磷化镍纳米粒子可为制氢反应提速
美国宾夕法尼亚州立大学化学教授雷蒙德!萨克领导的研究团队近日发现,由储量丰富且廉价的磷和镍构成的磷化镍纳米粒子可以成为制氢反应的催化剂,为该反应提速,最新研究将让更廉价的清洁能源技术成为可能,相关论文将发表在《美国化学会志》上。
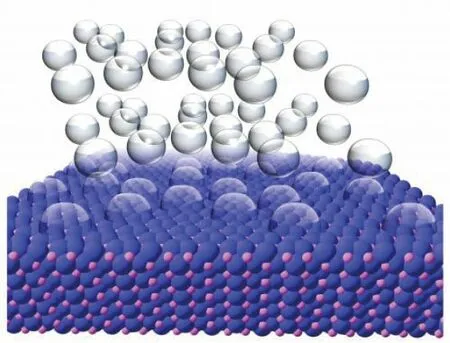
为了制造出磷化镍纳米粒子,研究团队使用经济上可行的金属盐进行试验。他们让这些金属盐在溶剂中溶解,并朝其中添加了另外一些化学元素,然后加热溶液,最终得到了一种准球形的纳米粒子——其并非完美的球形,因为拥有一些平的暴露的边角。萨克解释道:“纳米粒子个头小,但表面积很大,而且,暴露的边缘上有大量的点可以为制氢反应提速。”
接下来,加州理工学院化学系教授内森!刘易斯领导的科研团队对这种纳米粒子在反应中的催化表现进行了测试。研究人员首先将该纳米粒子放在一块钛金属薄片上,并将薄片没入硫酸溶液中,随后施加电压并对生成的电流进行了测试。结果表明,化学反应不仅按照他们所希望的那样发生了,效率也非常高。
萨克解释道,磷化镍纳米粒子的主要作用是帮助人们从水中制造出氢气,这一反应对很多能源生产技术,包括燃料电池和太阳能电池来说都很重要。水是一种理想的燃料,因为其廉价且丰富,但我们需要将氢气从中提取出来。氢气的能量密度很高且是很好的载能体,但产生氢气会耗费能量。
科学家们一直在寻找廉价的催化剂以便让水制氢反应更加实用且高效。萨克表示:“铂可以很好地完成这件事,但铂昂贵且稀少。我们一直在寻找替代铂的材料。此前有科学家预测,磷化镍会是好的‘替身’,我们的研究结果也表明,在制氢反应中,磷化镍纳米粒子的表现的确可以和目前铂的效果相媲美。”
萨克说:“纳米粒子技术有望让我们获得更廉价且更环保的能源。接下来,我们打算进一步改进这些纳米粒子的性能并厘清其工作原理。最新技术有望启发我们发现其他也由储量丰富的元素组成的催化剂,甚至其他更好的催化剂。”
Nanoparticle opens the door to clean-energy alternatives
Cheaper clean-energy technologies could be made possible thanks to a new discovery.Research team members led by Raymond Schaak,a professor of chemistry at Penn State University,have found that an important chemical reaction that generates hydrogen from water is effectively triggered—or catalyzed—by a nanoparticle composed of nickel and phosphorus,two inexpensive elements that are abundant on Earth.The results of the research will be published in the Journal of the American Chemical Society.
Schaak explained that the purpose of the nickel phosphide nanoparticle is to help produce hydrogen from water,which is a process that is important for many energy-production technologies,including fuel cells and solar cells."Water is an ideal fuel,because it is cheap and abundant,but we need to be able to extract hydrogen from it," Schaak said.Hydrogen has a high energy density and is a great energy carrier,Schaak explained,but it requires energy to produce.To make its production practical,scientists have been hunting for a way to trigger the required chemical reactions with an inexpensive catalyst.Schaak noted that this feat is accomplished very well by platinum but,because platinum is expensive and relatively rare,he and his team have been searching for alternative materials."There were some predictions that nickel phosphide might be a good candidate,and we had already been working with nickel phosphide nanoparticles for several years," Schaak said."It turns out that nanoparticles of nickel phosphide are indeed active for producing hydrogen and are comparable to the best known alternatives to platinum."
To create the nickel phosphide nanoparticles,team members began with metal salts that are commercially available.They then dissolved these salts in solvents,added other chemical ingredients,and heated the solution to allow the nanoparticles to form.The researchers were able create a nanoparticle that was quasi-spherical—not a perfect sphere,but spherical with many flat,exposed edges."The small size of the nanoparticles creates a high surface area,and the exposed edges means that a large number of sites are available to catalyze the chemical reaction that produces hydrogen," Schaak explained.
The next step was forteam members at the California Institute of Technology to test the nanoparticles' performance in catalyzing the necessary chemical reactions.Led by Nathan S.Lewis,the George L.Argyros Professor of Chemistry at the California Institute of Technology,the researchers performed these tests by placing the nanoparticles onto a sheet of titanium foil and immersing that sheet in a solution of sulfuric acid.Next,the researchers applied a voltage and measured the current produced.They found that,not only were the chemical reactions happening as they had hoped,they also were happening with a high degree of efficacy.
"Nanoparticle technology has already started to open the door to cheaper and cleaner energy that is also efficient and useful," Schaak said."The goal now is to further improve the performance of these nanoparticles and to understand what makes them function the way they do.Also,our team members believe that our success with nickel phosphide can pave the way toward the discovery of other new catalysts that also are comprised of Earth-abundant materials.Insights from this discovery may lead to even better catalysts in the future."

