Effect of pH,temperature and heating time on the formation of furan in sugar-glycine model systems
Jielun Hu Ynn Zhng Sunn Wng Chng Li Mssimo Mrcone Mingyong Xie
a State Key Laboratory of Food Science and Technology,Nanchang University,Nanchang,Jiangxi 330047,China
b Department of Food Science,University of Guelph,50 Stone Road East,Ontario N1G 2W1,Canada
Abstract Furan(C4H4O)has been classifie as a possible animal and human carcinogen by many international agencies.The formation of furan in three sugar-glycine models using glucose,fructose,and sucrose was investigated using headspace gas chromatography mass spectrometry method(HSGC-MS)with various dual combinations of three important heat processing conditions,i.e.pH,temperature,and heating time.Results indicated that furan levels from sugar-glycine model systems during the thermal processing can be attributed to selective sugar types,pH,temperature,and heating time.In glucose-glycine and fructose-glycine system,the lowest furan level was detected in acid condition but in sucrose-glycine system furan formed significantl lower(P <0.05)in acidic conditions the lowest furan level was found in alkaline conditions.The furan levels were observed to increase with heating time in all three model systems.Furthermore,less furan was generated in non-reducing sugar system(sucrose)than in reducing sugar system(glucose and fructose).Therefore,they demonstrate the possibility of limiting the formation of furan in heat processed foods by both the careful selection of carbohydrates (i.e.non-reducing sugars and reducing sugars) ingredients and appropriate processing conditions.
Keywords: Furan;Heat processing;Sugar-glycine model systems;GC-MS
1.Introduction
Maillard reactions refer to a chemical reaction between an amino acid and a reducing sugar such as glucose and fructose in the presence of heat.These reactions between the carbonyl group of sugar and the nucleophilic amino group of amino acid create a wide range of fl vor,odor,and/or noticeable browning color compounds in foods.Some compounds such as the melanoidins,the brown polymers formed via Maillard reaction during coffee roasting,are highly desirable.Besides of favorable aroma and fl vor components,several unpleasant compounds such as furan may also be produced from Maillard reactions,with detectable furan levels up to 65 g/kg formed in coffee during roasting [1].The average level of furan was approximately 170 ng/g based on a FDA screening of 340 food samples[2].
Furan (C4H4O) has been classifie as a possible animal and human carcinogen by many international agencies including the International Agency for Research on Cancer[3]and has been placed on the list of potential carcinogens by the US Department of Health and Human Service [4]and the National Toxicology Program [5].The occurrence of furan in a large number and variety of commercially thermal treated foods has brought intensive international attention[2,6].
Five main mechanisms for furan formation have been proposed (Fig.1),including:(1) the thermal degradation of carbohydrates such as glucose,lactose,and fructose [7];(2)the Maillard reaction involving the reaction of a specifi amino acid with a reducing sugar in the presence of heat;(3) the oxidation of polyunsaturated fatty acids;(4)the decomposition of ascorbic acid or its derivatives;and(5)the thermal oxidation of carotenoids[8-11].
Typically,the Maillard reaction involved three stages [12],i.e.:the initial,the intermediate,and the final The firs stage involves the sugar-amine condensation and the Amadori rearrangement to the so-called Amadori product.The second stage involves sugar dehydration and fragmentation and amino acid degradation especially at high temperatures.In the fina browning stage,the intermediates polymerize and unsaturated to form,fluorescen and colored polymers,known as melanoidins are formed.The chief reactions involved in the fina stage are thought to be aldol condensation,aldehyde-amine polymerization with the formation of heterocyclic nitrogen compounds.Previous studies have suggested that furan is produced or synthesized or made another work[8,13].
Since Maillard reactions are one potential route of the formation of furan[9-11],factors affecting the Maillard reactions have been thought possibly influenc the furan formation[13].These factors include temperature,duration of heating,pH,water content,type of reactant,the amino acid to sugar ratio,oxygen,and presence of metals,the type of buffer,and the presence of any reaction inhibitors such as sulfur dioxide.Among these,temperature,duration of heating and pH are believed to play crucial roles[14].
The objective of this study was to investigate the effects of various factors,i.e.initial pH,temperature,and heating time on the formation furan from Maillard reaction based on sugars and glycine.Three typical sugars (glucose,fructose and sucrose)were selected as reaction precursors.
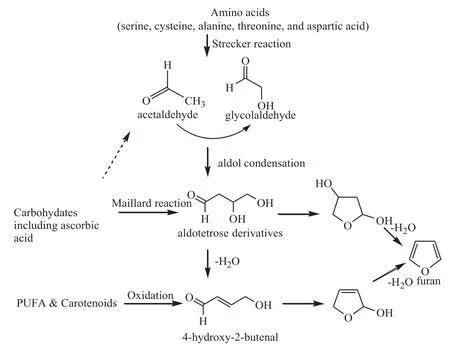
Fig.1.Proposed pathways and precursors of furan[8-10,22].
2.Materials and methods
2.1.Chemicals and reagents
D-(+)-glucose (≥99%),D-(-)-fructose (≥99%),sucrose(≥99.5%),glycine (≥99%),furan (≥99%),d4-furan(≥99%) and methanol (HPLC-grade),were purchased from Sigma-Aldrich (St.Louis,MO,USA).Sodium dihydrogen phosphate (NaH2PO4·2H2O),disodium hydrogen phosphate(Na2HPO4·12H2O),and other chemicals were of analytical grade.
2.2.Sugar-glycine model systems
Three equimolar sugar-glycine model systems (glucoseglycine,fructose-glycine,and sucrose-glycine)were prepared for this investigation.The sodium phosphate buffers with pH of 4.18,7.00,and 9.40 were prepared by using sodium dihydrogen phosphate(50 g/L,pH 4.10)and disodium hydrogen phosphate(50 g/L,pH 9.40).
2.3.Effect of pH,temperature and heating time on furan formation in sugar-glycine model systems
Equimolar amounts of sugar and glycine (50 mmol each)were placed in a 250 mL volumetric flas and then dissolved in the sodium phosphate solutions(pH 4.18,7.00,and 9.40)by stirring.Each sugar-glycine model system had 3 different pH aqueous model systems(acidic,neutral and alkaline).
For each reaction,an aliquot of the homogenous solution(5 mL)was transferred into a reaction vessel(a 20-mLheadspace vial)and then sealed with a crimp cap.The vials were heated for 30 min at 80,90,100,110,120,130,140 and 150°C in oil bath.Immediately after the heat treatment,the samples were cooled down in an ice bath(about 0°C)for 15 min to stop the reaction.Subsequently,40 μL working solution ofd4-furan(2.5 μg/mL)was added through the septum with a gas tight syringe and the vial was vortexed.The samples were kept at room temperature for at least 30 min before analysis.
To determine the effect of heating time on the furan formation,equimolar amounts of sugar and glycine (50 mmol each) were dissolved in the sodium phosphate solution(pH 9.40).The vials were heated at 120°C for 10,20,30,40,50 and 60 min in the oil bath.Similarly,after the heat treatment,the samples were cooled down in an ice bath(about 0°C)for 15 min to stop the reaction.Then,40 μL working solution ofd4-furan(2.5 μg/mL)was added through the septum with a gas tight syringe and the vial was vortexed.The samples were kept at room temperature for at least 30 min before analysis.
2.4.Furan analysis by headspace gas chromatography-mass spectrometry(HS-GC-MS)
Furan was analyzed and optimized HS-GC-MS as method using thed4-furan as internal standard.Furan andd4-furan were extracted from the samples by an Agilent model G1888 automated headspace sampler and were separated and identifie by Agilent model 7890A/7000 GC mass spectrometer equipped with a capillary column 19091P-Q04 HP-PLOT/Q(30 m×0.32 mm×20 μm).
Stock,working and standard solutions of furan andd4-furan were prepared according to previous report [15].Calibration standards(5,50,125,250,500,800,1000,1200 and 2400 ng)were prepared by injecting 20-960 μL working solution of furan(0.25 or 2.5 μg/mL) and 40 μL working solution ofd4-furan(2.5 μg/mL)through the septumof 20 mLsealed headspace vials containing 5 mL water,respectively.Headspace operating conditions were set as follows:Oven temperature was set at 70°C,loop at 110°C,transfer line at 130°C;30 min thermal equilibrations with low shake on;0.5 min pressurization,1 min injection with vial pressurized to 10 psi.
The GC oven temperature was initially set at 50°C and held for 1 min.It was then increased to 200°C at a rate of increase of 10°C/min and then held for 10 min at 200°C.The injector temperature was set at 200°C with a 3:1 split ratio and a constant fl w rate of 1.0 mL/min(UHP helium).The high sensitivity EI ion source and transfer line temperatures were set at 230°C and 225°C,respectively,with the quadrupole temperature set at 150°C.The MS operating conditions were as follows:positive electron ionization mode(EI+)using automatic gain control with 70 eV of electron energy.Furan andd4-furan were identifie by comparison of spectra of sample compounds with those of standards and by comparing retention times of the samples with those of the standards.The mass spectrometer was operated in selected-ion monitoring mode (SIM) by recording the current of the following ions:m/z68 [M]+andm/z72 [M]+for furan andd4-furan determination,respectively.For confirmatio of furan andd4-furan,the ions,m/z39 [M-CHO]+andm/z42[M-C2HO]+were monitored.
2.5.Statistical analysis
Analyses were performed in triplicate with the data expressed as mean±standard error.The least significan difference(LSD)test using the general linear model was performed to test for significan differences among the means by SAS(Version 8.2,SAS Institute,Cary,NC,USA).Differences among means atP<0.05 were considered to be significan differences.
3.Results and discussion
3.1.Effects of pH and temperature on furan formation in glucose-glycine model system
Furan levels formed at various pH and temperatures in the glucose-glycine model system are presented in Fig.2A.It was observed that temperature (≤110°C) may not lead to furan formation regardless of pH,but pH had a significan effect on thermally induced furan formation that temperature greater than 110°C.At pH 7.00,the solution produced significantl(P<0.05)more furan than that prepared in solution at pH 9.40 and pH 4.18,suggesting that pH is an important factor influenc ing furan formation in the glucose-glycine system as a function of thermal treatment and also greatly depends on the temperature.For instance,after 30 min of heating at 150°C,304,238 and 40 ng/mL furan were formed in this model at pH 7.00,9.40 and 4.18,respectively.Fan[16]also reported that less furan was formed at pH 3 than at pH 7 for glucose solution.
Furthermore,temperature also played an important role in furan formation in the glucose-glycine model system.In general,furan content increased with temperature(>110°C)for all three pH systems.At pH 4.18,furan level increased slowly from 15 to 39 ng/mL as temperature increased from 120 to 150°C.However,at pH 9.40,as temperature elevated from 120 to 150°C,furan content increased rapidly from 20 to 238 ng/mL.Similarly,at pH 7.00,furan content increased rapidly from 34 to 304 ng/mL with temperature increasing from 120 to 150°C.These results suggested that temperature is also a major factor affecting furan formation in this model system.
3.2.Effects of pH and temperature on furan formation in fructose-glycine model system
Furan levels with the variation of the pH and temperature in the fructose-glycine model system were shown in Fig.2B.It was observed that temperature(≤110°C)may not lead to significan amounts of furan formation regardless of pH.But the results suggested that pH of solutions had a profound effect on thermally induced furan formation when temperature is >110°C.At pH 7.00,the solution produced significantl (P<0.05)more furan than that prepared in solution at pH 9.40 and pH 4.18.For example,after heating at 150°C for 30 min,447 ng/mL furan was formed in this model at pH 7.00,302 ng/mL furan was formed at pH 9.40,and 137 ng/mL furan was formed at pH 4.18,respectively.However,the results also showed(Fig.2B)that pH had different effects on furan formation at different temperature regions,suggesting that pH and temperature play a combined effect on furan generation in this model and temperature is still the major factor.Earlier studies [17,18]have showed that pH influence the formation of furan derivatives as a result of the thermal treatment.

Fig.2.(A) Furan content with the variation of pH and temperature in the glucose-glycine model system.(B)Furan content with the variation of pH and temperature in the fructose-glycine model system.(C)Furan content with the variation of pH and temperature in the sucrose-glycine model system.
3.3.Effects of pH and temperature on furan formation in sucrose-glycine model system
The effects of pH and temperature on the formation of furan in sucrose-glycine model system due to thermal processing were showed in Fig.2C.The results indicated that temperature(≤120°C)may not lead to significan amounts of furan formation regardless of pH.It was shown that pH had a significaneffect on thermally induced furan formation when temperature is >120°C.At pH 4.18,the solution produced significantl(P<0.05)more furan than that prepared in solution at pH 9.40 and pH 7.00.The results indicated that pH makes main contributions to the formation of furan in this model system and also greatly depends on the temperature.For instance,after 30 min of heating at 150°C,94 ng/mL furan was formed in this model at pH 4.18,23 ng/mL furan was formed at pH 7.00,and 15 ng/mL furan was formed at pH 9.40,respectively.

Table 1 Furan formation in the glucose-glycine,fructose-glycine and sucrose-glycine heating model systems at 120°C for 30 min simulating sterilization conditions.a
In addition,temperature also played a crucial role in furan formation in the sucrose-glycine model system.In general,furan content increased with temperature(>120°C)for all three different pH systems.At pH 9.40,furan level increased relatively slowly from 2 to 15 ng/mL as temperature increased from 130 to 150°C.However,at pH 7.00,as temperature elevated from 130 to 150°C,furan content increased rapidly from 2 to 23 ng/mL.Similarly,at pH 4.18,furan content increased rapidly from 18 to 94 ng/mL with temperature increasing from 130 to 150°C.These results showed that temperature is also a major factor affecting furan formation in this model system.In summary,pH and temperature play a combined effect on furan generation in this model system.
3.4.Effect of heating time on furan generation in the glucose-glycine,fructose-glycine and sucrose-glycine heating model systems
To test whether furan formation from solutions in the glucose-glycine,fructose-glycine and sucrose-glycine model systems with the variation of heating time,it were investigated that furan levels in all solutions of pH 9.40,which were heated at 120°C for 10,20,30,40,50 and 60 min in the oil bath (Table 1).In general,the furan levels significantl elevated with heating time for all heating model systems.For the glucose-glycine system,as heating time increased from 10 to 60 min,furan level increased from 1 to 832 ng/mL.Likewise,for the fructose-glycine system,similar results about the formation of furan could be found (Table 1).For example,the amount(1522 ng/mL)of furan formed after 60 min of heating at 120°C was significantl higher than that(7 ng/mL)heated for 10 min.However,for sucrose-glycine heating system,no furan was formed with heating time less than 30 min,while only a little furan was produced as the heating time was no less than 40 min,which was a bit different from the occurrence of furan in the other two systems.It suggested that the non-reducing sugar system(sucrose)can produce less furan than those reducing sugar systems(glucose and fructose).Yaylayan et al.[19,20]reported furan formation from simple sugar/amino combinations heated at 250°C.Serine produced about 30% of this quantity when heated with sucrose,and about 10%-25% when heated with fructose or ribose.The disagreement with these reports may due to the different amino acids used.From the results above,it could conclude that long time heating also promotes furan formation,suggesting that heating time is also an important factor affecting furan formation by Maillard-type reactions.
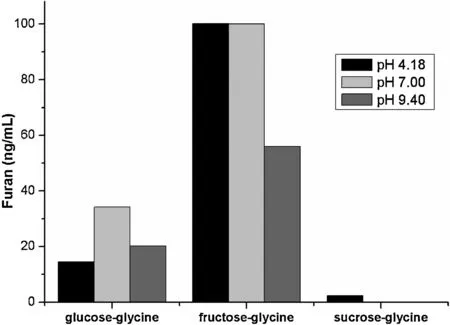
Fig.3.Furan formation in the glucose-glycine,fructose-glycine and sucrose-glycine heating model systems at 120°C for 30 min simulating sterilization conditions.
In addition,it seems that the formation of furan by Maillard reactions is dependent on the amino acids and sugars used.Limacher et al.[21]reported that addition of phenylalanine to glucose resulted in an increase of about 50%of furan,whereas the furan amount decreased by 20%in the binary mixture of fructose and phenylalanine.In contrast,the presence of the amino acids alanine,threonine,and serine all resulted in higher furan amounts.
3.5.Furan formation in the glucose-glycine,fructose-glycine and sucrose-glycine model systems at 120°C for 30 min simulating sterilization conditions
Fig.3 presented furan levels in the glucose-glycine,fructose-glycine and sucrose-glycine model systems at 120°C for 30 min that simulated sterilization conditions.Under sterilization conditions,fructose-glycine system produced the higher levels of furan,while less furan (P<0.05) significantl was formed in glucose-glycine than that in fructose-glycine system.However,little furan was generated from sucrose-glycine heating model system.
In conclusion,the results indicated that the formation of furan determined by the pH,temperature,heating time and their interactions in sugar-glycine heating systems.Optimized processing parameters and eliminate precursors of furan formation have been proposed for reducing unpleasant furan in certain foods [8-10,22].For example,key intermediates such as 2,3-diketogulonic acid (DKG),aldotetrose derivatives and 4-hydroxy-2-butenal could be eliminated under the change of processing conditions.Therefore,the work of this paper can be considered as a research basis for further reducing or eliminating the formation of furan in heat processed foods.In other words,we can reduce or eliminate furan formation by modifi cation of processing conditions and change of heat processing methods according to this study.The control of important processing parameters may be regarded as the most direct way to reduce furan level.These parameters include temperature,heating time,pH,etc.It was shown in this study that pH and temperature are important factors influencin furan formation from solutions of carbohydrates and amino acids.In addition,the furan concentrations were observed to increase with heating time.Therefore,furan reduction may be achieved by low temperature,short time heating and adjusting pH by citric acid,etc.
Carbohydrates and amino acids are also commonly used as additives in food products.The results of this study can be used for better formulation to minimize the formation of furan due to thermal processing.For example,if carbohydrates and amino acids are required together in formulation,sucrose would be a better choice than glucose and fructose to reduce the accumulation of furan by Maillard-type reactions as a function of heating.The mechanisms behind the impact of these factors on furan formation needs further study.
4.Conclusion
Taken together,the finding suggest that the amount of furan from sugar-glycine model systems during the thermal processing can be attributed to selective sugar types,pH,temperature,and heating time.For glucose-glycine system,furan formed significantl lower (P<0.05) in acidic conditions in comparison with neutral and alkaline conditions when temperatures were greater than 140°C.In the fructose-glycine system,the lowest level of furan was detected in acid condition whereas the sucrose-glycine system had the lowest furan formation in alkaline conditions.In addition,the furan levels were observed to increase with heating time in all three model systems.Furthermore,less furan was generated in non-reducing sugar system(sucrose)than in reducing sugar system(glucose and fructose).These results demonstrate the possibility of limiting the formation of furan in heat processed foods by both the careful selection of carbohydrates(i.e.non-reducing sugars and reducing sugars)ingredients and appropriate processing conditions.
Acknowledgements
The financia support for this study by National Natural Science Foundation of China (No.30960242),National Basic Research Program of China (973 program) (No.2012CB720805) and Training Project of Young Scientists of Jiangxi Province (Stars of Jing gang) is gratefully acknowledged.
- 食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Anti-inflammator and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats
- Composition and antioxidant activity of anthocyanins isolated from Yunnan edible rose(An ning)
- Health risk from fluorid exposure of a population in selected areas of Tamil Nadu South India
- Radical-scavenging activity,ACE-inhibiting capability and identificatio of rapeseed albumin hydrolysate
- Red onion extract(Allium cepa L.)supplementation improves redox balance in oxidatively stressed rats

