Effects of Low-temperature and Herbicide on Membrane Stability,Antioxidant Capacity, and Product of Metabolism in Barley Seedlings
Kong Zhi-you, Qin Peng, Liu Ye-ju, Chen Jia, and Wang Shuo
1 College of Resource and Environment, Baoshan University, Baoshan 678000, Yunnan, China
2 College of Agronomy and Biotechnology, Yunnan Agricultural University, Kunming 650201, China
3 Postgraduate Administration Off i ces, Yunnan Agricultural University, Kunming 650201, China
Introduction
Prometryn (2,4-bis (isopropylamino)-6-(methylthio)-s-triazine, a triazine herbicide)and isoproturon (N,N-dimethyl-N'-(4-(1-methylethyl)phenyl)-urea, a phenylurea herbicide), are extensively used herbicides in the world for pre- and post-emergence controls of grass and many broad leaf weeds in cereal crops acting by inhibition of photosynthetic electron transfer and oxidative phosphorylation (Diez et al., 2010; Miran et al., 2008).
Most of the researches were concentrated to the herbicide absorptive kinetics and toxicity. The herbicide sorption was rapid and sorption equilibrium was achieved within a short period of time, and the desorption of highly sorbed herbicides exhibited stronger hysteresis than the weakly sorbed herbicides(Baskaran et al., 1999). Increasing exposure temperature and herbicide toxicity, such as irgarol, diuron,atrazine, and ametryn reduced growth rate and 96 h cell density of the phytoplankton species Dunaliella tertiolecta. The toxicity of atrazine was higher when microorganisms (exception of N. pelliculosa)were acclimated to lower temperatures (Chalifour et al.,2011). Antioxidant enzymes, such as ascorbate pero-xidase (APX), dehydroascorbate reductase (DHAR),and glutathione reductase (GR), were generally more active in wheat than in Italian ryegrass, and malondialdehyde (MDA)content was only found in ryegrass, which indicated that the herbicides in the plants showed higher detoxification rates in wheat than in the grass. In barley root cells, activity for oxidizing protoporphyrinogen to protoporphyrin(protoporphyrinogen oxidase)is much less sensitive to inhibition by diphenylether herbicides (Jacobs et al.,1991). The high rate of the induction of synergistic and antagonistic effects in condition of the combined action of low concentrations of 2, 4-D herbicide on spring barley, which substantially determined the yield of cytogenetic disturbances in a certain range(Geras'kin et al., 2002).
However, the weed controlling effects of herbicide are influenced not only by their concentrations, but also by environment conditions, such as temperature,precipitation. In 2011, the low temperature after herbicides resulted in a certain degree of decrease of barley production in Yunnan Province, the growth potential, plant height, valid ear number, ripe ear rate, total grain number, actual grain number, fruit bearing rate, and production decreased. Up to now,little is known about oxidative damage induced in the metabolism of barley after the herbicide and lowtemperature treatment. Therefore, the aim of the research was to investigate the variability of some physiological and biochemical characteristics of barley seedlings under low-temperature and herbicides so as to evaluate the compound injury degree and the resistance of barley seedlings to herbicides and lowtemperature.
Materials and Methods
Plant material
In order to simulate the living environment, tillering barley seedlings planted in plastic cups were cultured in illumination incubator at 15℃ and 12 h-light per day for 7 days, and then subjected to herbicide treatment, Prometryn (with the concentrations of 0,0.15%, 0.30%, and 0.45%)or isoproturon (with the concentrations of 0, 0.30%, 0.60%, and 0.90%). Barley seedlings sprayed herbicides were put in refrigerator with -2, 0 and 2℃ respectively for 4 or 8 h. The leaves of treated barley seedlings were cut and stored in a refrigerator at -80℃ for physiological and biochemical characteristics determining (except for membrane permeability).
Membrane permeability
About 0.3 g of leaves were segmented to 1 cm and soaked in 50 mL deionised water, and vacuumed for 60 min, the electrical conductivities were determined by using a direct reading conductivity meter (DDS-11A)and expressed in μS · cm-1(recorded for D1,and the value of deionised water recorded for D0).The samples tested were put in boiling water (95℃,1 640 m above the sea level)for 15 min. Determined the electrical conductivity (recorded for D2). The membrane permeability was characterized by percentage rate of the electrical conductivity.
Malondialdehyde (MDA)content
About 0.3 g of leaves were homogenised with 6.0 mL of 10% trichloroacetic acid (TCA)at 4℃ and centrifuged at 4 000 r · min-1for 10 min. The supernatant was employed for the MDA content determination according to the methods of Zhao (1993)and Liu(1994).
Extraction and estimation of enzymes, rate ofand soluble protein content
The determination of superoxide dismutase (SOD)and peroxidase (POD)was performed using the extract of 0.3 g of leaves and 6 mL Tris-HCl buffer at pH 7.0. The same extract was used for determination of protein content that was utilized in expressing activities of tested enzymes. The obtained homogenate was centrifuged at 6 000 r · min-1and 4℃ for 15 min.Then, the supernatant was employed for the determination.
The SOD activity [U · mg-1(protein)]was determined using the spectrophotometric method described by Giannopolitis (1977). This method is based on the photochemical reduction of nitroblue tetrazolium(NBT), where a single unit of SOD is defined as the level of enzyme activity that inhibited the photoreduction of NBT to blue formazan by 50%.
The POD activity [U · mg-1(protein)]was assayed according to the method of Shannon et al. (1966),where a def i nite unit of POD is def i ned as a 0.1 change in O.D · min-1. The change in absorbance was recorded at 470 nm at a time interval of 5 s for 30 s.
The soluble protein content was estimated with the Bradford method according to Bradford (1976).
The rate of O2-was determined according to the method of Xiao (2005).
Proline content
About 0.3 g of leaves were homogenized with 6 mL of 75% ethylalcohol, and heated at 80℃ for 20 min,added 0.1 g active carbon and stood after shaken,then centrifugalized at 5 000 r · min-1for 10 min.The supernatant was employed for proline content determination according to the methods of Xiao(2005).
Statistics
All the experimen ts were performed in twice for each sample. Statistic analysis on two-way variance analysis (ANOVA), correlation coefficient, and stepwise regression were performed by SPSS.
Results
Two-way ANOVA analysis of the effects of the kinds of herbicide, the herbicide concentration, the treatment-treatment-time of low-temperature and the low-temperature on the SOD activity, soluble protein content, the POD activity, proline content, the rate of O2
-, and electrical conductivity are shown in Table 1.MDA content was only significantly affected by the treatment-treatment-time of low-temperature (p<0.001),the herbicide concentration /the low-temperature interaction (p<0.05), and the treatment-time of lowtemperature/the low-temperature interaction (p<0.05);the SOD activity was signif i cantly changed except of the kinds of herbicide/the treatment-treatment-time of low-temperature interaction, the POD activity was not signif i cantly affected by the kinds of herbicide/the lowtemperature interaction, soluble protein content was not significantly affected by the kinds of herbicides and the treatment-treatment-time of low-temperature;the kinds of herbicides, the kinds of herbicides/the treatment-treatment-time of low-temperature interaction, and the treatment-treatment-time of low-temperature/the low-temperature interaction had no significant effect on proline content, the rate of O2-was signif i cantly different for the herbicide concentrations,the treatment-treatment-time of low-temperature, the low-temperature, and the kinds of herbicides/the lowtemperature interaction. Electrical conductivity was signif i cantly affected by all independent variables and their interactions.
The effects of the herbicide concentration are shown in Table 2. With the increasing of the herbicide concentration, the POD activity and the rate ofincreased, the SOD activity decreased at fi rst and then increased, soluble protein content and the electrical conductivity increased at first and then decreased,proline content was reduced, but the MDA content had no signif i cant change.
The effects of the temperature are shown in Table 3.The SOD activity and soluble protein content increased with the increasing of temperature, but the POD activity, proline content, and electrical conductivity decreased. The rate ofincreased at first and then decreased with the increasing of temperature; however,the MDA content remained unchanged.
The correlations of the physiological indices and the treatments are shown in Table 4. All of the physiological and biochemical indices were not signif i cantly correlated to the kinds of herbicides and the herbicide concentrations. The MDA content and the rate ofwere extremely significant positive correlated to the treatment-time of low-temperature.There were extremely signif i cant positive correlations of the POD activity and electrical conductivity to the low-temperature, and that of proline content and the rate ofto the low-temperature were significantly positive.
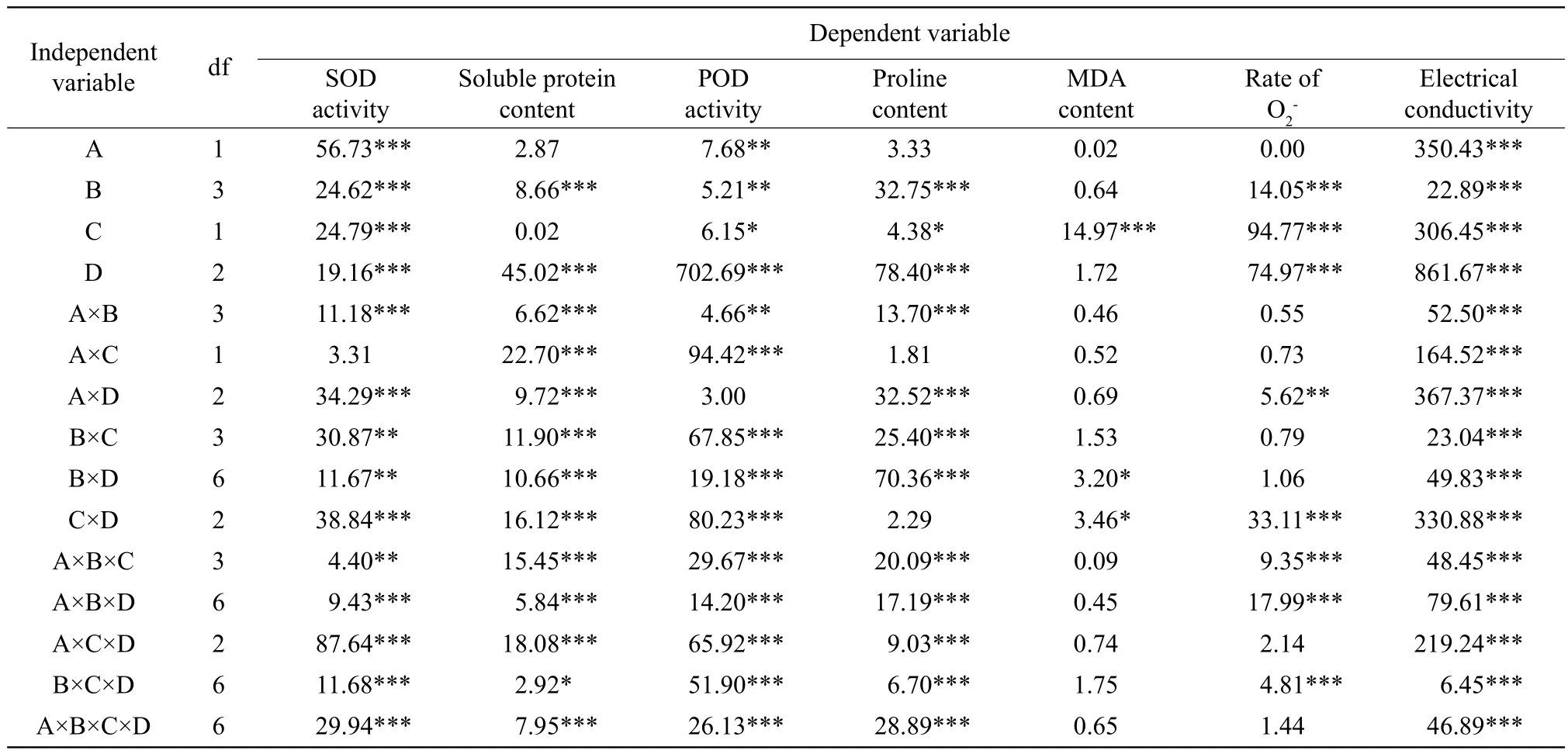
Table 1 Two-way variance analysis (ANOVA)of low-temperature and herbicides for physiological indice

Table 2 Signif i cance of difference among concentrations of herbicide
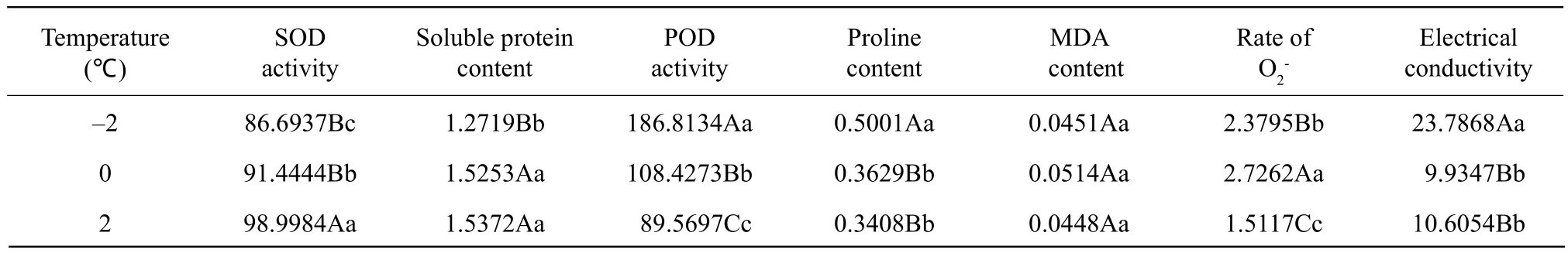
Table 3 Signif i cance of difference among temperatures
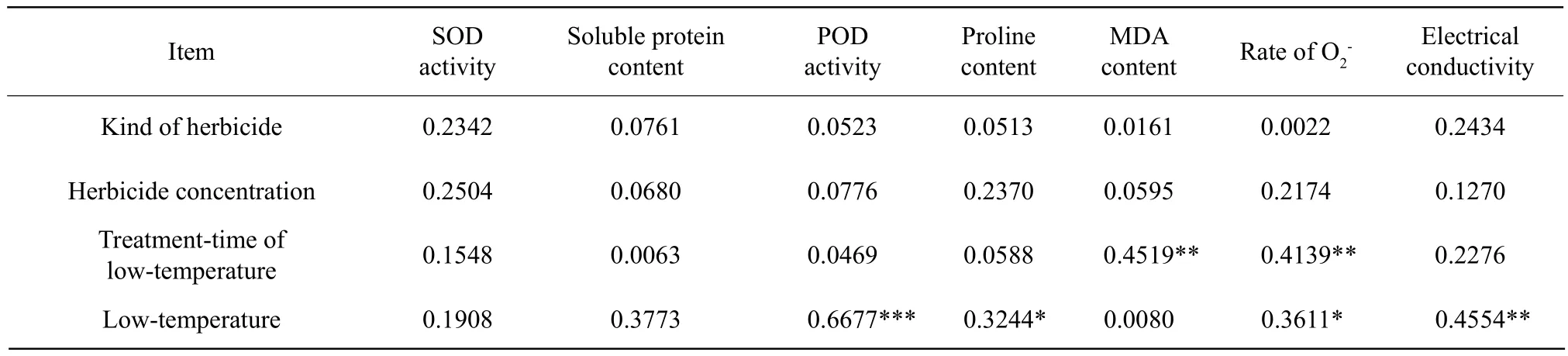
Table 4 Correlation of physiological indices and treatments
The results of stepwise regression between physiological indices (Y)and the treatments (X)are shown in Table 5. In the equation of stepwise regression, X4(low-temperature)was the dominant factor for Y2(protein content), Y3(POD activity), Y4(proline content),Y6(rate of O2), and Y7(electrical conductivity). Moreover, X3(treatment-time of low-temperature)was the most important factor for Y5(MDA content)and Y6(rate of. There was a linear correlation between the low-temperature and the protein content, the POD activity, the proline content, and the electrical conductivity. With the increasing of the temperature,the POD activity, the proline content, and the electrical conductivity decreased, but the protein content increased. The MDA content was linearly correlated to the treatment-time of low-temperature. The rate ofwas negatively affected by the treatment-time of low-temperature and the low-temperature. The SOD activity was not affected by all the treatment indices such as the herbicide, the herbicide concentration,the treatment-time of low-temperature and the lowtemperature.
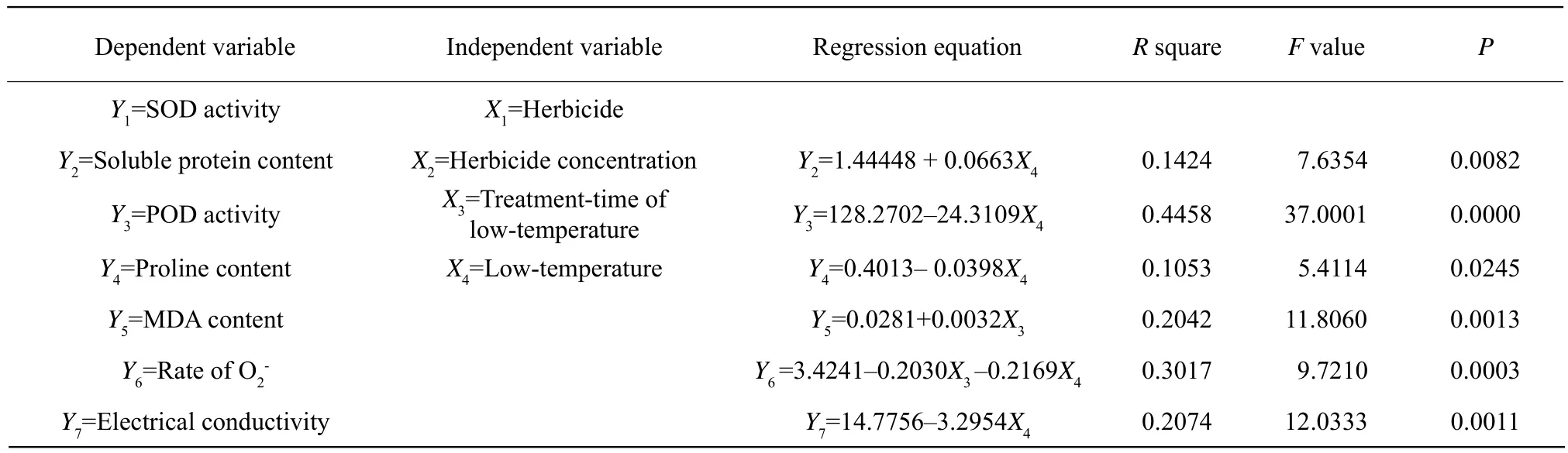
Table 5 Results of stepwise regression between physiological indices (Y)and treatments (X)
According to the stepwise regression and correlation between the physiological indices and the treatments, the low-temperature was the most important factor for the physiological indices which reported the injured degree of barley, and its effect on the physiological indices of barley is shown in Fig. 1. With the increasing of temperature (from -2℃ to 2℃), soluble protein content increased, the POD activity decreased except of isoproturon treatment for 8 h (decreased at first and then increased), proline content decreased except of prometryn treatment for 8 h (increased), the rate ofincreased at fi rst and then decreased except of prometryn treatment for 8 h (maintained unchanged), and electrical conductivity decreased.
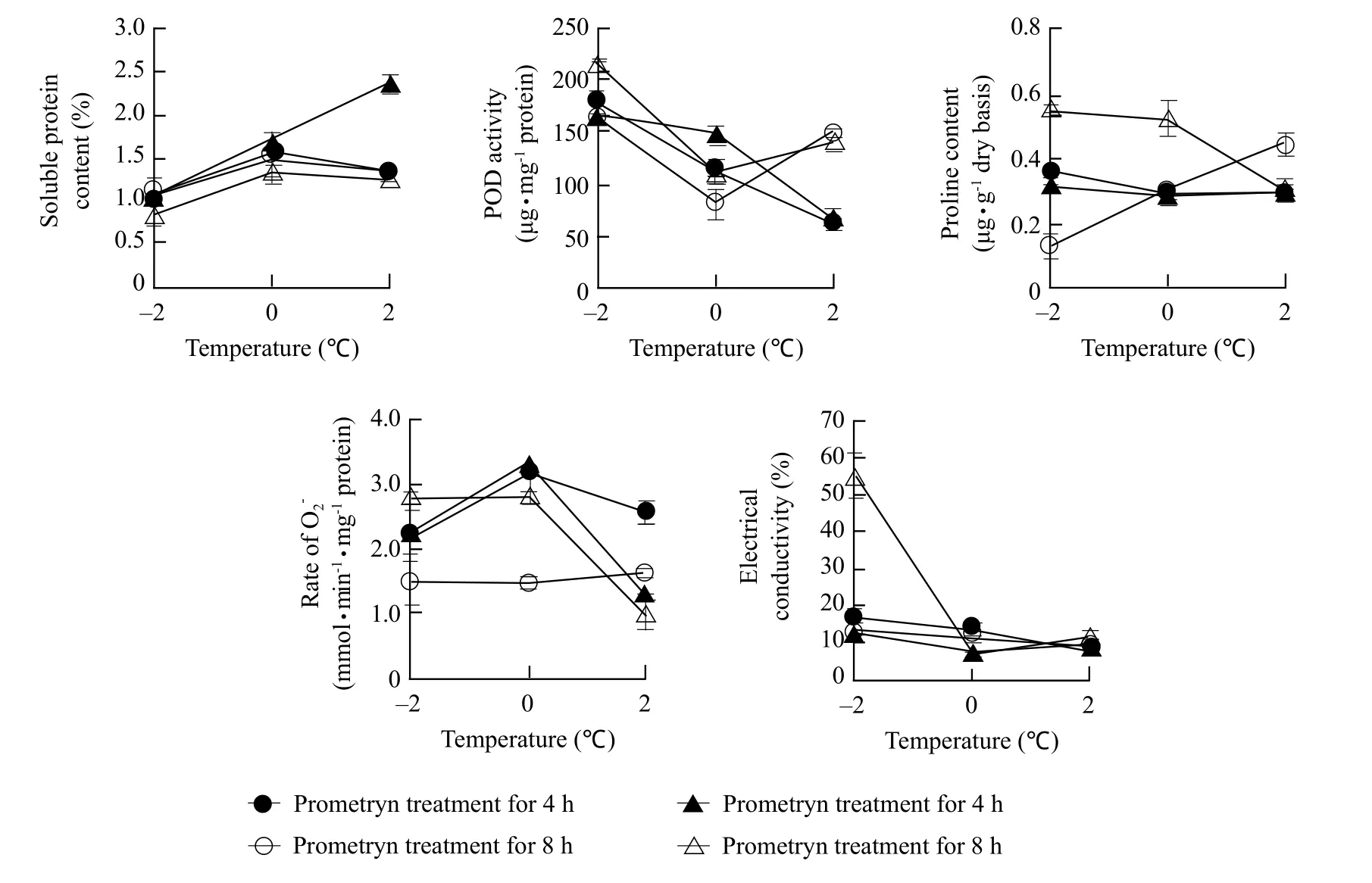
Fig. 1 Effect of low-temperature on the physiological and biochemical characteristics at normal herbicide concentrations Numbers represent Mean±Std.Dv
Discussion
External conditions, such as temperature, humidity,soil moisture, salinity, air pollution, the UV radiation,certain pesticides, and pathogens always changed dramatically in crop growth, and large amounts of free radicals, such as superoxide anions, hydroxyl radical,and hydrogen peroxide, would be produced and then oxidative damage would be occurred in plant. There were two extraction systems of free radical, enzymatic and non-enzymatic antioxidant system in the organism.The SOD, POD, CAT, APX as the enzymatic antioxidant system, and ascorbate, glutathione, mannitol,soluble sugar, and flavonoid as the non-enzymatic antioxidant system played an obviously crucial roles for extracting of free radicals in the plant (Vijayakumar et al., 2009; Narayan et al., 1999; Arulselvan et al.,2007; Ben Sghaier et al., 2011). As to this research,some of the physiological and biochemical indices,the SOD activity, the CAT activity, the POD activity,the MDA content, proline content, soluble protein content, electrical conductivity, and the rate ofwere determined and analyzed suffering the stress of low-temperature and herbicides.
The low-temperature was the most important, and the treatment-time of low-temperature was another signif i cant inf l uencing factor on the physiological and biochemical indices of barley seedlings. However,all of the physiological and biochemical indices determined were not affected by the kinds of herbicides and the herbicide concentrations, according to the stepwise regression and correlation. The rate of O2-was the highest treated at 0℃, but the POD activity was not inducted in a short time, which indicated that barley seedlings suffered the hardest damage. Electrical conductivity was increased with the decreas-ing of the temperature, which showed that the damage of barley cell membrane was aggravated, and the temperature below 0℃ had the failure functions on barley cell membrane. The soluble protein decreased with the decreasing of temperature, and it indicated that the soluble protein should be biological degraded at lowtemperature.
In enzymatic antioxidant system, the variation scope of the POD activity under low-temperature treatment was more than the SOD activity, which extremely significant positive correlated with the temperature.The result showed that the SOD was stable and should play the more prominent role on extracting of free radicals.
The study was applied in a narrow variation scope of the herbicide concentrations, the results could not truthfully reflect the effects of the herbicide concentrations on the physiological and biochemical indices of barley seedlings, so the herbicide concentrations should be expanded in the future research.
Acknowledgements
We wish to thank Zhang Xiao-fang, Zhai Xu-ming, Yang Yan-fei, Liu Xin, Li Yuan, and Li Tian-feng for their assistance in experimentation.
Arulselvan P, Subramanian S P. 2007. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic beta-cells in experimental diabetes in rats.Chemico-Biological Interactions, 165: 155-164.
Baskaran S, Kennedy I R. 1999. Sorption and desorption kinetics of diuron, fluometuron, prometryn and pyrithiobac sodium in soils.Journal of Environmental Science and Health, Part B, 34: 943-963.
Ben Sghaier M, Bhouri W, Neffati A, et al. 2011. Chemical investigation of different crude extracts from Teucrium ramosissimum leaves. Correlation with their antigenotoxic and antioxidant properties. Food and Chemical Toxicology, 49: 191-201.
Ben Sghaier M, Boubaker J, Skandrani I, et al. 2011. Antimutagenic,antigenotoxic and antioxidant activities of phenolic-enriched extracts from Teucrium ramosissimum: combination with their phytochemical composition. Environmental Toxicology Pharmacology, 31: 220-232.Bradford M M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye binding. Analytical Biochemistry, 72: 248-254.
Chalifour A, P Juneau. 2011. Temperature-dependent sensitivity of growth and photosynthesis of Scenedesmus obliquus, Navicula pelliculosa and two strains of Microcystis aeruginosa to the herbicide atrazine. Aquatic Toxicology, 103: 9-17.
Couée I, Cécile S, Gwenola G, et al. 2006. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany, 57: 449-459.
Del Buono D, Ioli G, Nasini L, et al. 2011. A comparative study on the interference of two herbicides in wheat and italian ryegrass and on their antioxidant activities and detoxification rates. Journal of Agricultural and Food Chemistry, 59: 12109-12115.
Diez C, E Barrado. 2010. Soil-dissipation kinetics of twelve herbicides used on a rain-fed barley crop in Spain. Analytical and Bioanalytical Chemistry, 397: 1617-1626.
Geras'kin S A, Dikarev V G, Dikareva N S. 2002. Effect of combined radioactive and chemical (heavy metals, herbicides)pollution on the yield of cytogenetic disruptions in the intercalary meristem of spring barley. Radiats Biol Radioecol, 42: 369-383.
Giannopolitis C N, Ries S K. 1977. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiology, 59: 309-314.
Jacobs J M, Jacobs N J, Sherman T D, et al. 1991. Effect of diphenyl ether herbicides on oxidation of protoporphyrinogen to protoporphyrin in organellar and plasma membrane enriched fractions of barley. Plant Physiology, 97: 197-203.
Liu Z Q, Zhang S C. 1994. Resistant physiology of plant. Chinese Agriculture Press. Beijing.
Miran B, Renata O, Polona K, et al. 2008. Metabolic acidosis in prometryn (triazine herbicide)self-poisoning. Clinical Toxicology,46: 270-273.
Narayan M S, Naidu K A, Ravishankar G A, et al. 1999. Antioxidant effect of anthocyanin on enzymatic and non-enzymatic lipid peroxidation. Prostaglandins Leukot Essent Fatty Acids, 60: 1-4.
Shannon L M, Kay E, Law J Y. 1966. Peroxidase isoenzyme from horse radish roots: isolation and physical properties. Journal of Biological Chemistry, 241: 2166-2172.
Vijayakumar R, Zhao C X, Gopal R, et al. 2009. Non-enzymatic and enzymatic antioxidant variations in tender and mature leaves of Strychnos nux-vomica L. (Family: Loganiaceae). Comptes Rendus Biologies, 332: 52-57.
Xiao L T, Wang S G. 2005. Experimental technique of plant physiology.China Agricultural Press, Beijing. pp. 167-170.
Zhao K F. 1993. Plant physiology of resistance to salt. Science and Technology Press, Beijing.
 Journal of Northeast Agricultural University(English Edition)2013年1期
Journal of Northeast Agricultural University(English Edition)2013年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Ecuador Export Potential Agricultural Products to China
- Effects of Sub-chronic Aluminum Exposure on Renal Pathologic Structure in Rats
- SWOT Analysis and Development Strategies of Maize Industry in Heilongjiang Province
- Research on DSP-Based Automatic Excitation Regulator in Small Rural Hydropower Station
- Expression of a Lysine-rich Gene in Bacillus subtilis 168
- Isolation, Purif i cation and Cryopreservation of Cells from Neonatal Bovine Testis
