自体荧光技术在早期肠癌诊断中的应用*
刘丽娜,李步洪,谢树森
(医学与光电科学与技术教育部重点实验室,福建省光子技术重点实验室,福建师范大学,福建福州 350007)
肠癌的发病率和死亡率居世界第三,在我国其发病率仅次于胃癌和食道癌,并且呈逐年上升趋势,威胁着人们的生命健康[1]。早期发现和切除癌变组织是有效预防肠癌发展,降低死亡率的关键。白光内镜(WLE)检查是当前临床诊断肠癌的主要方法,医生根据反射白光图像对形态或颜色发生变化的可疑病灶进行活检取样和病理分析,但往往难以发现粘膜下的病变组织和早期原位癌微小病灶,容易误诊或漏诊,从而导致错过最佳的治疗时间[2]。为了提高早期肠癌的诊断率,人们一直致力于寻找一种能有效地正确指导或替代手术活检的诊断方法。经过多年的研究与发展,出现了如色素内镜(CE)[3]、放大内镜(ME)[4]、窄带成像内镜(NBI)[5]、荧光内镜(AF)[6-8]、共聚焦激光内镜(CLE)[9]等新型消化内镜,这些内镜可以观察WLE尚无法判断的特殊微小结构变化,甚至观察到细胞水平的变化,使早期诊断内镜向微观化方向发展。目前,荧光内镜以无损、实时和灵敏度高等优点已成为早期肠癌诊断的研究热点[10-12]。
人体组织自体荧光主要来源于基质或细胞中的氨基酸、结构蛋白、酶和辅酶、脂肪、维生素和卟啉等五大类物质。如图1所示,在特定波长光的激发下,人体组织自体荧光光谱是多种不同物质成分同时诱发产生的光谱叠加,其强度和形状不仅取决于组织的生化特性和形态结构,还受到组织吸收和散射的影响。组织在癌变过程中,细胞新陈代谢的变化将引起荧光物质的浓度、血液浓度、细胞核大小和上皮层厚度等发生变化,因此可根据组织自体荧光特性的差异区分正常和癌变组织[13-17]。
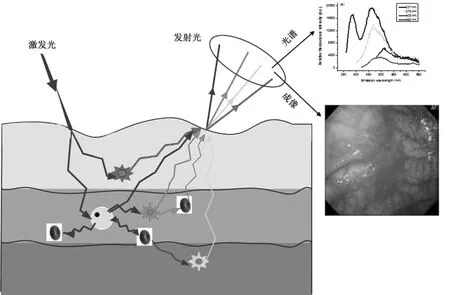
图1 自体荧光光谱与成像诊断癌变组织的示意图Fig.1 Autofluorescence spectroscopy and imaging for cancer diagnosis
为了揭示肠道组织癌变过程中自体荧光特性改变的机制,相关的主要研究工作包括:(1)分别开发自体荧光光谱和成像系统,测量肠道离体或活体正常与病变组织的自体荧光光谱和图像,并利用组织形态学、病理学以及生化学等研究肠道正常与癌变组织的自体荧光差异机制;(2)采用荧光显微技术监测肠道组织病变过程中各层组织结构和自体荧光强度分布的变化,从微观层次分析比较正常与癌变组织的差异;(3)应用逐步回归、判决分析、强度比值等算法对自体荧光光谱或图像数据进行处理与分析,进一步提高诊断的灵敏度和特异性,更加有效地鉴别不同类型的癌变组织;(4)采用Monte Carlo模拟方法,深入研究组织的厚度、样品的大小、激发光的入射角度和荧光收集角度等参数对自体荧光光谱测量结果的影响。本文总结了自体荧光光谱技术在早期肠癌诊断应用中的研究进展,重点阐述了荧光激发波长的选择,荧光光谱数据处理方法,以及正常和癌变肠道组织光谱差异的来源。在分析肠道组织中内源性荧光物质及其分布的基础上,回顾了自体荧光成像系统的临床应用进展。最后指出自体荧光光谱与成像技术在早期肠癌诊断中的应用和发展趋势。
1 自体荧光光谱技术
1.1 激发波长的选择
激发波长的选择是开发自体荧光光谱诊断系统的关键参数之一。优化选取激发波长应综合考虑荧光物质激发效率、穿透深度等因素,使之能够有效地激发组织的荧光物质,最大限度地反映不同类型组织荧光光谱之间的差异。如表1所示,各研究小组选择的激发波长主要在紫外光到蓝绿光范围,这些激发波长都采用了现有商业化激光器的激光输出波长。与蓝绿光相比,紫外光波长在组织中穿透深度较小,如337 nm的穿透深度约200μm,而442 nm的穿透深度约为600μm[18],但紫外光可激发的物质成分较多,当激发波长在325 nm-370 nm波段时,主要的荧光峰出现在390 nm和460 nm附近,同时还可能出现630 nm 和690 nm 的卟啉发射峰[17,19-23]。Chwirot等指出激发波长(325 nm-337 nm)的微小变化对内源性荧光物质的激发没有显著的影响,但各激发波长的有效穿透深度不同[24]。Cothren等则认为与短波长相比,370 nm能够更加有效地激发荧光信息,特别是 680 nm 的荧光[21,25]。Eker等指出应用337nm激发自体荧光光谱可较好地区别肠道正常和癌变组织,而采用405 nm和436 nm激发的正常和癌变组织自体荧光光谱均没有显著差异,区分组织的效果不理想[17]。采用近红外光也可以激发肠道腺瘤性息肉和增生组织的荧光,与紫外和可见光相比,近红外光更安全且在组织中的穿透深度更深[11,26]。特别需要指出的是,最佳的激发波长的选择可以通过测量肠道组织的自体荧光激发-发射矩阵(EEM)来实现。Richards-Kortum等利用250 nm-500 nm的光激发肠道组织的自体荧光,分析15个样品(4个正常组织,11个腺瘤组织)的EEM的比值图和差值图获得最佳激发波长为330±10 nm、370±10 nm和430±10 nm,并采用370 nm作为激发光,利用480 nm的荧光强度区分正常与腺瘤组织的灵敏度和特异性分别为100%和93%,但研究病例数较少[15]。Wang等认为300 nm,320 nm,330 nm和340 nm是肠道组织的最佳激发波长,以330 nm为激发波长,区分正常和癌变组织的灵敏度和特异性分别为85%和90%[27]。李步洪等采用260 nm-540 nm,步长20 nm的激发光对肠道组织的自体荧光光谱进行检测,并将所获得的系列荧光光谱转换为EEM,比较正常和腺癌组织在某些特定波长激发下的荧光光谱确定结肠癌诊断的最佳激发波长为340 nm,380 nm,460 nm和 540 nm[12]。
1.2 光谱数据的处理方法
如何正确提取光谱的有效信息是提高早期肠癌自体荧光光谱诊断准确率的关键。目前,应用于组织自体荧光光谱处理的方法主要有:逐步回归分析、主成分分析法、双峰比值法、人工神经网络判别法和模式识别法等。
1.2.1 逐步回归分析 逐步回归是一种从众多变量筛选重要变量的多元线性回归,它按照变量对因变量影响的显著程度从大到小地将变量逐个引入回归方程并剔除影响不显著的变量,因此该方法的计算量比较大。Schomacker等采用选取12个荧光发射波长的荧光强度值作为参量,采用逐步回归分析,对91个肠道瘤性和非瘤性组织样品进行区分的灵敏度和特异性分别为80%和92%。Kapadia等对325 nm激发的肠道组织自体荧光光谱选取6个荧光发射波长的荧光强度值进行逐步回归分析,区分正常粘膜、增生性组织和腺瘤性组织的准确率分别为100%、94%和100%[16]。Marchesini等采用410 nm激光激发83例离体样品,对450 nm-800 nm范围的自体荧光光谱提取9个荧光发射波长处的光强或光强比值作为参数进行逐步回归分析,区分癌变与正常组织的灵敏度和特异性分别为80.6%和90.5%,而区分腺瘤与正常组织的灵敏度和特异性分别为88.2%和95.2%[28]。
1.2.2 主成分分析法(PCA)PCA是一种降低维数的多元统计分析方法,可从全谱数据中提取少数几个波长或波段作为综合指标,实现对组织类型的区分。PCA的不足在于:选取主成分时容易漏掉一些相关性很小的有用变量,使得预测模型可靠性下降。此外,承载有效信息的主成分往往多于3个,不易于直接计算灵敏度和特异性,因此主成分分析法常被作为数据压缩的手段,与其它分类算法相结合进行组织类型的区分[22]。
1.2.3 双峰比值法 双峰比值法则根据光谱的形状选取某两个特定波段,通过计算比值获得鉴别不同组织的阈值,是一种简单的分析方法。与比值法相比,基于全谱的数据处理方法如多元回归分析等对光谱局部的变化比较敏感,可移植性不如比值法[24]。Mayinger等采用双峰比值法I(500-549)/I(657-700)诊断腺癌的灵敏度和特异性分别为96%和93%,诊断腺瘤伴异常增生的灵敏度和特异性分别为98%和 89%[10]。
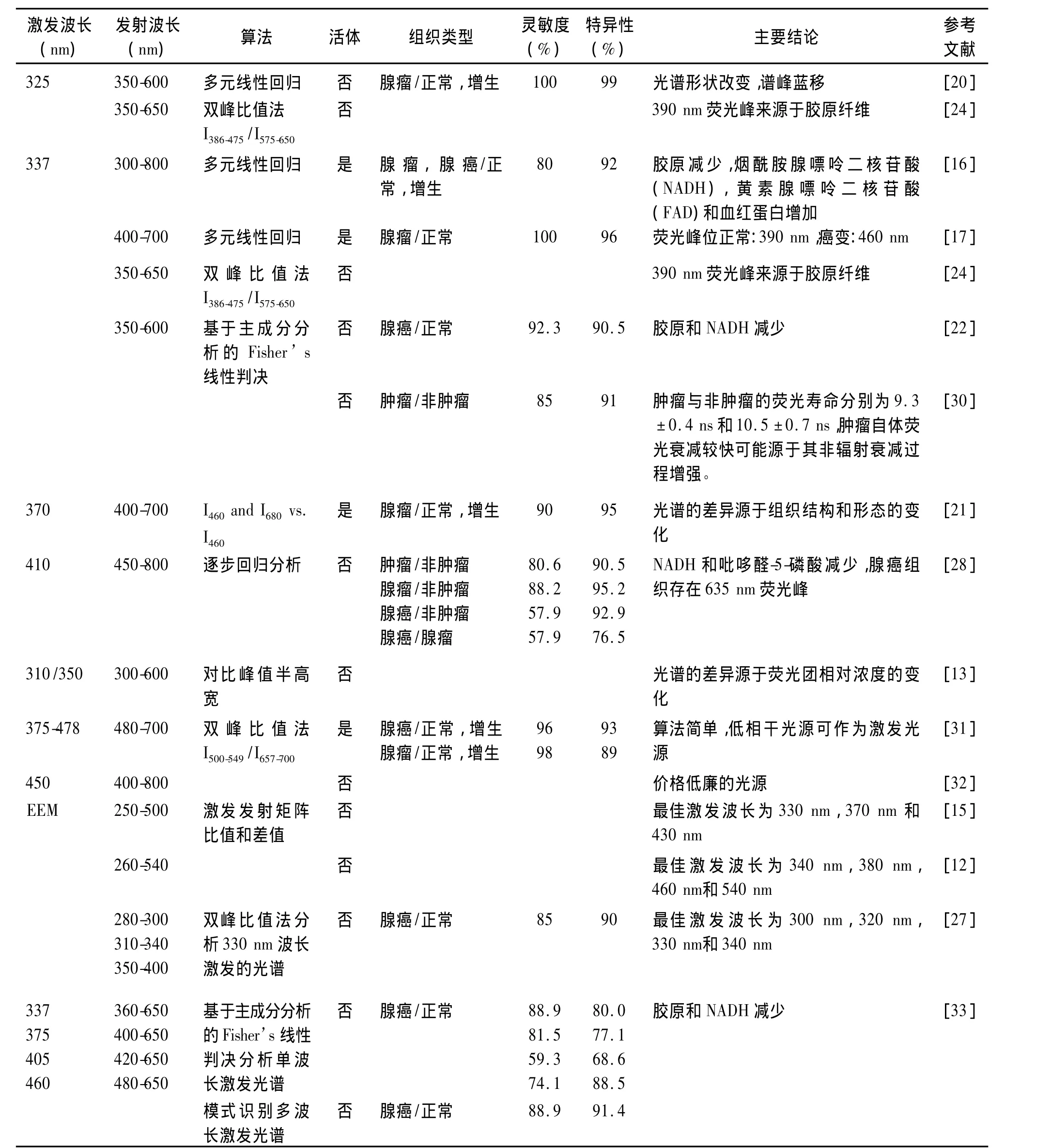
表1 不同激发波长的肠癌自体荧光光谱诊断结果Tab.1 Autofluorescence spectroscopy for the diagnosis of colorectal cancer using different excitation wavelengths
1.2.4 人工神经网络法(ANN) ANN是一种用来模拟人脑思维过程的计算模型。神经网络是由大量的、功能比较简单的神经元互相连接而成的复杂网络系统,每个神经元从其邻近神经元接受和发送信息。整个网络的信息处理通过这些神经元的相互作用完成。神经网络识别法具有解决模糊问题的优势,容错性比较好,但这种算法要求通过大量样本的训练以完成鉴别任务。罗湘健等应用PCA结合ANN法对结直肠的自体荧光光谱进行分析,区分结直肠腺癌和正常组织的灵敏度和特异性分别为100%和90%[29]。
1.2.5 模式识别多波长激发光谱 截止目前,各种光谱处理算法主要针对单波长激发的荧光光谱进行分析,尽管有研究指出EEM比单波长激发的光谱具有更丰富的诊断信息[34],但是并未见其在肠道组织诊断中应用的报道,主要是因为EEM检测与数据处理比较耗时,难以完成实时检测。为了充分利用多波长激发荧光光谱的丰富信息,又尽可能缩短检测的时间,我们优化选取337 nm、375 nm、405 nm和460 nm作为激发波长,并对所获得的荧光光谱应用基于特征提取的模式识别法进行综合处理。如图2所示,结肠正常和腺癌组织的自体荧光光谱存在显著差异。如图3所示,采用模式识别法分析多波长激发的光谱区分肠道正常和腺癌的灵敏度、特异性和准确率分别为 88.9%、91.4%和 90.3%,与单一采用337 nm激发波长获得的灵敏度相同,但特异性和准确率均有所提高。
1.3 肠道正常和癌变组织自体荧光差异的来源
1.3.1 组织结构的改变 Schomacker等采用337 nm作为激发波长发现正常和腺癌组织均有390 nm和460 nm两个荧光峰以及425 nm的血液吸收,但390 nm处正常组织的荧光强度比较高,而630 nm和690 nm是腺瘤组织特有的荧光峰。他们认为390 nm和460 nm荧光峰分别来自肠壁的胶原和NADH,仅有不到5%的荧光来自FAD。组织癌变后粘膜层厚度增加使粘膜下层的胶原荧光产出减少,因此组织形态结构改变是自体荧光光谱改变的主要原因[16]。
1.3.2 内源性荧光物质含量的改变 Banerjee等测量了肠道正常、异型增生和肿瘤组织的自体荧光光谱以及荧光物质纯品的光谱,发现不同癌变阶段的肠道组织自体荧光光谱形状没有差异,但荧光物质的相对浓度不同导致荧光峰值强度不同,同时指出新陈代谢较快的组织细胞较密集,细胞核质较多,而结缔组织的密度较低(如胶原变少)。将肠道组织光谱的四个荧光峰(331 nm、365 nm、385 nm、453 nm)与各荧光物质纯品荧光峰的峰值强度和半高宽进行比较分析,认为331 nm荧光峰来自色氨酸,365 nm荧光峰主要来自胶原蛋白,同时有弹性蛋白、酪氨酸的贡献,385 nm荧光峰主要来自IV型胶原,同时有苯丙氨酸、弹性蛋白的贡献,453 nm荧光峰最有可能来自NADH,苯丙氨酸、维生素B6和IV型胶原[13]。
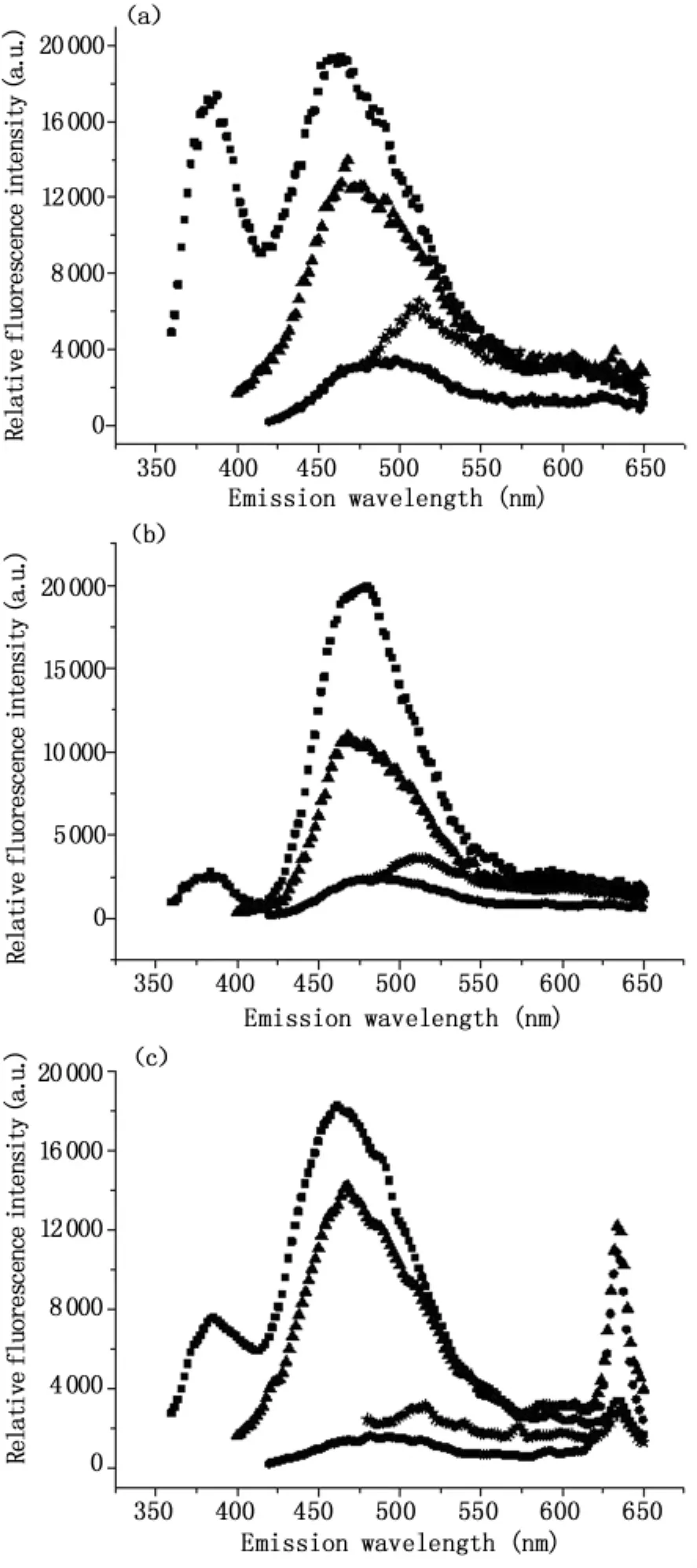
图2 多波长激发的人体结肠自体荧光光谱(a)正常组织(b)腺癌组织(c)含630 nm荧光峰的腺癌组织。激发波长分别为:337 nm(■),375 nm(▲),405 nm(★),460 nm(●)Fig.2 Autofluorescence spectra of human colonic tissues(a)normal(b)adenocarcinoma(c)adenocarcinoma with 630 nm fluorescence peak.Excitation wavelengths:337 nm(■),375 nm(▲),405 nm(★),460 nm(●)
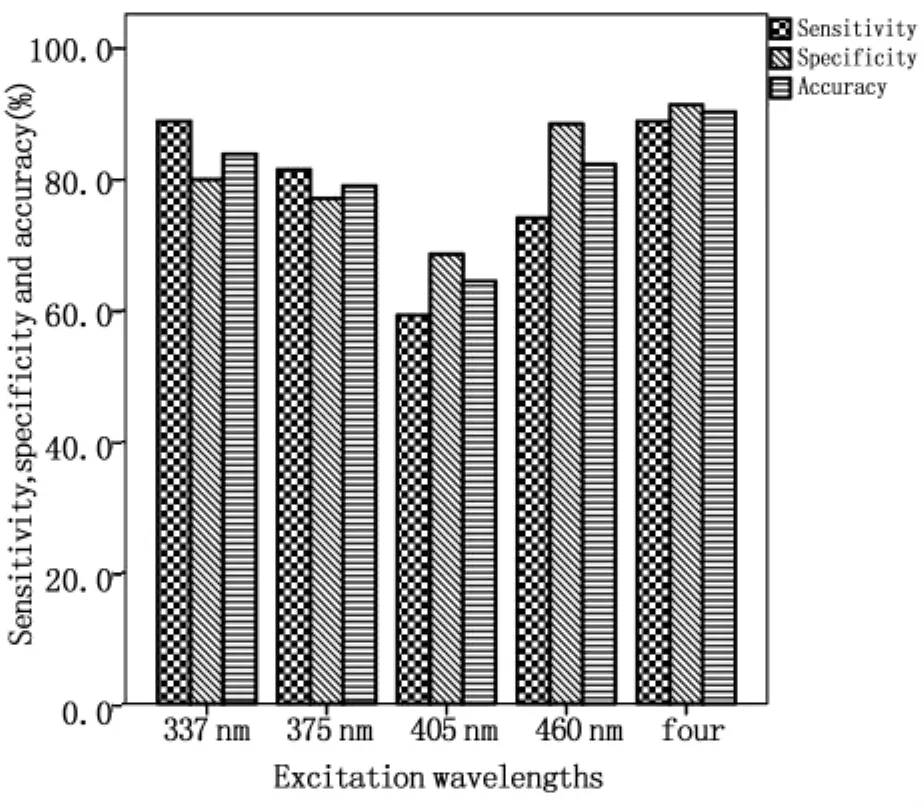
图3 多波长激发区分结肠正常和腺癌组织的灵敏度、特异性和准确率Fig.3 Sensitivity,specificity and accuracy achieved by monoexcitation and multiple-wavelength excitation for the classification of colonic normal and adenocarcinoma tissues,respectively
我们的前期研究表明部分肠癌患者的肠道组织特异地积聚有PpIX。如图2(c)所示。李步洪等采用时间分辨光谱技术研究发现正常和癌变组织635 nm荧光发射峰的平均寿命分别为4.32±0.12 ns和 18.45 ±0.05 ns,具有显著差异,表明肠癌组织中PpIX含量比正常组织高[35]。肿瘤组织中亚铁螯合酶(FECH)不足或活性降低[36,37]、胆色素原脱氢酶和FECH的失衡[38]、血红素合成过程中二价铁离子的不足或者PepT1表达高于正常组织[39]均有可能导致PpIX选择性积聚,但确切的分子机制尚有待于未来的进一步研究。
1.3.3 组织结构和内源性荧光物质含量改变Richards-Kortum等认为正常和癌变组织的光谱差异不仅取决于组织结构的差异,还可能源于癌变过程中组织的NADH和维生素B6的含量减少[15]。Mayinger等发现正常与癌组织自体荧光光谱均有绿色(480 nm-570 nm)和红色(600 nm-700 nm)两个特征峰,正常组织绿色荧光峰的强度明显高于癌组织,红色荧光峰的强度则低于腺瘤与结直肠癌。Brigitte认为绿色荧光可能来自胶原,而红色荧光则可能来自内源性卟啉。癌变组织粘膜厚度增加、粘膜下层被癌细胞取代使粘膜下层胶原的荧光发射减少以及正常和癌细胞中色氨酸和NADH的荧光强度比值不同,从而导致正常与癌变组织的荧光光谱存在差异,此外新陈代谢、炎症和血红素的含量及血液的吸收都会对荧光强度产生影响[31]。
2 自体荧光成像技术
2.1 内源性荧光物质在组织中的分布
如图4所示,不同病变阶段肠道组织的结构和病理特征存在显著差异。采用荧光显微系统可以观察到病变过程中各层组织结构和自体荧光强度分布情况,从分子水平上揭示肠癌与正常组织的差异。表2总结了离体肠道正常和病变组织的内源性荧光物质来源,以及正常组织与病变组织荧光差异及潜在原因。一般而言,正常与癌变组织荧光特性差异的主要因素包括:正常组织结构完整,而癌变组织或者结构不完整,粘膜厚度增加或者层结构被破坏。与正常组织相比,癌变组织的上皮层腺窝形状改变,杯状细胞减少,细胞核增大,细胞核质比发生变化。内源性荧光物质在正常和癌变组织中的分布存在较为显著的差异。
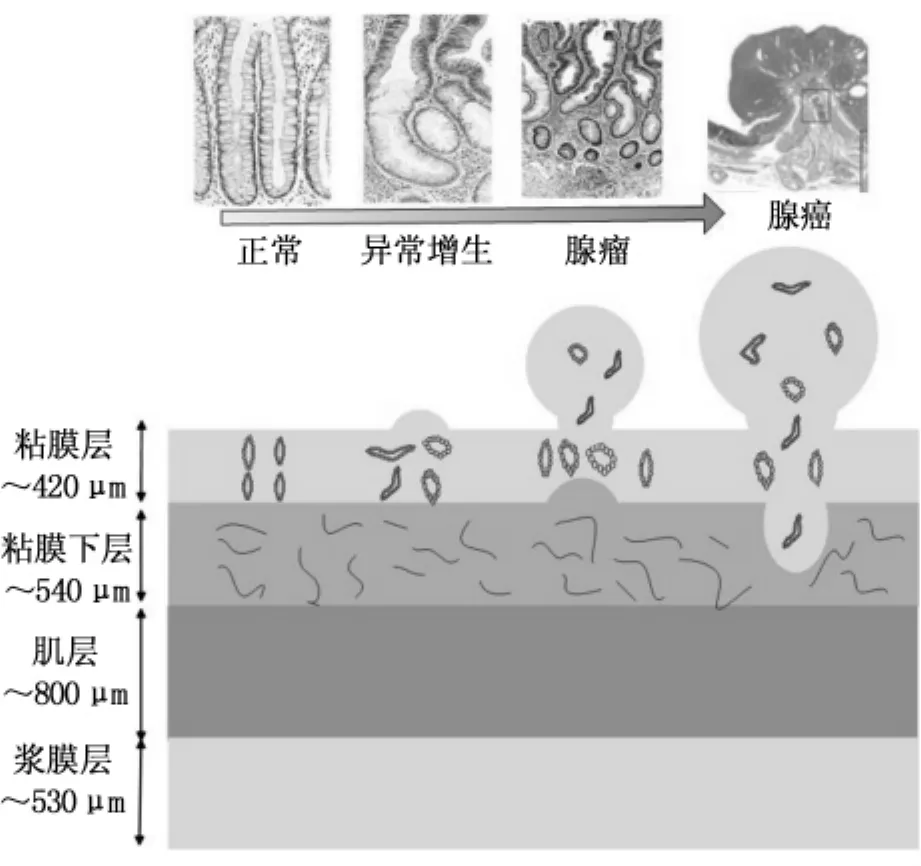
图4 不同病变阶段肠道组织的结构和病理特征Fig.4 The structure and histopathologic characteristics of colorectal tissues during neoplastic progress
2.2 自体荧光成像系统的临床应用
应用于临床的商业化肠道内镜荧光成像系统包括:(1)Xillix-LIFE-GI(Xillix Technologies Corp,Richmond,BC,Canada)(激发波长 400 nm-450 nm,中心波长437 nm);(2)D-light System(Karl Storz,Tuttlingen,Germany)(激发波长 375 nm-440 nm);(3)WavSTAT,Optical biopsy system(SpectraScience,San Diego,USA)(激发波长337 nm-410 nm);(4)Evis Lucera Spectrum或AFI(Olympus,Japan)(激发波长395-475 nm)。这些系统兼有荧光内镜和WLI两种诊断模式,并可以在两种模式之间实时切换。前三个系统通过内窥镜的活检通道实现激发光的传输和荧光收集,而Olympus的AFI则是白光与荧光同光路的视频内镜,采用两个CCD分别采集白光和荧光图像。Xillix-LIFE-GI系统分别检测绿色和红色荧光,根据红绿荧光强度的比值进行组织类型的区分。D-light系统主要用于肠癌的药物荧光诊断研究。新型的AFI可在普通白光、自体荧光和窄带成像模式之间快速切换,不仅可以实现大面积的组织荧光检查,还可以通过共焦微探头获得显微图像[52]。表3总结了这些自体荧光成像系统的临床诊断应用结果,表明自体荧光技术能够反映人体组织中内源性荧光物质和形态结构的微小变化,提高早期肠癌诊断的灵敏度。

表2 离体肠道组织的自体荧光物质来源和差异Tab.2 The origination and characteristics of autofluorescence for ex vivo colorectal tissues
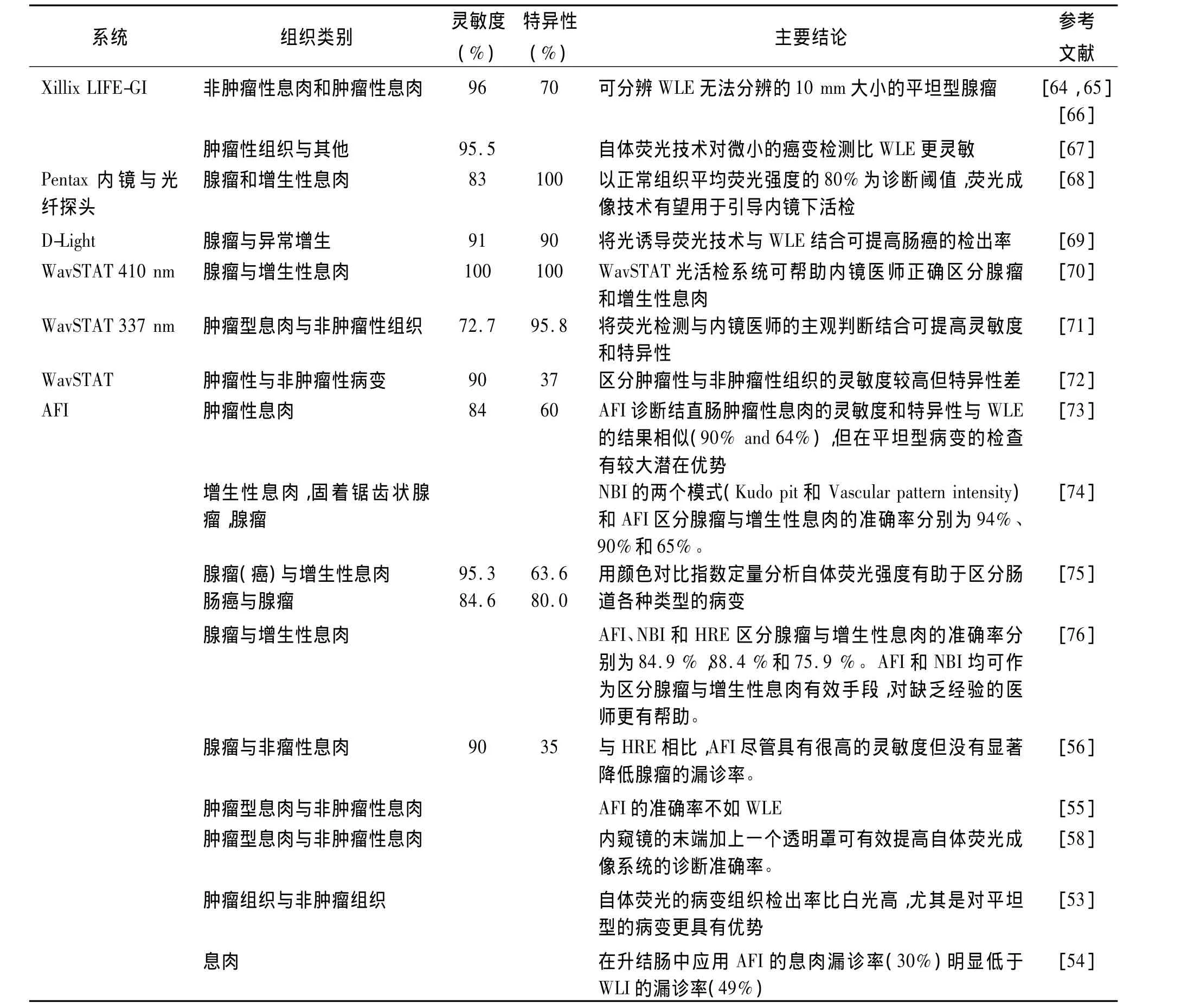
表3 自体荧光成像系统诊断肠癌的临床应用Tab.3 Autofluorescence imaging system for clinical diagnosis of colorectal cancer
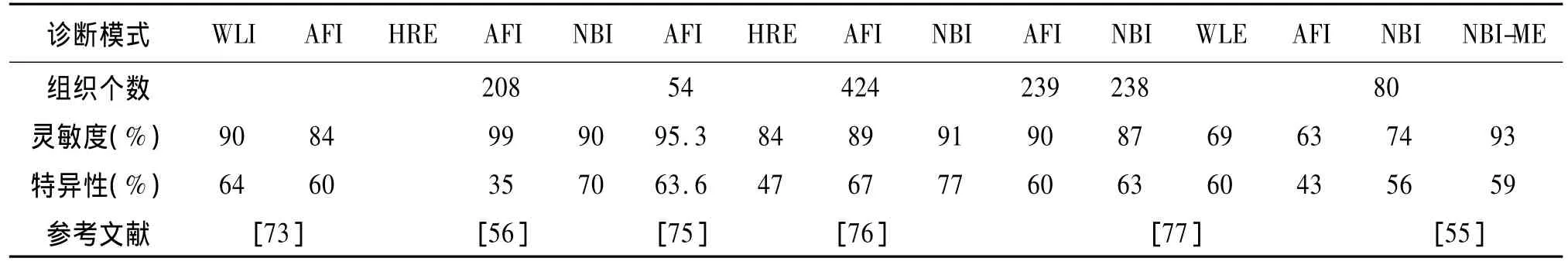
表4 自体荧光成像与其它光学技术诊断早期肠癌的性能比较Tab.4 Diagnostic sensitivity and specificity of AFI and other emerging optical modalities
应用AFI与WLI等系统诊断肠道癌变和非癌变组织的结果对比如表4所示。一些研究报道称AFI的病变组织检出率比WLI高,尤其是对平坦型的病变更具有优势[53],且对升结肠的息肉漏诊率(30%)明显低于 WLI(49%)[54]。然而另有研究表明 AFI在肠道病变组织的检测中并不占优势[55-58]。van den Broek等认为与高分辨内镜(HRE)相比,AFI虽具有高灵敏度,但并没有显著降低腺瘤的漏诊率[56]。应用一定的算法把AFI和NBI的信息相结合则可获得较高的诊断准确率,而且经验不同的医师诊断结果也比较一致[57]。Takeuchi等[58]认为各个研究小组应用AFI诊断肠癌的结果不一致可能由于所检查的部位差异引起的。与升结肠相比,乙状结肠的肠腔比较弯曲且狭窄,AFI的成像质量不如WLI,因此在这些部位进行检查时,没有明显的优势。为了改善AFI在狭窄的肠腔处的诊断效果,他们在内窥镜的末端加上一个透明罩,使内窥镜末端与粘膜表面保持足够的距离,确保CCD可以接收组织表面的自体荧光。研究结果表明加上透明罩后,AFI的病变诊出率明显提高,且明显优于WLI。
3 自体荧光技术的应用和发展趋势
自体荧光技术能够反映人体组织中内源性荧光物质和形态结构的微小变化,对于提高早期肠癌诊断的灵敏度具有十分重要的临床意义。其次,自体荧光技术在临床上的另外一个重要应用是引导手术,在患者实施肿瘤切除的手术过程中实时地确定病灶组织的边界,有效避免组织切除的盲目性,同时降低病灶的术后复发率[59]。最后,随着荧光分子探针及高分辨分子成像技术的发展,荧光标记分子成像技术在临床中的应用也备受关注。研究表明将荧光标记的抗体或肽等外源性探针靶向到癌组织的过表达生物分子,应用荧光成像系统可观察组织病变的生物过程,如上调生长因子、蛋白质水解酶和细胞粘附分子的表达等,可实现个性化的诊断和治疗,大大提高平坦型肠道肿瘤的诊断率和肿瘤边界定位精确度[60]。在该项技术中,人体组织自体荧光成为检测荧光标记分子探针信号的主要噪声来源。根据组织的自体荧光特性,对所检测的信号进行实时去噪,最大限度地提高荧光标记分子探针的检测信噪比,是开发早期肿瘤“荧光标记分子探针”诊断技术的前提。因此,揭示正常和癌变肠道组织的自体荧光光谱差异,以及内源性物质来源对于开展“荧光标记分子探针”也具有十分重要的研究意义和应用价值。
但是,由于自体荧光信号比较微弱,而且临床医生对颜色判别存在主观性等客观原因,自体荧光技术对早期肠癌的诊断特异性较低(<67%),这是制约该技术在临床上应用推广的瓶颈之一。光谱技术如自体荧光光谱、漫反射光谱可给出组织内源性荧光物质的变化、光学特性、形态结构和血的含量及其氧合特性等信息,具有较高的诊断特异性,将自体荧光成像技术与光谱技术相结合是提高早期肠癌诊断灵敏度和特异性的发展趋势之一[61]。应用多波长激发和图像处理算法提高荧光图像的对比度是提高早期肠癌诊断灵敏度和特异性的另一发展趋势。将325 nm波长激发的色氨酸荧光图像除以555 nm绿光的反射图像,突出血管的微小变化以及肿瘤部位的不均匀性,可大大提高内镜成像的对比度[62]。Imaizumi等研制了双波长激发的成像系统,连续采集由365 nm和405 nm激发的自体荧光图像并计算二者的强度比值从而减少血红蛋白吸收的影响,提高了肠道微小型腺瘤的诊断率[63]。与此同时,如何把快速、超分辨率显微技术引入现有的荧光成像内镜系统,以实现细胞以及亚细胞水平的显微诊断是自体荧光技术发展的新趋势[78]。
[1]A JEMAL,F BRAY,M M CENTER,et al.Global cancer statistics[J].CA:A Cancer Journal for Clinicians,2011,61(2):69-90.
[2]F JENKINSON and R STEELE.Colorectal cancer screeningmethodology[J].The Surgeon,2010,8(3):164-171.
[3]K TOGASHI,D G HEWETT,G L RADFORD-SMITH,et al.The use of indigocarmine spray increases the colonoscopic detection rate of adenomas[J].Journal of Gastroenterology,2009,44(8):826-833.
[4]S KATO,T FUJII,I KOBA,et al.Assessment of colorectal lesions using magnifying colonoscopy and mucosal dye spraying:Can significant lesions be distinguished?[J].Endoscopy,2001,33(4):306-310.
[5]S OBA,S TANAKA,Y SANO,et al.Current status of narrowband imaging magnifying colonoscopy for colorectal neoplasia in Japan[J].Digestion,2011,83(3):167-172.
[6]T MATSUMOTO,S NAKAMURA,T MORIYAMA,et al.Autofluorescence imaging colonoscopy for the detection of dysplastic lesions in ulcerative colitis:A pilot study[J].Colorectal Disease,2010,12(10 Online):e291-e297.
[7]T MATSUMOTO,M ESAKI,R FUJISAWA,et al.Chromoendoscopy,narrow-band imaging colonoscopy, and autofluorescence colonoscopy for detection of diminutive colorectal neoplasia in familial adenomatous polyposis[J].Disease of Colon & Rectum,2009,52(6):1160-1165.
[8]T MATSUMOTO,T MORIYAMA,T YAO,et al.Autofluorescence imaging colonoscopy for the diagnosis of dysplasia in ulcerative colitis[J].Inflammatory Bowel Disease,2007,13(5):640-641.
[9]X XIE,C LI,X ZUO,et al.Differentiation of colonic polyps by confocal laser endomicroscopy[J].Endoscopy,2011,43(2):87-93.
[10]B MAYINGER,M JORDAN,P HORNER,et al.Endoscopic light-induced autofluorescence spectroscopy for the diagnosis of colorectal cancer and adenoma[J].Journal of Photochemistry and Photobiology B:Biology,2003,70(1):13-20.
[11]X SHAO,W ZHENG,Z HUANG.In vivo diagnosis of colonic precancer and cancer using near-infrared autofluorescence spectroscopy and biochemical modeling[J].Journal of Biomedical Optics,2011,16(6):067005.
[12]B LI,S XIE.Autofluorescence excitation-emission matrices for diagnosis of colonic cancer[J].World Journal of Gastroenterology,2005,11(25):3931-3934.
[13]B BANERJEE,B MIEDEMA,H R CHANDRASEKHAR.E-mission spectra of colonic tissue and endogenous fluorophores[J].The American Journal of the Medical Sciences,1998,316(3):220-226.
[14]T J ROMER,M FITZMAURICE,R M COTHREN,et al.Laser-induced fluorescence microscopy of normal colon and dysplasia in colonic adenomas:Implications for spectroscopic diagnosis[J].The American Journal of Gastroenterology,1995,90(1):81-87.
[15]R RICHARDS-KORTUM,R P RAVA,R E PETRAS,et al.Spectroscopic diagnosis of colonic dysplasia[J].Photochemistry and Photobiology,1991,53(6):777-786.
[16]K T SCHOMACKER,J K FRISOLI,C C COMPTON,et al.Ultraviolet laser-induced fluorescence of colonic tissue:Basic biology and diagnostic potential[J].Lasers in Surgery and Medicine,1992,12(1):63-78.
[17]C EKER,S MONTAN,E JARAMILLO,et al.Clinical spectral characterisation of colonic mucosal lesions using autofluorescence and δ aminolevulinic acid sensitisation[J].Gut,1999,44(4):511-518.
[18]Z HUANG,W ZHENG,S XIE,et al.Laser-induced autofluorescence microscopy of normal and tumor human colonic tissue[J].International Journal of Oncology,2004,24(1):59-63.
[19]C R KAPADIA,F W CUTRUZZOLA,K M O’Brien,et al.Laser-induced fluorescence spectroscopy of human colonic mucosa.Detection of adenomatous transformation[J].Gastroenterology,1990,99(1):150-157.
[20]P YAKSHE,R BONNER,R PATTERSON,et al.Laser induced fluorescence spectroscopy(LIFS):Can it be used in the diagnosis and treatment of colonic malignancy?[J].The A-merican Journal of Gastroenterology,1989,84:1199(abstract).
[21]R M COTHREN,M V SIVAK Jr,J VAN DAM,et al.Detection of dysplasia at colonoscopy using laser-induced fluorescence:A blinded study[J].Gastrointestinal Endoscopy,1996,44(2):168-176.
[22]L LIU,B LIU,W LI,et al.Discriminant analysis for classification of colonic tissue autofluorescence spectra[C].Proceedings of SPIE,2010,7845,78450P.
[23]张阳德,万小平,范春,等.大肠早癌自体荧光检测系统研究 ⅰ.大肠癌自体荧光光谱诊断研究[J].中国内镜杂志,1995,1(1):6-8.ZHANG Yangde.WAN Xiaoping,FANG Chun,et al.Research on diagnosis of large intestine carcinoma with autofluorescence spectra analysis[J].China Journal of Endoscopy,1995,1(1):6-8.
[24]B W CHWIROT,M KOWALSKA,N PLOCIENNIK,et al.Variability of spectra of laser-induced fluorescence of colonic mucosa:Its significance for fluorescence detection of colonic neoplasia[J].Indian Journal of Experimental Biology,2003,41(5):500-510.
[25]R M COTHREN,R RICHARDS-KORTUM,M V SIVAK Jr.et al.Gastrointestinal tissue diagnosis by laser-induced fluorescence spectroscopy at endoscopy[J].Gastrointestinal Endoscopy,1990,36(2):105-111.
[26]X SHAO,W ZHENG,Z HUANG.Near-infrared autofluorescence spectroscopy for in vivo identification of hyperplastic and adenomatous polyps in the colon[J].Biosensors and Bioelectronics,2011,30(1):118-122.
[27]C Y WANG,J K LIN,H K CHIANG.Autofluorescence spectroscopy to identify normal and cancerous colorectal tissues[C].Proceedings of the 20th Annual International Conference of the IEEE,Engineering in Medicine and Biology Society.1998,967-969.
[28]R MARCHESINI,M BRAMBILLA,E PIGNOLI,et al.Lightinduced fluorescence spectroscopy of adenomas,adenocarcinomas and non-neoplastic mucosa in human colon.I.in vitro measurements[J].Journal of Photochemistry and Photobiology B:Biology,1992,14(3):219-230.
[29]X J LUO,B ZHANG,J G LI,et al.Autofluorescence spectroscopy forevaluating dysplasia in colorectaltissues[J].Zeitschrift für Medizinische Physik,2012,22(1):40-47.
[30]M A MYCEK,K T SCHOMACKER,N S NISHIOKA.Colonic polyp differentiation using time-resolved autofluorescence spectroscopy[J].Gastrointestinal Endoscopy,1998,48(4):390-394.
[31]B MAYINGER,P HORNER,M JORDAN,et al.Light-induced autofluorescence spectroscopy for tissue diagnosis of GI lesions[J].Gastrointestinal Endoscopy,2000,52(3):395-400.
[32]L HORAK,J ZAVADIL,V DUCHAC,et al.Auto-fluorescence spectroscopy of colorectal carcinoma:ex vivo study[J].Journal of Optoelectronics and Advanced Materials,2006,8(1):396.
[33]L LIU,Y NIE,L LIN,et al.Pattern recognition of multiple excitation autofluorescence spectra for colon tissue classification[J].Photodiagnosis and Photodynamic Therapy,2012.http://dx.doi.org/10.1016/j.pdpdt.2012.07.003
[34]R A ZANGARO,L SILVEIRA,R MANOHARAN,et al.Rapid multiexcitation fluorescence spectroscopy system for in vivo tissue diagnosis[J].Applied Optics,1996,35(25):5211-5219.
[35]B LI,Z ZHANG,S XIE.Steady state and time-resolved autofluorescence studies of human colonic tissues[J].Chinese Optics Letters,2006,4(6):348-350.
[36]R VAN HILLEGERSBERG,J W VAN DEN BERG,W J KORT,et al.Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats[J].Gastroenterology,1992,103(2):647-651.
[37]H A DAILEY,A SMITH.Differential interaction of porphyrins used in photoradiation therapy with ferrochelatase[J].Biochemical Journal,1984,223(2):441-445.
[38]P HINNEN,F W DE ROOIJ,M L VAN VELTHUYSEN,et al.Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin ix accumulation:A study in patients with(pre)malignant lesions of the oesophagus[J].British Journal of Cancer,1998,78(5):679-682.
[39]C M ANDERSON,M JEVONS,M THANGARAJU,et al.Transport of the photodynamic therapy agent 5-aminolevulinic acid by distinct H+-coupled nutrient carriers coexpressed in the small intestine[J].Journal of Pharmacology and Experimental Therapeutics,2010,332(1):220-228.
[40]K IZUISHI,H TAJIRI,T FUJII,et al.The histological basis of detection of adenoma and cancer in the colon by autofluorescence endoscopic imaging[J].Endoscopy,1999,31(7):511.
[41]G BOTTIROLI,A C CROCE,D LOCATELLI,et al.Natural fluorescence of normal and neoplastic human colon:A comprehensive“ex vivo”study[J].Lasers in Surgery and Medicine,1995,16(1):48-60.
[42]B W CHWIROT,M KOWALSKA,N SYPNIEWSKA,et al.Spectrally resolved fluorescence imaging of human colonic adenomas[J].Journal of Photochemistry and Photobiology B:Biology,1999,50(2):174-183.
[43]B W CHWIROT,Z MICHNIEWICZ,M KOWALSKA,et al.Detection of colonic malignant lesions by digital imaging of uv laser-induced autofluorescence[J].Photochemistry and photobiology,1999,69(3):336-340.
[44]G I ZONIOS,R M COTHREN,J T ARENDT,et al.Morphological model of human colon tissue fluorescence[J].IEEE Transactions on Biomedical Engineering,1996,43(2):113-122.
[45]T D WANG,J VAN DAM,J M CRAWFORD,et al.Fluorescence endoscopic imaging of human colonic adenomas[J].Gastroenterology,1996,111(5):1182-1191.
[46]G S FIARMAN,M H NATHANSON,A B WEST,et al.Differences in laser-induced autofluorescence between adenomatous and hyperplastic polyps and normal colonic mucosa by confocal microscopy[J].Digestive Diseases Sciences,1995,40(6):1261-1268.
[47]H W WANG,J WILLIS,M CANTO,et al.Quantitative laser scanning confocal autofluorescence microscopy of normal,premalignant,and malignant colonic tissues[J].IEEE Transactions on Biomedical Engineering,1999,46(10):1246-1252.
[48]R S DACOSTA,L D LILGE,J KOST,et al.Confocal fluorescence microscopy,microspectrofluorimetry,and modeling studies of laser-induced fluorescence endoscopy(LIFE)of human colon tissue[C].Proceedings of SPIE,Laser-Tissue Interaction VIII,1997,2975,98.
[49]张阳德,刘蔚东,杨川,等.结肠早癌自体荧光内镜诊断系统研究ⅰ.结肠组织显微自体荧光图像分析[J].中国内镜杂志,2000,6(1):77-79.ZHANG Yangde,LIU Weidong,YANG Chuan,et al.A study on autofluorescence endoscopic diagnostic system for early colonic cancer i.microscopic autofluorescence imaging of colonic tissues[J].China Journal of Endoscopy,2000,6(1):77-79.
[50]S ZHUO,J YAN,G CHEN,et al.Label-free monitoring of colonic cancer progression using multiphoton microscopy[J].Biomedical Optics Express,2011,2(3):615-619.
[51]M YING,S ZHUO,G CHEN,et al.Real-time noninvasive optical diagnosis for colorectal cancer using multiphoton microscopy[J].Scanning,2012,34(3):181-185.
[52]H AIHARA,H TAJIRI AND T SUZUKI.Application of autofluorescence endoscopy for colorectal cancer screening:Rationale and an update[J].Gastroenterology Research and Practice,2012,971383.
[53]K INOUE,N WAKABAYASHI,Y MORIMOTO,et al.Evaluation of autofluorescence colonoscopy for diagnosis of superficial colorectal neoplastic lesions[J].International Journal of Colorectal Disease,2010,25(7):811-816.
[54]T MATSUDA,Y SAITO,K I FU,et al.Does autofluorescence imaging videoendoscopy system improve the colonoscopic polyp detection rate?-a pilot study[J].The American Journal of Gastroenterology,2008,103(8):1926-1932.
[55]A IGNJATOVIC,J EAST,T GUENTHER,et al.What is the most reliable imaging modality for small colonic polyp characterization?Study of white-light,autofluorescence,and narrowband imaging[J].Endoscopy,2011,43(2):94.
[56]F J C VAN DEN BROEK,P FOCKENS,S VAN EEDEN,et al.Clinical evaluation of endoscopic trimodal imaging for the detection and differentiation of colonic polyps[J].Clinical Gastroenterology and Hepatology,2009,7(3):288-295.
[57]F J C VAN DEN BROEK,E J VAN SOEST,A H NABER,et al.Combining autofluorescence imaging and narrow-band imaging for the differentiation of adenomas from non-neoplastic colonic polyps among experienced and non-experienced endoscopists[J].The American Journal of Gastroenterology,2009,104(6):1498-1507.
[58]Y TAKEUCHI,N UEDO,M HANAFUSA,et al.Endoscopic diagnosis of colorectal neoplasms using autofluorescence imaging[J].Intestinal Research,2012,10(2):142-151.
[59]A PROBST,D GOLGER,H ARNHOLDT,et al.Endoscopic submucosal dissection of early cancers,flat adenomas,and submucosal tumors in the gastrointestinal tract[J].Clinical Gastroenterology and Hepatology,2009,7(2):149-155.
[60]T D WANG.Targeted imaging of flat and depressed colonic neoplasms[J].Gastrointestinal Endoscopy Clinics of North America,2010,20(3):579-583.
[61]K LIN,W ZHENG,Z HUANG.Integrated autofluorescence endoscopic imaging and point-wise spectroscopy for real-time in vivo tissue measurements[J].Journal of Biomedical Optics,2010,15(4):040507.
[62]B BANERJEE,T RENKOSKI,L R GRAVES,et al.Tryptophan autofluorescence imaging of neoplasms of the human colon[J].Journal of Biomedical Optics,2012,17(1):016003.
[63]K IMAIZUMI,Y HARADA,N WAKABAYASHI,et al.Dualwavelength excitation of mucosal autofluorescence for precise detection of diminutive colonic adenomas[J].Gastrointestinal Endoscopy,2012,75(1):110-117.
[64]J HARINGSMA,G N TYTGAT,H YANO,et al.Autofluorescence endoscopy:feasibility of detection of GI neoplasms unapparent to white light endoscopy with an evolving technology[J].Gastrointestinal Endoscopy,2001,53(6):642-650.
[65]J HARINGSMA,G N TYTGAT.The value of fluorescence techniques in gastrointestinal endoscopy:better than the endoscopist’s eye?I:the european experience[J].Endoscopy,1998,30(4):416-418.
[66]M VAN LERLAND-VAN LEEUWEN,G TYTGAT.Detection of dysplasia using fluorescence in vivo using the Xillix-LIFE-GI system[J].Endoscopy,1996,28 S44-45.
[67]W CEBULA,W ZIELEZNIK,A SIERON,et al.Laser-induced fluorescent endoscopy(LIFE)in detection of malignant lesions of the colon[C].Proceedins of SPIE,2001,4156,272-276.
[68]T D WANG,J M CRAWFORD,M S FELD,et al.In vivo identification of colonic dysplasia using fluorescence endoscopic imaging[J].Gastrointestinal Endoscopy,1999,49(4):447-455.
[69]S BRAND,H STEPP,T OCHSENKÜHN,et al.Detection of colonic dysplasia by light-induced fluorescence endoscopy:A pilot study[J].International Journal of Colorectal Disease,1999,14(1):63-68.
[70]Z BENES,Z ANTOS.Optical biopsy system distinguishing between hyperplastic and adenomatous polyps in the colon during colonoscopy[J].Anticancer Research,2009,29(11):4737-4739.
[71]C SCHMIDT,I PETERSEN,A STALLMACH.Su1488 laserinduced fluorescence to distinguish adenomatous from non-adenomatous colorectal polyps[J].Gastrointestinal Endoscopy,2012,75(4):AB350.
[72]T KUIPER,Y A ALDERLIESTE,K M TYTGAT,et al.Sa1601 differentiation of small colorectal lesions;laser-induced autofluorescence using the wavstat[J].Gastrointestinal Endoscopy,2012,75(4):AB216.
[73]N UEDO,K HIGASHINO,R ISHIHARA,et al.Diagnosis of colonic adenomas by new autofluorescence imaging system:A pilot study[J].Digestive Endoscopy,2007,19(s1):S134-S138.
[74]K S BOPARAI,F J C VAN DEN BROEK,S VAN EEDEN,et al.Hyperplastic polyposis syndrome:A pilot study for the differentiation of polyps by using high-resolution endoscopy,autofluorescence imaging,and narrow-band imaging[J].Gastrointestinal Endoscopy,2009,70(5):947-955.
[75]K ARITA,K MITSUYAMA,H KAWANO,et al.Quantitative analysis of colorectal mucosal lesions by autofluorescence endoscopy:Discrimination of carcinomas from other lesions[J].Oncology Reports,2011,26(1):43-48.
[76]R SATO,M FUJIYA,J WATARI,et al.The diagnostic accuracy of high-resolution endoscopy,autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma[J].Endoscopy,2011,43(10):862.
[77]T KUIPER,F J C VAN DEN BROEK,A H NABER,et al.Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy[J].Gastroenterology,2011,140(7):1887-1894.
[78]R SHUKLA,W ABIDI,R RICHARDS-KORTUM,et al.Endoscopic imaging:How far are we from real-time histologhy[J].World Journal of Gastrointestinal Endoscopy,2011,3(10):183-194.

