邻菲罗啉铜与人血清白蛋白相互作用的研究
王 丽,高 勇,吴 丹,苏海艳,邓赛鹏,张业中,戴 捷
(长江大学 化学与环境工程学院,湖北 荆州 434023)

1 实验部分
1.1 仪器与试剂
LS-55荧光分光光度计(美国Perkin 公司),AY-120M 电子分析天平(日本岛津公司),SYC-15超级恒温水浴(南京桑力电子设备厂,控温精度±0.1 ℃),Jasco J-810 圆二色谱仪(Jasco,Tokyo,Japan).
合成邻菲罗啉铜参照文献[6],表征后配成不同浓度溶液,HSA(Sigma公司提供,浓度:1.0×10-5mol/L),NaCl溶液(0.5 mol/L),Tris-HCl缓冲溶液(pH 7.40),所用试剂均为分析纯,实验用水为二次蒸馏水,经检测均无荧光杂质.
1.2 实验方法
1.2.1 荧光猝灭光谱设定激发波长为λex=285nm,激发狭缝宽度为15.0nm,发射狭缝宽度为4.5nm,扫描速度为300nm/min.在模拟生理条件下测定体系温度分别为292,298,304,310K时不同浓度的邻菲罗啉铜溶液与人血清白蛋白相互作用的荧光猝灭图谱,记录波长在300~450nm 范围内的荧光光谱.


2 实验结果与讨论
2.1 荧光猝灭机制


图1 加入不同浓度的 之后HSA 的荧光发射光谱,插图为298K 时的Stern-volmer关系图Fig.1 Fluorescence emission spectra of HSA in the presence of various concentrations of (T=298K,λex=285nm).c(HSA)=1×10-5 mol/L;c /(10-6 mol·L-1),
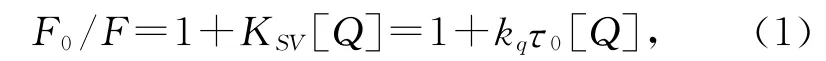
式中,F0和F分别表示不存在和存在猝灭剂时体系中荧光物质的荧光强度;KSV是Stern-Volmer猝灭常数,[Q]是猝灭剂的浓度,kq是生物大分子的猝灭速率常数,τ0为不存在猝灭剂时荧光分子的平均寿命.当T=298 K 时,以F0/F对[Q]作图(如图1中插图所示),具有良好的线性关系.利用方程(1)计算4个不同温度下的猝灭常数,结果列于表1.

表1 不同温度下 与HSA 相互作用的猝灭反应常数和热力学参数Tab.1 Quenching constants and thermodynamic parameters for the interaction between and HSA at four different temperatures.

静态猝灭过程遵循Lineweaver-Burk方程[10]:

式中,(F0-F)是加入猝灭剂前后荧光强度的差值,KLB是静态荧光猝灭结合常数.以(F0-F)-1对[Q]-1作图,如图2所示,再根据方程(2)算出4个温度下的结合常数,结果列于表1.

图2 不同温度下HSA- 体系的Lineweaver-Burk关系图Fig.2 Lineweaver-Burk plots for the quenching of HSA by at four different temperatures
2.2 热力学参数及结合作用力
一般情况下,小分子与生物大分子之间的作用力包括疏水作用力、氢键、范德华力和静电作用力.根据Ross等人的观点[11]:ΔH>0,ΔS>0为典型的疏水作用力;ΔH<0,ΔS>0为静电引力;ΔH<0,ΔS<0为氢键和范德华力.温度变化不大时,可认为焓变ΔH是一个常数.焓变ΔH和熵变ΔS可以用van't Hoff方程计算:

吉布斯自由能变ΔG可由下列关系算出:

式中,K是相应温度下的结合常数;R是气体摩尔常数.根据方程(3)和(4)可求出ΔH,ΔS,ΔG等热力学参数(见表1).

2.3 结合位点



图3 位点探针对-HSA 体系的影响(图中分别插入了相应探针的分子结构)Fig.3 Effect of site markers to -HSA system(T=298K,λex=285nm)c(HSA)=1.0×10-5 mol/L;(a)c(Warfarin)=1.0×10-5 mol/L;(b)c(Ibuprofen)=1.0×10-5 mol/L;c /(10-6 mol/L),A~K:0,1.0,2.0,3.0,4.0,5.0,6.0,7.0,8.0,respectively.The inserts correspond to the molecular structures of site markers.
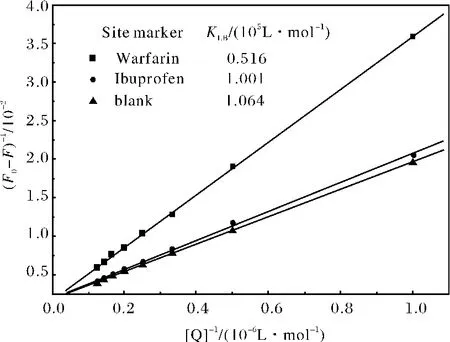
图4 位点竞争实验的Lineweaver-Burk曲线Fig.4 Lineweaver-Burk for site marker competitive experiments of -HSA system


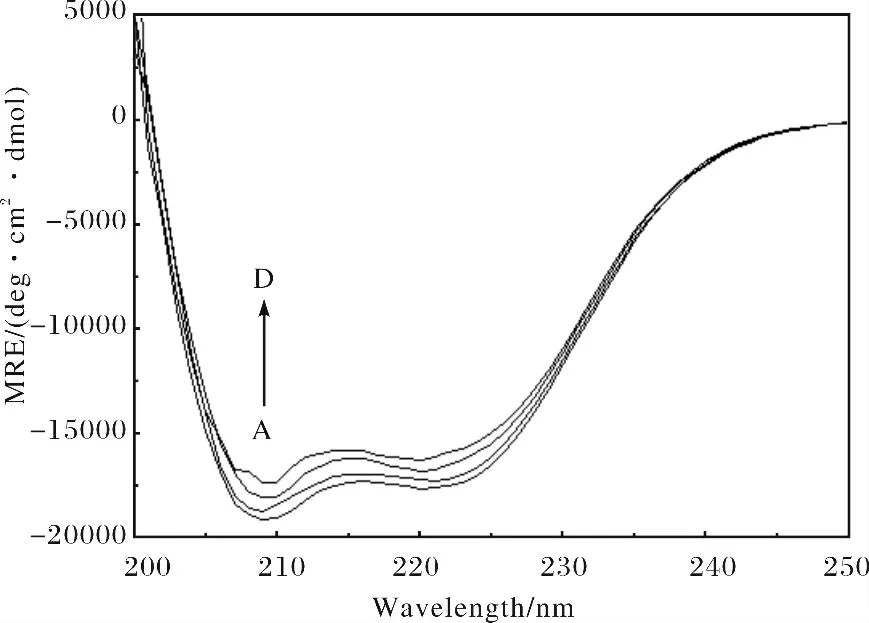
图5 -HSA 体系的圆二色谱图Fig.5 The CD spectra of the HSA- system c(HSA)=1.0×10-5 mol/L;c( )/(10-5 mol·L-1)A~D:0,2.0,6.0,10.0,respectively.
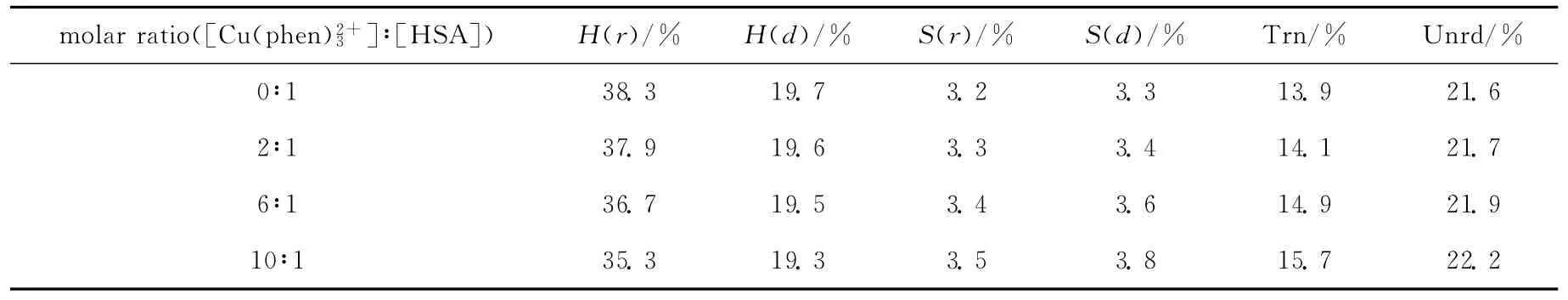
表2 根据SELCON3程序解析的HSA中不同二级结构含量Tab.2 Fractions of different secondary structures of HSA determined by SELCON3
3 结论

[1]Kragh-Hansen U.Molecular aspects of ligand binding to serum albumin[J].Pharmacol Rev,1981,33:17-53.
[2]Ahmad B,Parveen S,Khan R H.Effect of albumin con-formation on the binding of ciprofloxacin to human serum albumin:a novel approach directly assigning binding site[J].Biomacromolecules,2006,7:1350-1356.
[3]Zhang J,Sun H H,Zhang Y Z,et al.Interaction of human serum albumin with indomethacin:Spectroscopic and molecular modeling studies[J].J Solut Chem,2012,41:422-435.[4]Zhang Q L,Liu J,Liu J Z,et al.Effect of intramolecular hydrogen-bond on the DNA binding and photocleavage propertiesof polypyridy cobalt(Ⅲ)complexes[J].Inorg Chim Acta,2002,339:34-40.
[5]Tabassum S,Sharma G C,Arjmand F.New modulated design and synthesis of chiral CuII/SnIVbimetallic potential anticancer drug entity:In vitro DNA binding and pBR322DNA cleavageactivity[J].Spectroc Acta Part A:Molec Biomolec Spectr,2012,90:208-217.
[6]Inskeep R G.The spectra of the tris complexes of 1,10-phenanthroline and 2,2-bipyridine with the transition metals iron(II)through zinc(II)[J].J Inorg Nucl Chem,1962,24:763-776.
[7]Kathiravan A,Chandramohan M,Renganathan R,et al.Spectroscopic studies on the interaction between phycocyanin and bovine serum albumin[J].J Mol Struct,2009,919:210-214.
[8]Lakowicz J R.Principles of Fluorescence Spectroscopy(2nd ed)[M].New York:Plenum Press,1999:237-265.
[9]Lakowicz J R,Weber G.Quenching of fluorescence by oxygen.Probe for structural fluctuations in macromolecules[J].Biochemistry,1973,12:4161-4170.
[10]Zhang H X,Mei P,Yang X X.Optical,structural and thermodynamic properties of the interaction between tradimefon and serum albumin[J].Spectrochimica Acta,Part A:Mol Biomol Spectrosc,2009,72:621-626.
[11]Ross D P,Subramanian S.Thermodynamics of protein association reactions:forces contributing to stability[J].Biochemistry,1981,20:3096-3102.
[12]Vetri V,Librizzi F,Leone M,et al.Thermal aggregation of bovine serum albumin at diverent pH:comparison with human serum albumin[J].Eur Biophys J,2007,36:717-725.
[13]Carter D C,He X M,Munson S H,et al.Three-dimensional structure of human serum albumin[J].Science,1989,244:1195-1196.
[14]Sudlow G,Birkett D J,Wade D N.The characterization of two specific drug binding sites on human serum albumin[J].Mol Pharmacol,1975,11(6):824-832.
[15]Wanwimolruk S,Birkett D J,Brooks P M.Structural requirements for drug binding to site II on human serum albumin[J].Mol Pharmacol,1983,24(3):458-463.
[16]Zhang Y Z,Zhou B,Zhang X P,et al.Interaction of malachite green with bovine serum albumin:Determination of the binding mechanism and binding site by spectroscopic methods[J].Journal of Hazardous Materials,2009,163(2-3):1345-1352.
[17]Cui F T,Fan J,Hu Z D.Interactions between 1-benzoyl-4-p-chlorophenyl thiosemicarbazide and serum albumin:investigation by fluorescence spectroscopy[J].Bioorg Med Chem,2004,12(1):151-157.

