Electrochemical study and application on rutin at chitosan/graphene films modified glassy carbon electrode
Jing An, Ying-Yn Bi, Chun-Xi Yng, Fng-Di Hu,*, Chun-Ming Wng
aSchool of Pharmacy, Lanzhou University, Lanzhou 730000, China
bCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China
1. Introduction
Rutin (Fig.1), a kind of the most abundant bioactive flavonoid, is the main active ingredient in Sophora japonica L.
Known as vitamin P, rutin has a broad range of physiological activities such as anti-oxidant [1], antiviral [2], anti-inflammatory[3,4],and anti-depression[5],and it has potential to treat diabetes[6]and hypertension [7].Some analytical methods,including high performance liquid chromatography[8,9],capillary electrophoresis[10-12], spectrophotometry [13], have been employed for the determination of rutin. However, some of these methods are time-consuming, expensive or need complicated pre-treatment,which hamper their further application. Compared with these methods, electrochemical determination shows advantages of satisfactory reliability,fast response,inexpensive instrument,low energy consumption, simple operation, time saving, high sensitivity and selectivity, especially in situ determination. In recent years,with the development of nanoscience and nanotechnology,many nanomaterial-based electrodes, which can dramatically enhance the signal intensity of electrochemical sensor and lead to ultrasensitive determination, have been applied for the electrochemical determination of rutin [14,15]. The electrochemical measurement has also been applied to analyze rutin, whose construction contains electrochemical active group [16-19].However, to the best of our knowledge, it was still a challenge for the fabrication of novel electrochemical sensors using graphene to achieve sensitive, fast and facile detection of rutin.

Fig.1 Chemical structure of rutin.
Graphene (G), owing to its unique two-dimensional nanostructure and excellent electrical conductivity, has attracted enormous attention following the discovery of nanotube. As the thinnest carbon material until now,graphene has exhibited potential applications in electrode modifying materials [20],sensors [21], and drug carrier [22,23].
Chitosan (Chit), a natural polymer, has many admirable properties such as non-toxicity, biodegradability, and good compatibility. Bringing with quantity of amino and hydroxyl active group, chitosan has been widely used due to its absorption enrichment to some certain organic composites and chelation of metal ion and silver nano-particle has been widely employed for the modification of electrode because of its high surface area and outstanding electrical conductivity.
In this paper, a Chit/G/GCE, integrating the absorption enrichment of Chit, excellent electrical conductivity of graphene and high surface area of nano-metal, was fabricated and the electrochemical behavior of rutin on this Chit/G/GCE was investigated.The result demonstrated that both the metal particle and Chit/G contributed to the sensitive detection of rutin to a certain degree. Compared with the existing electrochemical methods, this research has some innovative results in the biocompatibility of graphene and the selectivity and sensitivity of rutin analysis.Furthermore,this novel method was successfully used for the determination of rutin content in samples with satisfactory results,providing a basis for its application to the measurement of active substance in vivo.
2. Materials and methods
2.1. Apparatus and reagents
CHI1220 electrochemical workstation (Shanghai Shenhua Instrument Co., Ltd., Shanghai, China) was employed for all the voltammetric measurement, and 7821 magnetic stirring apparatus was used to prepare all the solutions. A conventional three-electrode system was used, including Chit/G modified GCE as working electrode, a saturated calomel electrode as reference electrode and a platinum wire electrode as auxiliary electrode. Chit (deacetylation degree >90%, Sinopharm Chemical Reagent Co., Ltd.), graphite (AR, Shanghai Chemical Reagent Co., Ltd.), rutin (100080, National Institute of China for the Control of Pharmaceutical and Biological Products.),hydrogen peroxide (10-30 nm, Bill Technology Co., Ltd. Shenzhen), potassium permanganate (AR, Shanghai Hui Shisheng Reagent Co., Ltd.), sodium nitrate (AR), sodium hydroxide(AR), sodium dihydrogen phosphate (AR), disodium hydrogen phosphate(AR)and redistilled water were used.Phosphate buffer solution was prepared by NaH2PO4-Na2HPO4and pH value was adjusted by NaOH and H3PO4. All of the experiments were processed at room temperature (~20°C).
2.2. Preparation of graphene
Natural graphite powders were oxidized to graphite oxide using a modified Hummers method. In a typical synthesis process,graphite powder and sodium nitrate were put into concentrated H2SO4(in an ice bath).Afterward,KMnO4was gradually added.The mixture was then transferred to room temperature and stirred for about 5 h, forming a thick paste. Subsequently, de-ionized water and 30% H2O2were added to reduce the residual KMnO4.The solution was then treated by ultrasonication for 5 h and washed with de-ionized water until the pH was 7 and dried at 65°C under vacuum to obtain graphene oxide(GO)solid.G was obtained by the reduction of GO using hydrazine hydrate as a reducing agent. And then, the mixture was washed several times until the pH was 7 and dried under vacuum.
2.3. Fabrication of Chit/G/GCE
2.3.1. Pretreatment of bare GCE
Before the start of the electrochemical experiments and modification procedures, the GCE was burnished on the metallographic sandpaper, then polished to a mirror-like surface with 0.05 μm α-alumina slurries and finally ultrasonicated for 3 min in nitric acid (1:1), ethanol and redistilled water successively.Finally, the surface of electrode was dried by nitrogen.
2.3.2. Fabrication of electrochemical sensor
Chit was first dissolved in acetic acid (1 mL in 100 mL). This was stirred for 2 h to generate chitosan solution (0.5 mL in 100 mL, adjusted pH=5 by 0.1 M NaOH). Subsequently,1.5 mg G was cast into the solution (1.0 mL), followed by ultrasonication for 2 h. Then 4 μL of the as-prepared Chit/G composite (1.5 M) was dropped on the pretreated GCE using micropipette and dried in a desiccator for 2 h at room temperature. Meanwhile, 100 nanoAg was cast into the solution of Chit/G composite and the G/Chit/nanoAg was obtained after 2 h ultrasonic dispersion. Finally, this solution was dropped on the GCE using the same method. And the obtained electrodes were placed at 4°C.
2.4. Cyclic voltammetry (CV)
Unless otherwise stated, 0.1 M PBS (pH 4.0) was used as the supporting electrolyte.A certain volume of rutin standard solution and PBS was added into an electrochemical cell.The G/Chit/GCE was used as working electrode, a platinum wire as counter electrode and a saturated calomel electrode as reference electrode completing the cell assembly.Cyclic voltammograms were scanned from 0.2 to 0.8 V with the rate of 100 mV/s. The electrodes were modified again after each scan.
2.5. Differential pulse voltammetry (DPV)
Before the electrodes were used to analyze samples,a steady G volt-ampere characteristic (from -0. 2 to 0.8 V) should be achieved in a PBS (pH=4). A certain volume of rutin standard solution and PBS (10 mL totally) was added into an electrochemical cell. DPV was scanned from -0.2 to 0.8 V and the height of current peaks was recorded.
3. Results and discussion
3.1. Characteristics of graphene and the mixture of G and Chit
Fig.2(A) shows the scanning electron micrograph(SEM)image of graphene nanosheets, which exhibited a significant difference with GCE. Transmission electron microscope (TEM) can effectively prove the morphologies of the mixture of G and Chit.Fig.2(B) shows the TEM image of Chit/G composite, clearly clarifying the crumpled and wrinkled flake-like structure.
3.2. Electrochemical response of rutin on G/Chit/GCE
The electrochemical behavior of rutin (1×10-5M) on different electrodes (GCE, Chit/G/GCE, Chit/G/nanoAg/GCE and G/GCE) was studied by CV. As shown in Fig.3, rutin showed redox current peaks at 0.408 V and 0.482 V. The heights of the redox peaks were in agreement with this order: Ip(GCE)<Ip(G/GCE)<Ip(G/Chit/nanoAg/GCE)<Ip(G/Chit/GCE). The unique two-dimensional nanostructure of G could benefit to the electrical conductivity, which explains the increased peak currents and background. Furthermore, the redox peaks also rose after the presence of Chit. However, the addition of nanoAg resulted in an opposite effect. This phenomenon may be related to the complexing action between nanoAg and amino group of Chit, leading to fewer binding sites of rutin and Chit.

Fig.2 (A) SEM image of G and (B) TEM image of G/Chit film.
3.3. Optimization of experimental conditions
3.3.1. Effect of pH
Fig.4(A) displays the effect of different pH on the response of 1×10-5M rutin. When the pH changed from 2.0 to 9.0 (by NaOH), the anodic peak in CV moved towards the negative direction and the current response decreased. There was a linear relationship between the anodic peak potential and the pH value as follows (Fig.4(B)):


Fig.3 CVs of bare GCE(a),G/GCE(b),Chit/G/nanoAg(c)and Chit/G/GCE (d) with 1×10-5 M rutin in 0.1 M PBS (pH 4.0) at 100 mV/s.

Fig.4 (A)CVs of Chit/G modified GCE in 0.10 M PBS containing 1×10-5 M of rutin with pH values of 2.0,3.0,4.0,5.0,6.0,7.0,8.0,9.0 at scan rate of 100 mV/s and (B) plot of equilibrate potentials vs. pH values.
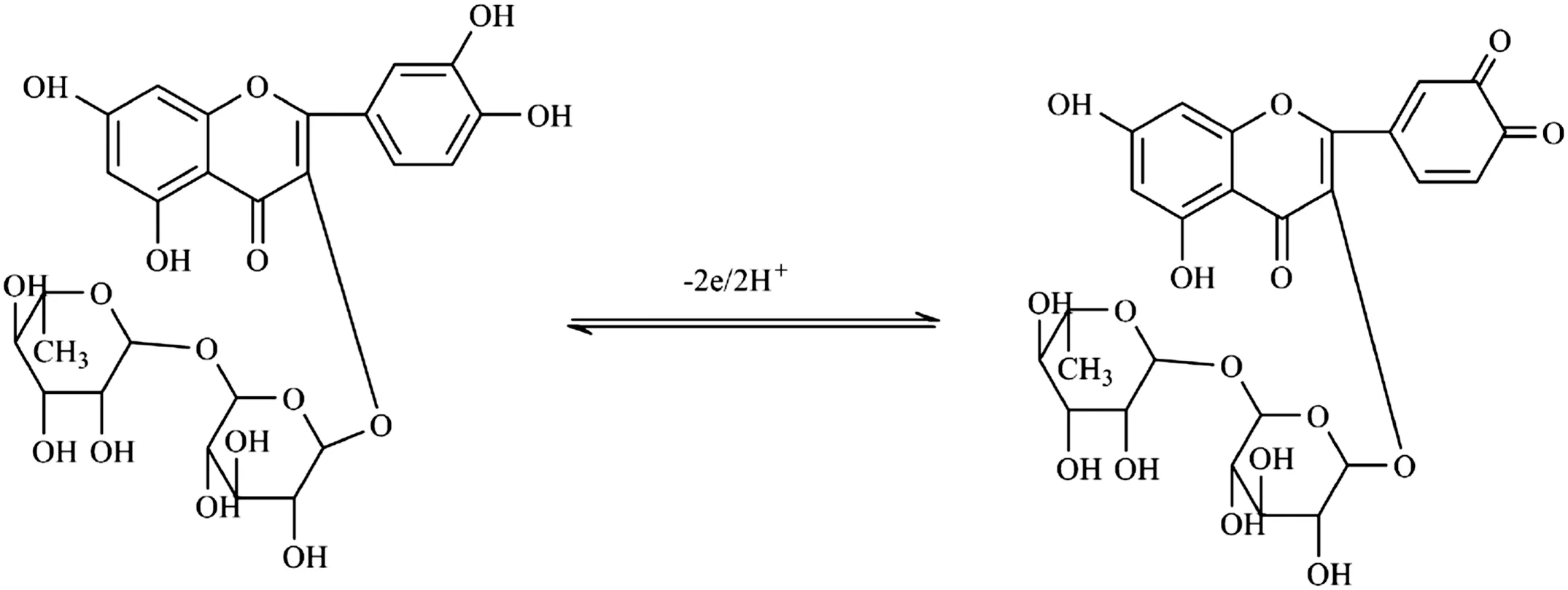
Fig.5 Mechanism of rutin redox processes.
According to the slope of 57 mV/pH,it could be deduced that H+participated in this reaction and the number of electrons and protons transferred was equal in the electrochemical reaction. When the pH was over 7.0, the anodic peak became very small and irreversible. These experimental phenomena were related to the proton involved in the electrochemical reaction. When pH exceeded 7.0, with the increase of negative ions, the electrostatic repulsion occurred between chitosan and rutin, leading to the reduction of current. Yang et al. [24] studied the electrochemical behavior of rutin on a Chit/G/GCE and the linear relationship between the anodic peak potential and pH was Epa=0.6838-0.056 pH (r=0.9984), whose rate of slope is similar to our own result. In the meantime, with regard to Faraday's law, they investigated the mechanism of this electrode reaction and deduced that the number of electrons and protons transferred was both two in this electrochemical action (Fig.5).
3.3.2. Effect of scan rate
The effect of scan rate on the electrochemical response of 1×10-5M rutin is shown in Fig.6(A). With the increase of scan rate,the redox peak currents increased simultaneously without significant change in peak potential.Both the cathodic and anodic peak currents increased linearly with the scan rate from 20 to 200 mV/s (Fig.6(B)), indicating an adsorptioncontrolled process. The regression equation was

3.3.3. Effect of the amount of modification
The effect of the amount of modification was also investigated in rutin solution. When the volume of Chit/G composite increased on the surface of electrode, the peak current increased first until it was up to 4 μL, and then decreased(Fig.7), indicating that rutin cannot be fully enriched on the less modified electrodes, nonetheless, the peak current decreased on over modified electrodes because increased electron transfer path would affect the sensitivity of electrodes.Therefore, 4 μL was selected as the amount of modification.
3.3.4. Effect of accumulation time
The effect of accumulation time on its reduction peak current was also investigated in 1×10-5M rutin solution for the Chit/G/GCE.With the increase of accumulation time(1,3,5 and 7 min), the anodic peak current increased gradually. And the peak current reached the maximum after 7 min and then tended to be stable, indicating that 7 min was sufficient to reach the rutin saturation on the Chit/G/GCE. Therefore,7 min was generally chosen as the accumulation time.

Fig.6 (A)CVs of Chit/G modified GCE in 0.10 M PBS(pH 4.0)containing 1×10-5 M of rutin at scan rate of 20,40,60,80,100,120,160,180 and 200 mV/s and (B) plot of peak current vs. scan rate.

Fig.7 Plot of peak current vs. modified amount.
3.4. Calibration curve
Under optimal conditions,DPV was explored for the amperometric response of rutin at the proposed electrochemical sensor. Fig.8(A) shows the typical DPV obtained at different concentrations of rutin. The peak currents had a good linear relationship with the rutin concentration in the range of 5×10-7-1.04×10-5M, as shown in Fig.8(B). The linear regression equation was

3.5. Reproducibility and stability
Reproducibility and stability are two important characteristics for the modified electrode, which should be investigated.The reproducibility of the proposed electrochemical sensor was evaluated by the determination of ten samples of 1.0×10-6M rutin solution using ten modified electrodes separately. The average peak current is 9.12 μA and the standard deviation(SD)was 4.7%which suggested acceptable reproducibility of the proposed electrochemical sensor(RSD=5.15%). Furthermore, the 1.0×10-6M rutin solution was also measured by modified electrodes which were placed for 1 week at 4°C. There were no significant changes in current.
3.6. Interference studies
The influence of some coexistent interference substances was examined in the presence of rutin solutions. The results showed that when the concentrations of ascorbic acid,glucose,uric acid, and glutamic acid were 50 times more than those of rutin, the concentrations of l-tryptophan, l-serine, l-histidine,and rhein were 100 times more,and no observable interference was observed in the determination of rutin according to the relative error <±5%. Furthermore, large quantity of sodium or potassium ions also do not interfere with the results.Therefore, the proposed method had excellent selectivity for rutin. However, some compounds, such as flavonoids and dopamine, whose peak potentials were very close to that of rutin, caused significant interferences. The recovery of this developed method was evaluated by the determination of six samples of rutin solution, which was prepared by ultrasound dissolving rutin tablets and whose concentration was controlled within detection limit. The content of rutin was detected via the peak current in 0.408 V. As shown in Table 1, under the optimized conditions, the recovery of six experiments varied from 94.47% to 100.99%. Finally, the developed method was used to determine the content of rutin in Flos Sophorae Immaturus. 10 g Flos Sophorae Immaturus was added in 25 times volume of water and decocted 3 times.The combined filtrate was concentrated and placed over night.Subsequently, the solution was filtrated under vacuum to obtain the initial extracted rutin. 0.067 g accurately weighed rutin extract was dissolved in ethanol and quantified in a 10 mL volumetric flask. Finally, 50 μL solution was added into 10 mL PBS (pH=4). The results are shown in Table 2.
4. Conclusion

Fig.8 (A)DPV of different concentrations of rutin(in the range of 5×10-7-1.04×10-5 M)and(B)concentration calibration curve of the DPV current response for rutin.

Table 1 Determination of rutin in rutin tablets.

Table 2 Determination of rutin in Flos Sophorae Immaturus.
In this study, a Chit/G/GCE has been introduced and the content of rutin in Flos Sophorae Immaturus has been determined with this modified electrode. The proposed sensor exhibited an excellent electrochemical activity for the reduction and oxidation of rutin and the electrode process was controlled by absorption effect under low scan rate. This sensitive and rapid method for the measurement of rutin proves that the good matrix structure of Chit contributes to the water-solubility of G and that the concentration of rutin adhered on the Chit/G/GCE is increased and the electrochemical response is enhanced rapidly owing to the amino group of Chit.
Acknowledgment
The authors acknowledge the support of the Twelfth Five-Year National Science and Technology Support Program(2011BAI05B02). This work is also supported by the Fundamental Research Funds for the Central Universities(lzujbky-2011-95), the Project of Science and Technology Agency of Lanzhou (No. 2011-1-67) and the item of scientific and technological research from Gansu province administration bureau of traditional Chinese medicine (GZK-2011-73),Gansu, China.
[1] J.X. Yang, J. Guo, J.F. Yuan, In vitro antioxidant properties of rutin, Food Sci. Technol. 41 (2008) 1060-1066.
[2] J. Tao, Q.X. Hu, J. Yang, et al., In vitro anti-HIV and -HSV activity and safety of sodium rutin sulfate as a microbicide candidate, Antiviral Res. 75 (2007) 227-233.
[3] L. Selloum, H. Bouriche, C. Tigrine, et al., Anti-inflammatory effect of rutin on rat paw oedema,and on neutrophils chemotaxis and degranulation, Exp. Toxicol. Pathol. 54 (2003) 313-318.
[4] J. Tian, S.G. Song, Relationship between protective effect and antioxidative action of rutosid on experimental acute pancreatitis in rats, Chin. J. Clin. Pharmacol. Ther. 04 (2004) 455-458.
[5] G.M. Daniele, E.B.B. Luis, P.C. Mauricio, Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L.in mice:evidence for the involvement of the serotonergic and noradrenergic systems, Eur. J. Pharmacol. 587 (2008)163-168.
[6] B.H. Jang, H.D. Su, F. Xu, Prevention of rutin for diabetic nephropathy, Heilongjiang Med. J. 12 (2005) 899-901.
[7] H.K. Hellerstein, J.L. Orbison, S. Rodbard, et al., The effect of rutin in experimental malignant hypertension, Am. Heart J. 42(1951) 271-283.
[8] K.Ishii, T.Furuta,Y.Kasuya,Determination of rutin in human plasma by high-performance liquid chromatography utilizing solid-phase extraction and ultraviolet detection, J. Chromatogr.B 759 (2001) 161-168.
[9] C.H.Wang,Y.X. Wang,H.J.Liu,Validation and application by HPLC for simultaneous determination of vitexin-200-O-glucoside, vitexin-200-O-rhamnoside, rutin, vitexin, and hyperoside,J. Pharm. Anal. 1 (2011) 291-296.
[10] Q.H. Lu, C.D. Ba, D.Y. Chen, Investigating noncovalent interactions of rutin-serum albumin by capillary electrophoresisfrontal analysis, Pharm. Biomed. Anal. 47 (2008) 888-891.
[11] G. Chen, H.W. Zhang, J.N. Ye, Determination of rutin and quercetin in plants by capillary electrophoresis with electrochemical detection, Anal. Chim. Acta 423 (2000) 69-76.
[12] H.T. Duan, Y. Chen, G. Chen., Far infrared-assisted extraction followed by capillary electrophoresis for the determination of bioactive constituents in the leaves of Lycium barbarum Linn, J.Chromatogr. A. 1217 (2010) 4511-4516.
[13] Z. Cai, J. Zhao, C.Y. Jang, Simultaneous determination of 2 constituents in compound rutoside tablets by PLS-UV spectrophotometry, Chin. Pharm. 20 (2009) 2454-2455.
[14] I.R. Zwirtes de Oliveira, S.C. Fernandes, I.C. Vieira, Development of a biosensor based on gilo peroxidase immobilized on chitosan chemically crosslinked with epichlorohydrin for determination of rutin, J. Pharm. Biomed. Anal. 41 (2006) 366-372.
[15] X.Q. Lin, J.B. He, Z.G. Zha, Simultaneous determination of quercetin and rutin at a multi-wall carbon-nanotube paste electrodes by reversing differential pulse voltammetry, Sens.Actuators B 119 (2006) 608-614.
[16] X.H. Liu, L. Li, X.P. Zhao, et al., Electrochemical behavior of rutin on a multi-walled carbon nanotube and ionic liquid composite film modified electrode, Colloids Surf. B 18 (2010)344-349.
[17] B.Z. Zeng, S.H. Wei, F. Xiao, et al., Voltammetric behavior and determination of rutin at a single-walled carbon nanotubes modified gold electrode, Sens. Actuators B 115 (2006) 240-246.
[18] J.L. He, Y. Yang, X. Yang, et al., β-Cyclodextrin incorporated carbon nanotube-modified electrode as an electrochemical sensor for rutin, Sens. Actuators B 114 (2006) 94-100.
[19] Y. Wang, Y.M. Li, L.H. Tang, et al., Application of graphenemodified electrode for selective detection of dopamine, Electrochem. Commun. 11 (2009) 889-892.
[20] H.S. Yin, Y.L. Zhou, Q. Ma, et al., Electrochemical oxidation behavior of guanine and adenine on graphene-nafion composite film modified glassy carbon electrode and the simultaneous determination, Process Biochem. 45 (2010) 1707-1712.
[21] K.P. Liu, J.J. Zhang, G.H. Yang, et al., Direct electrochemistry and electrocatalysis of hemoglobin based on poly(diallyldimethylammonium chloride) functionalized graphene sheets/room temperature ionic liquid composite film, Electrochem. Commun. 12(2010) 402-405.
[22] W. Wu, Z.H. Liu, A.J. Luis, et al., Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing, Sens. Actuators B 150 (2010) 296-300.
[23] L.M. Zhang, J.G. Xia, Q.H. Zhao, et al., Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs, Small 6 (2010) 537-544.
[24] S.L. Yang, L.B. Qu, G. Li, Gold nanoparticles/ethylenediamine/carbon nanotube modified glassy carbon electrode as the voltammetric sensor for selective determination of rutin in the presence of ascorbic acid, J. Electroanal. Chem. 645 (2010) 115-122.
 Journal of Pharmaceutical Analysis2013年2期
Journal of Pharmaceutical Analysis2013年2期
- Journal of Pharmaceutical Analysis的其它文章
- Application of analytical instruments in pharmaceutical analysis
- JPA Prize in 2012
- Reversed-phase fused-core HPLC modeling of peptides
- Establishment of inherent stability of pramipexole and development of validated stability indicating LC-UV and LC-MS method
- Determination of cefcapene acid by LC-MS and their application to a pharmacokinetic study in healthy Chinese volunteers
- New simple spectrophotometric method for determination of the binary mixtures (atorvastatin calcium and ezetimibe;candesartan cilexetil and hydrochlorothiazide) in tablets
