Effects of Sub-chronic Aluminum Exposure on Renal Pathologic Structure in Rats
Xia Shi-liang, Li Miao, Shao Bing, Bai Chong-sheng, Zhang Ji-hong, and Li Yan-fei
College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China
Introduction
Studies show that Al exert a wide range of biological toxicity (Wang et al., 1995). Al3+is absorbed by digestive system and accumulated in brain, bone,blood, reproductive system, liver and immune organs,etc. Al is excreted by kidney, and most of Al3+mainly discharged with urine (Liu et al., 2009). But the toxicity effect of Al on kidney is uncertain. In the experiment, rats were administrated orally with different concentrations of AlCl3for 120 days. Kidney coeff i cient, and Al concentrations in blood and kidney were determined and renal autopsy and histological changes were observed to investigate the effect of Al on kidney, which will provide basic data for the toxicity of Al on kidney.
Materials and Methods
Animal sectionalization and treatment
A total of 40 healthy Wistar rats, weighing 130-140 g,were randomly allocated into control group (GC), lowdose group (GL), middle-dose group (GM), and highdose group (GH). All of the rats were acclimatized for 1 week before the test, and then were orally administrated with 0, 64.18, 128.36, and 256.72 mg · kg-1BW AlCl3in drinking distilled water for 120 days, respectively. All rats had free access to water and were housed in cages at constant temperature (22-25℃)and certain relative humidity (55%±5%). The size of the cages was 470×300×150 mm, which was large enough for the growth of five rats. Throughout the experiment, wood chips were renewed every 3 days.The health status of rats was monitored every day.
Tissue sampling and processing
The rats were weighed, then sacrificed by ether anesthesia at the end of the experiment. Blood was collected from the caudal vein, and serum was separated and stored at -20℃ for assays of Al. The kidneys were carefully removed and sliced into two parts. One part was immediately fixed with 10%neutral formaldehyde solution for HE straining to observe renal structure, while the other part was triturated with PBS (pH 7.2)to detect Al concentration.
Determination of organ coeff i cient and observation of tissue
The kidneys were cut lengthways, decorticated quickly and weighted. The kidney coefficient was calculated as the renal weight/the body weight ×100.
Observation of histomorphology
The fixed renal tissues were cut into 2×1.5×0.3 (cm)blocks fi rstly, then the blocks were cut into slices for HE straining. Histopathology changes were observed under optical microscope (Li, 2012).
Determination of Al concentrations in blood and kidney
Serum or renal tissue was conducted with wet digestion and detected by graphite furnace atomic absorption spectrometry (Liang et al., 2011).
Data processing and analysis
Data was expressed as mean ± standard deviation(SD)and analyzed by one-way analysis of variance using the statistical package SPSS 17.0 for windows,P values of less than 0.05 were considered signif i cant and less than 0.01 were extremely signif i cant.
Results
Kidney coeff i cient
The kidney coefficient in Al-treated groups was significantly higher than that in GC (P<0.01),decreased with the increased Al doses, and there was a dose-effect relationship (Table 1).
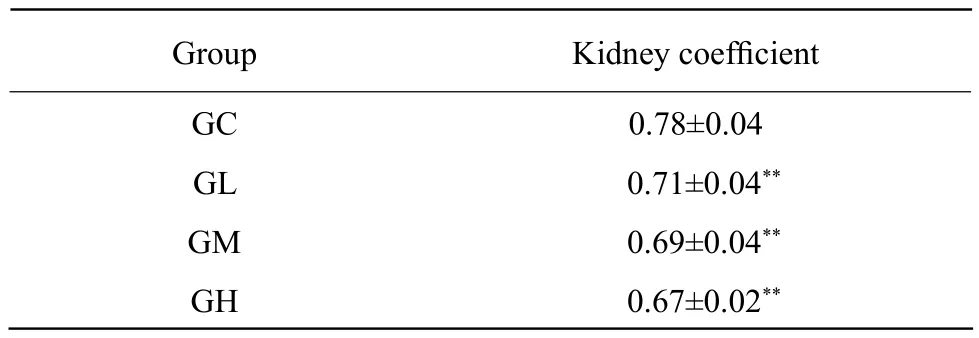
Table 1 Kidney coeff i cient in each group
Observation of renal pathologic autopsy
The kidneys in GC were auburnish red and glossy,section were smooth, and capsule thickness were normal (Fig. 1). While the kidneys in Al-treated groups were solid, lustreless and pale brown with white necrosis points on surface (Fig. 2, arrow), renal cortex was thin, and dividing line between cortex and medulla was unclear.

Fig. 1 Kidneys in GC

Fig. 2 Kidneys in GM
Histological changes of kidneys

Fig. 3 HE staining of kindneys (×400)
The structures of glomerulus and renal tubules were clear in GC (Fig. 3A). In all Al-treated rats, renal tubules narrowed, renal glomerulus atrophied, epitheliums dissolved and cell nucleus disappeared (Fig. 3B and C). The vessels of glomerulus were dilated and glomerulus were necrotic (Fig. 3D). Pathological changes aggravated gradually with Al dose increase in rats.
Al concentrations in serum and kidney
The Al concentrations in serum and kidney in Altreated groups were obviously higher than those in GC(P<0.01), and there were a dose-effect relationship(Table 2).

Table 2 Al concentrations in serum and kidney
Discussion
Under the normal condition, Al was excreted by kidney. High Al concentration caused function damage of renal (Tang et al., 2002). Organ coefficient was a sensitive index ref l ecting target organ damage caused by toxicant (Huang et al., 2003). In this study, the renal coefficient was lower in all Al-treated groups compared with GC, which indicated that the development of the kidney was restrained by Al.
Overmuch Al ingestion could cause renal damage.The swell of renal tubules, capillaries and the epithelial cells of proximal convoluted tubule were observed by HE straining in rats exposed to AlCl3(Zhuo, 2008).Ji et al. (2011)found that glomerulus shrank in rats treated with 100 mg · kg-1AlCl3through nasal drip. This experiment showed that in all Al-treated rats, renal tubules were narrowed, epitheliums dissolved and its nucleus disappeared, renal glomerulus atrophied. The vessels of glomerulus were dilated and glomerulus were necrotic. And the damages aggravated with Al dose increase.
Al is excreted mainly by kidney. Liu et al. (2007)reported that Al cumulated in serum and kidney with Al dose increase in rabbits. Zhuo (2008)found that Al contents in serum and kidney of rats orally administrated Al for 4 weeks were about 30 times higher than those in the control one. This experiment demonstrated that the Al concentrations in serum and kidney in Al-treated groups were obviously higher than that in GC. The above results showed that overmuch ingested Al could cumulated in kidney.
In conclusion, kidney was the target organ of Al toxicity. Sub-chronic Al exposure caused pathological damage of kidney, especial in renal tubules and glomerulus. The study results could provide morphological evidence for the study of renal Al toxicity.
Wang S H, Li K S, Wang X D. 1995. Metabolism and toxicity of aluminum. China Occupational Medicine, 5(22): 48-49.
Li L. 2012. Discussion about HE straining methods of old tissue.Journal of Qiqihar University of Medicine, 22(33): 3123-3124.
Liang R F, Niu Q, Li W Q, et al. 2011. Effect of aluminum-maltolate on learning and memory in rats. Chinese Journal of Public Health,27(11): 1429-1430.
Tang B Z, Liu X, Ma Y D, et al. 2002. Measurement of organ weight,organ coeff i cients and blood indices in HSF1 knockout mice. Chinese Journal of Laboratory Animal Science, 12(3): 153-156.
Huang Y Q, Zhang W C, Li H Y, 2003. Effect of cadmium on body weight and organ coefficient of ovaries in female rats. Occupation and Health, 19(7): 7-9.
Ji J W, Zhang Q L, Gao F P, et al. 2011. The basic pathological changes in mice induced by nano-alumina. Journal of Toxicology, 25(6):442-446.
Zhuo J H. 2008. The mechanism of DFP protecting against nervous system, liver and kidney in aluminum-exposed rats. Shandong University, Jinan.
Liu P. 2007. The synthesis and removing aluminum effect and the mechanism of DFP protecting against chronic aluminum-exposed animals. Shandong University, Jinan.
 Journal of Northeast Agricultural University(English Edition)2013年1期
Journal of Northeast Agricultural University(English Edition)2013年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Ecuador Export Potential Agricultural Products to China
- Effects of Low-temperature and Herbicide on Membrane Stability,Antioxidant Capacity, and Product of Metabolism in Barley Seedlings
- SWOT Analysis and Development Strategies of Maize Industry in Heilongjiang Province
- Research on DSP-Based Automatic Excitation Regulator in Small Rural Hydropower Station
- Expression of a Lysine-rich Gene in Bacillus subtilis 168
- Isolation, Purif i cation and Cryopreservation of Cells from Neonatal Bovine Testis
