Isolation, Purif i cation and Cryopreservation of Cells from Neonatal Bovine Testis
Zheng Peng, Hu Peng-fei, Tian Ya-guang, Huang He, and Zhang Gui-xue*
1 College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
2 Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China
Introduction
Spermatogonial stem cell research has become a new research focus since they were fi rst reported by Brinter and Eimmerman in 1994, which recipient mouse testes were recovered and restored normal spermatogenesis after spermatogonial stem cell transplantation (Brinster and James, 1994). Spermatogonial stem cell technology development and application will offer new opportunities and avenues for animal cloning, endangered species preservation, transgenic animal making, treatment of male sterility and gene therapy for human genetic diseases.
Spermatogonial stem cells from neonatal mouse(Kanatsu-Shinohara et al., 2003), 4-7-day-old mouse(Kubota et al., 2004; Hongyan et al., 2008), adult mouse (Kaomei et al., 2006), adults human (Sabine et al., 2008), sheep (Jose et al., 2006), neonatal pigs(Sandeep et al., 2007; Sandeep et al., 2009), and 3-7-month-old cattle (Izadyar et al., 2002)were successfully isolated and purified by different laboratories,respectively, and the cells were used for in vitro culture, transplantation and other related researches.
In this study, by using neonatal cattle as the experimental animal, isolation, purification and cryopreservation of spermatogenic epithelial cells were studied in order to provide theoretical and practical basis for establishment of stable and eff i cient system of isolation and cultivation of spermatogonial stem cells.
Materials and Methods
Animals and reagents
Testis were collected from 0-1 day neonatal cattle, and transported to the laboratory within 2 h. DMEM/F12 and FBS were from Gibco, other reagents were from Sigma, unless noted otherwise, antibodys were from Santa cruz.
Methods
Histological examination
Small pieces of testicular tissues were fixed in 2.5%glutaraldehyde at 4℃ overnight. Then they were evaluated under electron microscopy as described by Izadyar (Izadyar et al., 2002).
Isolation and collection of bovine testicular cells
Testis were fi rst decapsulated and dispersed in DPBS solution, then incubated in DPBS solution with 1.5 mg · mL-1collagenase IV and 10 μg · mL-1DNase I at room temperature for 5-10 min with gentle agitation until the tubules were separated. Dispersed seminiferous tubules were then washed 2 times in 10 volumes of PBS by centrifugation at 170 g for 3 min each time and removal of most of the interstitial cells. For the second digestion, seminiferous tubules fragments were incubated in DPBS solution with 2.5 mg · mL-1trypsin at room temperature for 2-5 min with vigorous aspiration until clumps of tubules were invisible.Digestion was then stopped by addition of Medium D(DMEM/F-12 supplemented with 10% FBS and 1%penicillin-streptomycin)and then filtered through a nylon mesh with 100 μm pore size to remove large clumps of cells. Cells were collected by centrifugation at 200 g for 5 min. Finally, the cells were suspended in Medium D. Cell suspension was plated on a cover glass pre-coated with poly-L-lysine for AKP staining.Cell selection and identif i cation
Suspension of spermatogenic epithelial cells were incubated in tissue culture plates at a density of 1-5 ×105cells · cm-2for 2-4 h. The fl oating cells were collected and transferred to another plate after gentle pipetting,and then they were cultured overnight in an incubator at 37℃ with a water-saturated atmosphere of 95% air and 5% CO2. The cells were collected by pipetting and centrifugation at 200 g for 5 min. Finally, they were suspended in Medium D. Cells were identified by means of AKP staining before and after differential adherent cell selection.
After differential adherent cell selection, the cells were used for immunocytochemical analysis using rabbit anti C-kit polyclonal antibody and goat anti OCT-4 polyclonal antibody.
Cryopreservation of spermatogonial stem cells
Seminiferous tubules were cut to small pieces with ophthalmic scissors for cryopreservation according to the stage of cooling method (4℃, 1 h; -20℃, 1 h;-80℃, overnight). Frozen liquid used was Medium D supplemented with 5%, 10%, and 15% of dimethyl sulfoxide (DMSO), ethylene glycol (EG)and glycerol(G), respectively. Tissues were thawed by 38℃ water bath for recovery, and then were digested into spermatogenic epithelial cells suspension and identif i ed by trypan blue staining.
Isolation, purification and identification of sertoli cells
Spermatogenic epithelial cell suspension were incubated for 2-4 h. After gentle pipetting, the cells were cultured with fresh medium overnight. Cells were digested, replated, and cultured for 24 h, and then they were used for HE staining, rathonum red staining and immunocytochemical analysis by using vimentin.Cryopreservation of sertoli cells
Cells were passaged after forming single layer. They were selected for cryopreservation according to stages of cooling method. Frozen liquid was Medium D supplemented with 10% DMSO, 10% EG, 0.1, 0.2 mmol · L-1of sucrose and trehalose, respectively.
Statistical analysis
All statistical analyses were conducted by using SPSS 13.0. The level of signif i cance was set at P<0.05. Data in text were presented as the mean ± SEM.
Results
Histological examination
Seminiferous tubules of neonatal bovine testis were comprised by myoid cells, basement membrane, sertoli cells and spermatogonial stem cells. Sertoli cells were attached to basement membrane (Fig. 1), there were round, conical and cylindrical myoid cells with diverse morphology, the cell diameter was about 15 μm, the cytoplasm was stained strong, and the nucleus shape varied with the cell shape. There were 1-3 nucleolus and the location of nucleus was not fi xed, which could distribute in the nuclear cytoplasm and nuclear intima.Spermatogonial stem cells could be divided into two types according to diameter: large spermatogonia stem cells with the diameter of about 15-20 μm, and the small spermatogonia stem cells with diameter of about 10-15 μm. The cytoplasm of spermatogonial stem cells was light staining and the nucleus was larger with 1-2 round nucleoli. Some of the spermatogonial stem cells were attached to the base membrane (Fig. 1A),and some were located in the central lumen (Fig. 1B).There were leydig cells and capillaries between the seminiferous tubules.
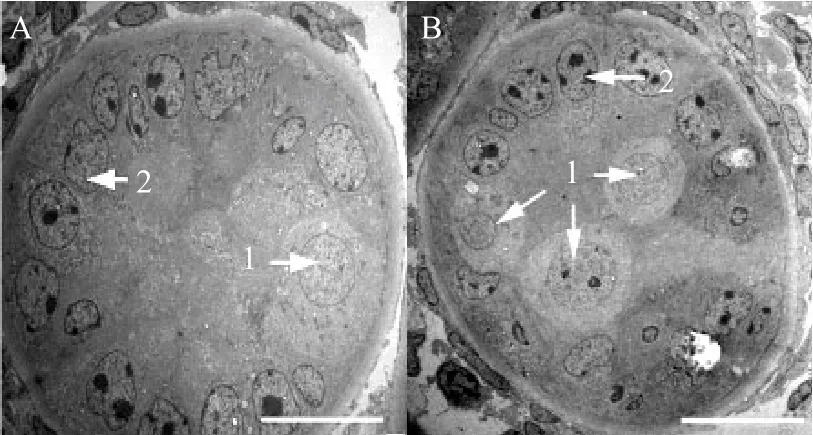
Fig. 1 Cross sections of seminiferous tubules
Isolation, purification and identification of spermatogonial stem cells
Seminiferous tubules were harvested after the first round digestion of testis and washed by low-speed centrifugation. Most of the leydig cells have been removed from testicular cell suspension. After the second round of digestion, fi ltration and centrifugation,spermatogonial stem cells and sertoli cells were the main components in the testicular cell suspension, the undigested tissues, leydig cells and cell debris were removed. It showed that a small number of cells were positive in testicular cell suspension by AKP staining,which could be judged as spermatogonial stem cells(Fig. 2A).
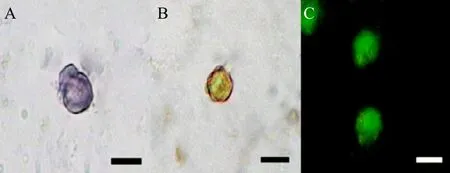
Fig. 2 Identif i cation of spermatogonial stem cells
AKP staining was carried out before and after differential adherent cell selection. The results showed that only a very few cells were positive before differential adherent cell selection, and the majority of cells were positive after differential adherent cell selection. The positive rate was 72% (Table 1). Immunohistochemical analysis showed that C-kit (Fig. 2B),and OCT-4 (Fig. 2C)were positive after differential adherent cell selection.
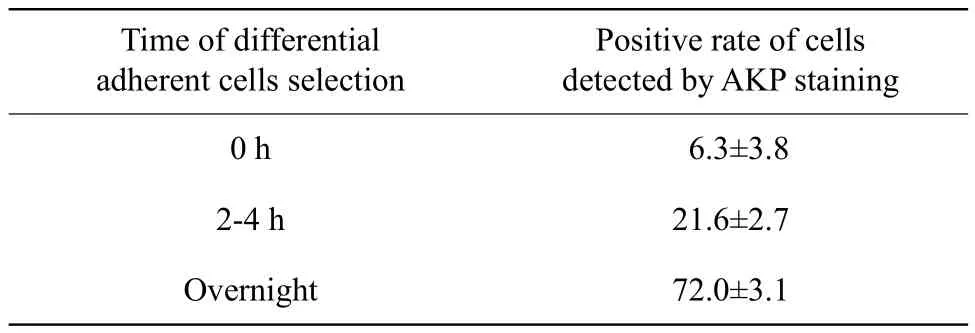
Table 1 Differential adherent spermatogonial stem cell selections (%)(n=10)
Cryopreservation of spermatogonial stem cells
There were nine kinds of frozen liquid used in our experiments for spermatogonial stem cells cryopreservation. The present results showed that Medium D supplemented with 10% of DMSO was the optimal choice for spermatogonial stem cells cryopreservation(Table 2).
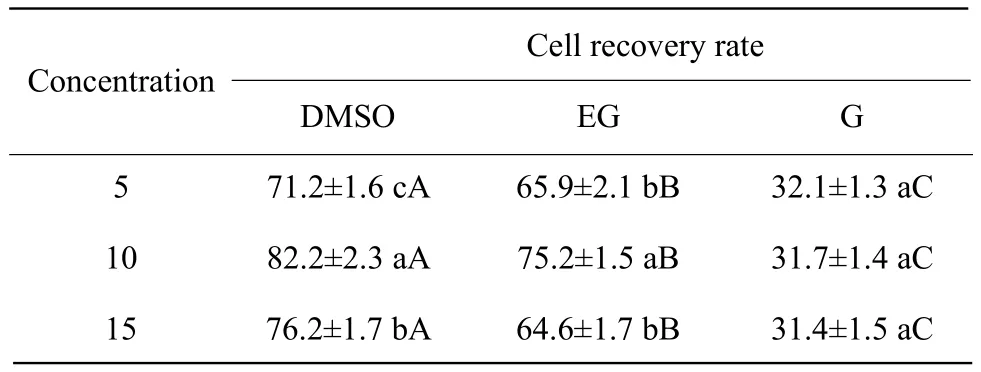
Table 2 Spermatogonia stem cell survival rate with three cryoprotectants (%)(n=10)
Isolation, purification and identification of sertoli cells
Spermatogenic epithelial cells were observed under the microscope after the cell suspension were incubated for 2-4 h. Adherent cells were determinated as sertoli cells, which had been polarized, with cell body extended and irregular morphology. After cultured overnight with fresh Medium D, adherent cells were identif i ed by HE staining, sertoli cells had irregular morphology with cytoplasm fully rolled out and stained lighter. Nuclei of sertoli cells were round or oval in the cytoplasm center, with prominent nucleoli which was stained strong (Fig. 3A). Round or oval lipid droplets were around the nucleus of sertoli cells, and they were stained in red or reddish-brown by rathonum red staining, while other parts of cells were not stained (Fig. 3B). Vimentin was specif i cally expressed in the sertoli cells of the seminiferous tubules. The expression of vimentin in cultured cells was shown by immunohistochemical staining (Fig. 3C).
Cryopreservation of sertoli cells
There were 10 kinds of the frozen liquid used in our experiments for the cryopreservation of sertoli cells.The present results showed that Medium D supplemented with 10% EG and 0.1 mmol · L-1trehalose had the best effect on sertoli cell cryopreservation (Table 3).

Fig. 3 Sertoli cell staining
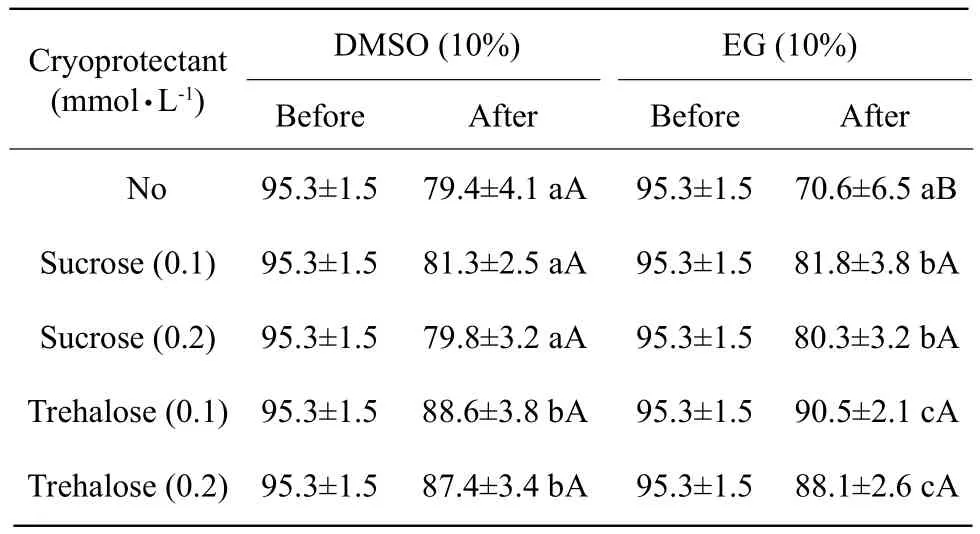
Table 3 Sertoli cell survival rate with some cryoprotectants(%)(n=10)
Discussion
Histological examination
Spermatogonial stem cells derived from primordial germ cells (PGCs). Male PGCs then migrated to the genital crest and became gonocytes. Gonocytes stopped in the G0/G1 phase after mitotic proliferation for a short period. Then gonocytes of postpartum pup males underwent a resumption of proliferation and migrated to basement membrane of seminiferous tubules. On the sixth day, gonocytes of mouse reached basement membrane and became spermatogonial stem cells (Malkov et al., 1998). In the experiment, we found that some of neonatal bovine spermatogonial stem cells had migrated to basement membrane. We were unable to determine them when gonocytes began resumption of proliferation and migrated to basement membrane, and it was presumed that it needed at least 25 weeks (Wrobel et al., 1995). The fate of the large spermatogonia stem cells and the small spermatogonia stem cells was in developing, and needed further research, too. It was presumed that the small spermatogonia stem cells were seldom differentiation(Wrobel et al., 1995). Other reports showed that the majority of large spermatogonia stem cells and only a very few cells were positive with c-kit examination(Izadyar et al., 2002).
Isolation, purif i cation of spermatogonial stem cells and sertoli cells
Collagenase, hyaluronidase, trypsin, and DNAse digestion enzyme combination method have been used in isolation of spermatogenic epithelial cells,laboratories could select different combinations according to their needs (Kanatsu-Shinohara et al.,2003; Kubota et al., 2004; Kaomei et al., 2006).Target cells could not be completely isolated from the spermatogenic epithelial cell suspensions, and could be only achieved at a certain degree in medium, therefore,this process was called enrichment. The methods used for isolation and purification were: discontinuous density gradient Percoll centrifugation (Izadyar et al.,2002), differential adherent cell selection (Kanatsu-Shinohara et al., 2003), plate method (Jose et al.,2006), fl uorescence activated cell sorter (Kubota et al.,2004; Hongyan et al., 2008), and magnetic-activated cell separation (Sabine et al., 2008). As the experiment described, our results demonstrated that the method of differential adherent cell selection was feasible and the positive rate of cells was 72%.
Cryopreservation of spermatogonial stem cells and sertoli cells
Sucrose and trehalose, as nonpenetrating cryoprotectants and preserving cell membrane integrity and vitrification, have been successfully used in cryopreservation (Jelinkova et al., 2002). EG, as low toxic cryoprotectants, was used in mammalian embryos and human embryonic stem cells cryopreservation (Peng et al., 2009). Whether DMSO could be replaced by EG or sucrose and trehalose supplemented in Medium D were studied in our research. It indicated that Medium D supplemented with 10% of DMSO had the best effect in spermatogonial stem cell cryopreservation,Medium D supplemented with 10% EG and 0.1 mmol · L-1trehalose had the best effect in sertoli cell cryopreservation.
Conclusions
Neonatal bovine seminiferous tubules were comprised by spermatogonial stem cells and sertoli cells, differential adherent cell selection methods could effectively separate these two cell types, medium supplemented with 10% of DMSO had the best effect in spermatogonial stem cell cryopreservation, medium supplemented with 10% of EG and 0.1 mmol · L-1trehalose had the best effect in sertoli cell cryopreservation.
Zheng Peng and Hu Peng-fei contributed equally to this work.
Brinster R L, James W. 1994. Zimmermann. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA, 91:11298-11302.
Hongyan L, He H, Dongxu L, et al. 2008. In vitro long-term culture and differentiation of mouse spermatogonia stem cells. Journal of Agricultural Science and Technology, 2(10): 1-5.
Izadyar F, Spierenberg G T, Creemers L B, et al. 2002. Isolation and purification of type A spermatogonia from the bovine testis.Reproduction, 124: 85-94.
Jelinkova L, Selman H A, Arav A, et al. 2002. Twin pregnancy after vitrification of 2-pronuclei human embryos. Fertility and Sterility,77(2): 412-414.
Jose R, Rodriguez S, Howard D, et al. 2006. Isolation and transplantation of spermatogonia in sheep. Theriogenology, 66: 2091-2103.
Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. 2003. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biology of Reproduction, 69: 612-616.
Kaomei G, Karim N, Lars S M, et al. 2006. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature, 440:1199-1203.
Kubota H, Avarbock M R, Brinster R L, et al. 2004. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA, 101: 16489-16494.
Malkov M, Fisher Y, Don J, et al. 1998. Developmental schedule of the postnatal rat testis determined by flow cytometry. Biology of Reproduction, 59: 84-92.
Peng Zh, Zhijun H, Zhonghua L, et al. 2009. Study of several factors affecting on preparation of mouse embryonic stem cells. Life Science Journal, 6(1): 1-4.
Sabine C, Markus R, Jorg H, et al. 2008. Generation of pluripotent stem cells from adult human testis. Nature, 456: 344-351.
Sandeep G, Mayako F, Kazuo T, et al. 2009. Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro.Reproduction, Fertility and Development, 21: 696-708.
Sandeep G, Miki S, Naojiro M. 2007. Identif i cation, isolation, and in vitro culture of porcine gonocytes. Biology of Reproduction, 77:127-137.
Wrobel K, Bickel D, Kujat R, et al. 1995. Evolution and ultrastructure of the bovine spermatogonia precursor cell line. Cell Tissue Res, 281:249-259.
 Journal of Northeast Agricultural University(English Edition)2013年1期
Journal of Northeast Agricultural University(English Edition)2013年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Ecuador Export Potential Agricultural Products to China
- Effects of Sub-chronic Aluminum Exposure on Renal Pathologic Structure in Rats
- Effects of Low-temperature and Herbicide on Membrane Stability,Antioxidant Capacity, and Product of Metabolism in Barley Seedlings
- SWOT Analysis and Development Strategies of Maize Industry in Heilongjiang Province
- Research on DSP-Based Automatic Excitation Regulator in Small Rural Hydropower Station
- Expression of a Lysine-rich Gene in Bacillus subtilis 168
