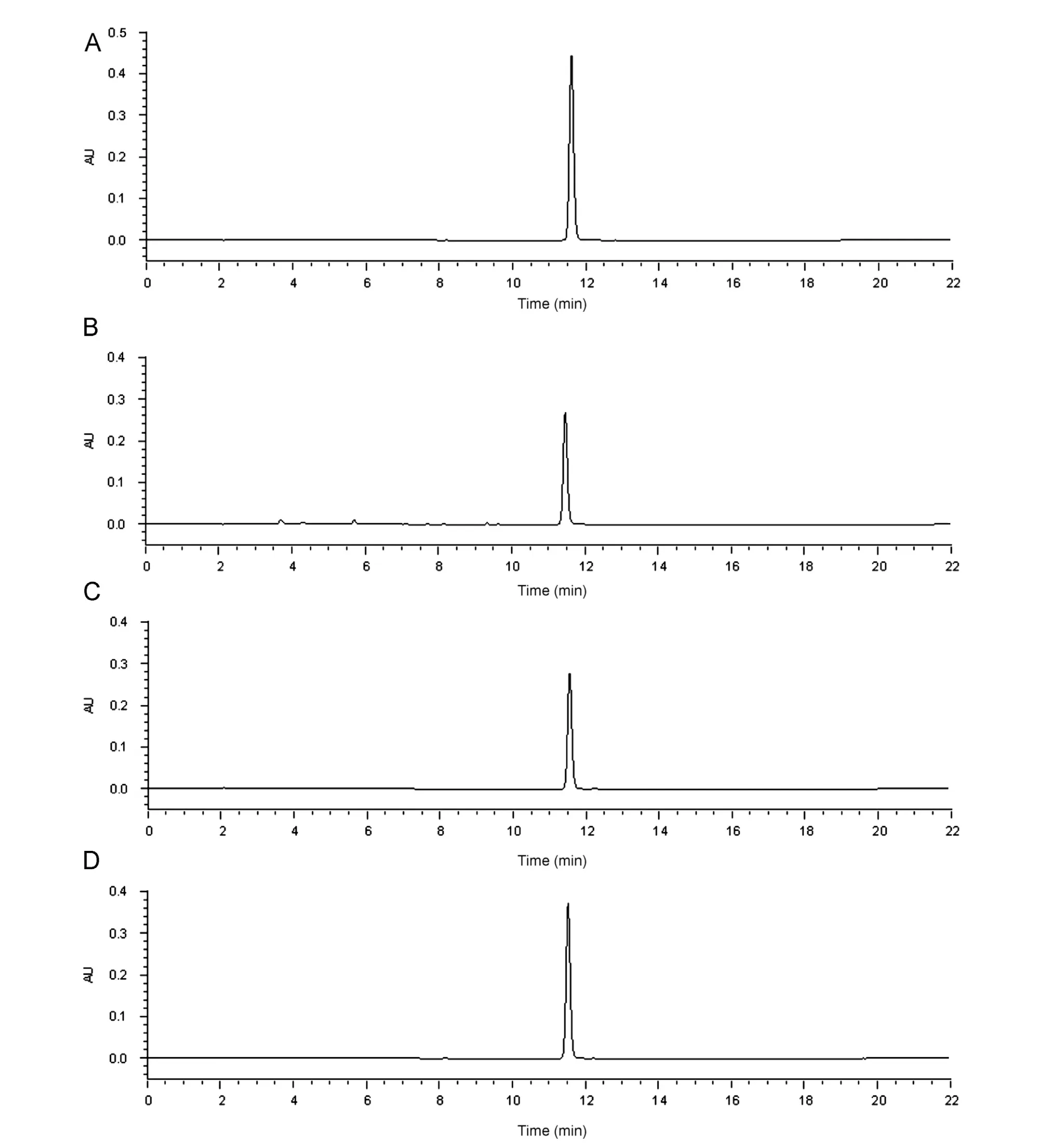
Fig.4 Chromatogram of standard CDRI 99/411(A)and representative chromatograms of CDRI 99/411 on acidic(B),alkaline(C)and oxidative(D) degradations.
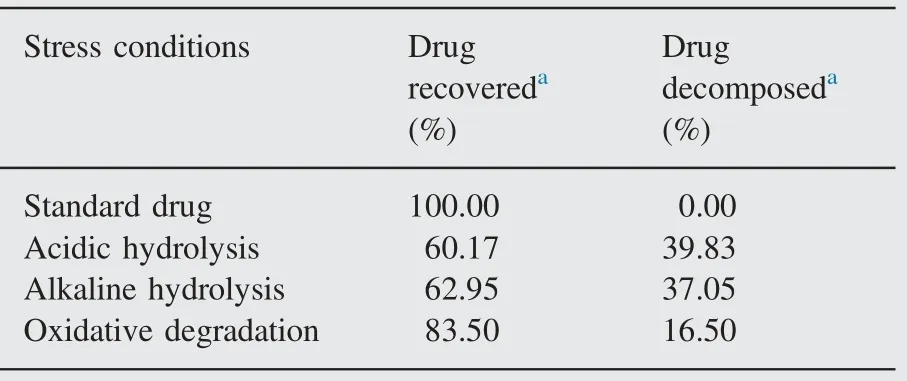
Table 3 Forced degradation studies of CDRI 99/411.
The purpose of this study was to develop an HPLC method for in process quality control and for stability studies.During the method development,priority was given for the complete separation of the compound of interest CDRI 99/411 from other starting material and intermediates involved in the process of synthesis of the final product.Optimum chromatographic conditions were obtained after running various combinations of acetonitrile and water with glacial acetic acid as the mobile phase with C18 (Lichrocart®column;250×4 mm, 5 μm, Merck), Cyno (Lichrocart®column;250×4 mm, 5 μm, Merck) and RP-select B (Lichrospher®column; 250×4 mm, 5 μm, Merck) columns. C18 and Cyno columns resulted in peak with tailing and higher flow rate was required. Finally RP-select B Lichrospher®column was used for the study as it gave sharp peak for compounds CDRI 99/411, 1, 2 and 3. Gradient elution was preferred over the isocratic method as separation of compounds 1 and 2 was not proper.
3.2. Selectivity and specificity
The retention times of compounds 1, 2, 3 and CDRI 99/411 were 5.4, 5.8, 8.35 and 11.93 min, respectively. On a RP-select B Lichrospher®column, compounds were separated on the basis of their polarity. Compound-3 contains hydroxyl group which makes it more polar thus eluting first i.e., with less retention time, next comes compound-1 which has a ketonic group followed by compound-2 which is a conjugated ester, less polar than ketone,and hence elutes after compound-1.CDRI 99/411 has a substituted peroxide bond and an ether linkage; consequently it is least polar and therefore it has the longest retention time.It should be recalled that compound 1 is the starting material, whereas compounds 2 and 3 are intermediates in the synthesis of final compound CDRI 99/411. None of the above compounds interfered with the peak corresponding to CDRI 99/411 or with each other (Fig.3).
3.3. Linearity, limits of detection and quantification
The calibration curves(n=3)showed a linear relationship between peak area and concentration over the ranges of 0.8—25.7, 0.75—24.0, 1.1—35.5 and 1.05—68.0 μg/mL for compounds 1, 2, 3 and CDRI 99/411, respectively. The correlation coefficient (r2) of the calibration curves for all the analytes was found to be more than 0.999. The limits of detection (LOD) and quantification (LOQ)were measured according to the ICH guidelines. The LOD and LLOQ of the analytes were determined based on a signal to noise ratio of 3 and 10 respectively (Table 1).
3.4. Accuracy and precision
Accuracy and precision data for intra- and inter-day assay are given in Table 2. The assay values on both occasions (intra- and inter-day) were found to be within the acceptable limits [14—16].
3.5. Stability studies
Chromatograms obtained from the assay of stressed samples are shown in Fig.4(A)—(D). CDRI 99/411 shows significant degradation in alkaline and acidic hydrolysis and oxidative stress conditions (Table 3).
4. Conclusions
The developed and validated HPLC method is reproducible and can be routinely used for quality control of CDRI 99/411 in bulk manufacture, with ongoing stability studies for drug development process. It provides base line separation of the compound with all the starting materials, intermediates and product of interest.Several bulk samples of compound 99-411 from the Medicinal and Process Chemistry Division and bulk preparation, required for pharmacological and toxicological studies, were analyzed by the reported method.
Financial support to Shakti Deep Pachauri and Kiran Khandelwal from the Council of Scientific and Industrial Research(CSIR)New Delhi, India, is gratefully acknowledged.
[1] S.Ray,P.B.Madrid,P.Catz,et al.,Development of a new generation of 4 Aminoquinoline antimalarial compounds using predictive pharmacokinetic and toxicology models,J.Med.Chem.53(9)(2010)3685—3695.
[2] WHO, World Health Organization, 10 Facts on Malaria. 〈www.who.int/features/factfiles/malaria〉, 2012 (accessed 11.03.13).
[3] WHO,World Malaria Report.〈http://www.who.int/malaria/world_ma laria_report_2010/en/index.html〉, 2010 (accessed 11.03.13).
[4] M. Imwong, S. Nakeesathit, N.P.J. Day, et al., A review of mixed malaria species infections in anopheline mosquitoes, Malar. J. 10(2011) 253.
[5] R.G. Ridley, Medical need, scientific opportunity and the drive for antimalarial drugs, Nature 415 (6872) (2002) 686—693.
[6] O.P. Asthana, J.S. Srivastava, N. Valecha, Current status of the artemisinin derivatives in the treatment of malaria with focus on arteether, J. Parasitic Dis. 21 (1) (1997) 1—12.
[7] S.Mishra,L.Manickavasagam,G.K.Jain,Determination of metabolic profile of anti-malarial trioxane CDRI 99/411 in rat liver microsomes using HPLC, Biomed. Chromatogr. 26 (1) (2012) 115—122.
[8] R.P. Singh, S.K. Singh, R.C. Gupta, A high throughput approach for simultaneous estimation of multiple synthetic trioxane derivatives using sample pooling for pharmacokinetic studies,J.Pharm.Biomed.Anal. 37 (1) (2005) 127—133.
[9] C.Singh,R.Kanchan,U.Sharma,et al.,New adamantane-based spiro 1,2,4-trioxanes orally effective against rodent and simian malaria, J.Med. Chem. 50 (3) (2007) 521—527.
[10] C. Singh, H. Malik, S.K. Puri, US Patent, 7495025B2, 24 February,2009.
[11] ICH Impurities In New Drug Substances Q3A(R2),in:Proceedings of the International Conference on Harmonization, 2006.〈http://www.ich.org〉.
[12] N.Kapoor,S. Khandavilli,R. Panchagnula,Simultaneous determination of lamivudine, stavudine and nevirapine in antiretroviral fixed dose combinations by high performance liquid chromatography,Anal.Chim. Acta 570 (1) (2006) 41—45.
[13] ICH Stability Testing of New Drug Substances and Products Q1A(R2), in: Proceedings of the International Conference on Harmonization, 2003.〈http://www.ich.org〉.
[14] ICH Validation of Analytical Procedures: Text and Methodology Q2(R1), in: Proceedings of the International Conference on Harmonization, 2005.〈http://www.ich.org〉.
[15] M.M. Annapurnan, S.V.S. Goutam, S. Anusha, et al., Development and validation of the stability-indicating LC—UV method for the determination of Cefditoren pivoxil, J. Pharm. Anal. 2 (6) (2012) 466—469.
[16] B.S.P. Kumarn, M.M. Annapurna, S. Pavani, Development and validation of a stability indicating RP-HPLC method for the determination of Rufinamide, J. Pharm. Anal. 3 (1) (2013) 66—70.
 Journal of Pharmaceutical Analysis2013年5期
Journal of Pharmaceutical Analysis2013年5期
- Journal of Pharmaceutical Analysis的其它文章
- A novel and rapid microbiological assay for ciprofloxacin hydrochloride
- Determination of sodium hyaluronate in pharmaceutical formulations by HPLC-UV
- Copper interactions with DNA of chromatin and its role in neurodegenerative disorders
- A critical quality parameter in quantitative fused-core chromatography: The injection volume
- Application of LC-MS/MS for quantitative analysis of glucocorticoids and stimulants in biological fluids
- In situ modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in its formulation
