Composition and antioxidant activity of anthocyanins isolated from Yunnan edible rose(An ning)
Qin Ge,Xiaojun Ma
School of Food Science and Technology,Jiangnan University,Wuxi,214122,China
Abstract Edible roses (An ning) are a good source of anthocyanins and grown widely in Yunnan Province of China.In this study,the contents of anthocyanins and total phenol as well as the antioxidant activity of methanol extract from specifi variety of rose were systematically investigated.The results showed that anthocyanins and total phenolic content of the petals were (353.56±2.50)mg cyanidin 3,5-diglucoside (Cy-3,5-diglu)equivalents and(2087.43±17.37)mg gallic acid equivalents(GAE)per 100 g fresh weight(FW),respectively.Totally,3 kinds of anthocyanins were detected and Cy-3,5-diglu was the predominant constituent which accounted for approximately 94.9%of total anthocyanins according to the analysis results of high performance liquid chromatography-photodiode array detection (HPLC-PAD).Data demonstrated that the extract from edible rose exhibited excellent ferric reducing capacity and free radical scavenging activity against both 2,2′-diphenyl-1-picrylhydrazyl(DPPH)and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS).The equivalents of anthocyanins from roses on DPPH,ABTS and ferric reducing ability were 2089,639 mg and 1400 mg GAE per 100 g FW,respectively.The high anthocyanins content and excellent antioxidant activity suggested that Yunnan edible roses could be applied in food industry as a good source of natural pigments.
Keywords: Yunnan edible rose;Anthocyanins;Structure identification Antioxidant activity
1.Introduction
Rose,of the family Rosaceae,with its brilliant colors,rich aroma and high trophic value,is an important raw material for the production of spices and functional food.Edible rose is widely grown in Yunnan Province of China.But up to now it still has been directly used to make sauce and the comprehensive utilization is relatively low.
Anthocyanins,a major material of natural food colorants,are a group of water-soluble pigments that are composed of an aglycone anthocyanidin and sugar moieties [1].They essentially belong to fl vonoid family and are basically responsible for the brilliant colors of numerous fruits,fl wers and vegetables [2].From the aspect of chemical structure,one or more sugar molecules can be linked to anthocyanidin through glycosidic bonds.The sugar molecules could be glucose,galactose,xylose,arabia sugar,rhamnose and a homogeneous or inhomogeneous which constitute of two or three monosaccharides[3].Anthocyanins often exist in the form of their acylate owing to acylation with organic acids,such as caffeic acid,pcoumaric acid,sinapic acid,p-hydroxybenzoic acid,ferulic acid,malonic acid,malic acid,succinic acid and acetic acid [4].It was reported that there are more than 600 types of anthocyanins distributing in nature [5].However,the common anthocyanins found in plants are pelargonidin(Pg),cyaniding(Cy),delphinidin(Dp),peonidin(Pn),petunidin(Pt)and malvdin(Mv)[6,7].
Free radicals can be define as molecules or molecular fragments containing one or more unpaired electrons in atomic or molecular orbitals,such as superoxide,hydroxyl,peroxyl(RO2·),alkoxyl (RO·),and hydroperoxyl (HO2·) radicals[8].Due to mitochondrial respiration,radicals derived from oxygen represent the most important class of radical species generated in living systems.Free radical metabolism seems to occupy a central and remarkably common position in causing potential biological damage,as the unpaired property usually gives a considerable degree of reactivity to the free radical.Numerous studies have indicated that free radical is to a large extent relevant to aging [9-11].Anthocyanins not only endow nature with attractive colors but also give plants varieties of physiological health and disease prevention effects.Previous studies have revealed that anthocyanin compounds have a high radical scavenging capacity and play an essential role in the prevention of cardiovascular disease,cancer,diabetes and other diseases[12].The high radical scavenging capacity of anthocyanins is mainly due to the phenolic hydroxyl groups in molecule.The phenolic hydroxyl groups can prevent peroxidation effectively by providing hydrogen atoms which can remove free radicals and consequently cut off the chain reaction of oxidation.
In recent years,researches on anthocyanins have attracted more and more attentions,especially their isolation and purifi cation[13-15].In contrast,there have been few studies focusing on chemical structures and antioxidant activity of anthocyanins in edible rose.Therefore,the aim of this study is to determine the structures and evaluate the antioxidant activity of anthocyanins extracted from rose so as to provide a theoretical basis for the effective exploitation of this feature natural resource.
2.Materials and methods
2.1.Plant materials
Edible roses(An ning)were collected from local gardens in the country of Yunnan during spring season.The petals were preserved at-20°C for latter analysis.
2.2.Chemicals
Cy-3,5-diglu(purity 97%),Cyanidin 3-glucoside(Cy-3-glu),DPPH and ABTS were obtained from Sigma-Aldrich(St.Louis,MO,USA).Methanol and acetonitrile for HPLC- PAD and HPLC-PAD-electrospray ionization (ESI)/mass spectrometry(MS) analysis were of chromatographic grade and purchased from Jiangsu Hambon Sci &Tech.Co.,Ltd (Jiangsu,China).All of other chemicals used were obtained from China National Medicines Corporation and were of analytical grade unless otherwise noted.
2.3.Methods
2.3.1.Extraction of anthocyanins
5 g petals were extracted with 150 mL acidic methanol solution(0.1%HCl,V/V)following the method of Zhang et al.[16]with slight modifications The procedure was conducted in a 250 mL conical breaker with its orific sealed by parafil in a shaking water-bath at 35°C in darkness for 10 h.After vacuum filtration the filtrat was then concentrated in a rotary evaporator at 35°C under reduced pressure to obtain aqueous extract by adding a certain amount of distilled water.
2.3.2.Anthocyanins purification
The crude extract of anthocyanins was successively extracted triply with petroleum ether and ethyl acetate so as to remove the fat as well as polyphenols and then subjected to AB-8 resin.The AB-8 resin was washed with distilled water and subsequently the absorbed anthocyanins were eluted with methanol acidifie with 0.1%HCl.The eluate was concentrated at 35°C and the residue was redissolved in acidic methanol for structure identificatio and antioxidant activity determination.
2.3.3.HPLC-PAD analysis
To identify the anthocyanins compounds,a Agilent 1100 Series HPLC system(Agilent Technologies,US)equipped with a Agilent HPLC pump,a photodiode array detector and an Eclipse XDB-C18 column (250 mm×4.6 mm i.d.,5 μm fil thickness) was used.Gradient elution program was applied for anthocyanins analysis.The mobile phase was:A (0.1%aqueous formic acid:acetonitrile=95:5,V/V)and B(0.1%aqueous formic acid:acetonitrile=50:50,V/V).The applied elution profil was:0-15 min,linear gradient from 0% to 100% B;15-20 min,100% B isocratic.Aliquot of 10 μL solution was injected.The fl w rate was 0.8 mL/min and the temperature was set at 30°C.Peak identificatio was grounded on retention time(tR)data and elution order as compared with the standard under the same conditions and was positively confirme with characteristic MS spectra detection.The relative proportion of each composition was calculated from the total identifie area.
2.3.4.HPLC-PAD-ESI-MS analysis
The HPLC-PAD-ESI/MS system was Waters Acquity UPLC chromatography equipped with a Waters Acquity PDA detector and Waters MALDI SYNAPT Q-TOF MS.The column was Acquity UPLC BEH C18with 1.7 μm particle size,50 mm×2.1 mm i.d.(Waters Corporation,US).The mobile phase was:A (0.1% aqueous formic acid:acetonitrile=95:5,V/V) and B (0.1% aqueous formic acid:acetonitrile=80:20,V/V).The applied elution profil was:0-10 min,linear gradient from 0%to 100%B;10-20 min,100%B isocratic.Aliquot of 1 μL solution was injected.The fl w rate was 0.3 mL/min and the temperature was 45°C.Chromatogram was obtained at 520 nm for anthocyanins,and photodiode array spectra was recorded from 200 to 800 nm.The MS conditions were:positive ion mode;capillary,3.5 kV;cone,45 V;source block temperature,100°C;desolvation temperature,400°C;desolvation gas fl w rate,500 L/h;cone gas f ow rate,50 L/h;collision energy,6 V,20 V;scan range,100-1000m/z;detector voltage,1.7 kV.
2.3.5.Analysis of anthocyanins quantification
The anthocyanins content was quantifie based on the modifie procedure described by Zheng et al.[17].Calibration curve was obtained with Cy-3,5-diglu at 7 different concentrations (2.5,5.0,7.5,10.0,15.0,20.0,30.0 μg/mL) and each absorbance was measured at 520 nm so as to get the following linear equation:Y(absorbance)=38.421X(Cy-3,5-diglu concentration,mg/mL)+0.005 (r=0.9967).The sample was appropriately diluted with acidic methanol solution until the absorbance was in the range of 0.200 to 0.800 at 520 nm.The anthocyanins content was finall calculated according to the standard curve and expressed as mg Cy-3,5-diglu equivalents per 100 g FW.
2.3.6.Determination of total phenolic content
Total phenolic content was determined based on Folin-Ciocalteu method [18]with some modifications 1 mL sample was mixed with 0.5 mL Folin-Ciocalteau reagent and 1.5 mL of 15% aqueous sodium carbonate solution.Then the mixture was brought up to 7 mL with distilled water.After incubating at 30°C for 2 h,the absorbance was measured at 765 nm.GA(0.066 mmol/L)was used as the standard compound.Total phenolic content was calculated from the calibration equation:Y(absorbance)=1.1753X(GA additive volume,mL)+0.0173(r=0.9995)and the result was expressed as mg GAE per 100 g FW.
2.3.7.DPPH radical scavenging activity
The DPPH assay was conducted according to the method described previously [19]with some modifications 30 μL anthocyanins sample with different concentrations was mixed with 2 mL DPPH methanolic solution.After incubation for 30 min at 40°C in darkness,each absorbance was measured at 517 nm.The scavenging rate of DPPH was calculated according to the following formula(1):

where:Iwas DPPH scavenging rate;Acwas the absorbance of blank;Aiwas the absorbance in presence of sample;Ajwas the absorbance without DPPH.GA was used as a calibration reader with concentrations ranging from 0.2 to 2.0 mmol/L (additive volume of 20 μL) and the obtained calibration wasY(scavenging rate)=2.373X(GA concentration,mg/mL)+0.0627(r=0.9989).The additive amounts of both sample and GA were calculated from the obtained formula and supposed to be equivalent when scavenging rate reached to 50%.Results were expressed in term of mg GAE per 100 g FW.Here,it should be very clearly pointed out that we are concerned on the additive amount rather than additive volume of both sample and calibration reader when they achieve the same scavenging rate.
2.3.8.ABTS radical scavenging activity
The ABTS assay was carried on following the method described by Re et al.[20].ABTS reagent was obtained by mixing 10 mL 7 mmol/L ABTS stock solution with 178 μL 140 mmol/L potassium persulfate.The solution was placed in darkness at room temperature for 15 h before use and should be appropriately diluted with methanol (with absorbance of 0.200-0.800 at 734 nm)prior to further analysis.10 μLsample at different concentrations was added into 2 mL fresh ABTS solution.The mixture was slightly shaken for 10 s and then allowed to stand at room temperature for 6 min.Afterwards,the absorbance at 734 nm was recorded immediately.The scavenging rate of ABTS was calculated on the basis of following formula(2):

where:Iwas ABTS scavenging rate;A0was the absorbance of blank;A1was the absorbance of test sample.GA was used as a calibration reader with concentrations ranging from 0.1 to 0.5 mmol/L (additive volume of 10 μL) and the calibration wasY(scavenging rate)=-222.14X2(GA concentration,mg/mL)+35.442X- 0.4283 (r=0.9985).Identical to DPPH assay,the additive amounts of both sample and GA were calculated from the obtained formula when scavenging rate reached to 50%and the results were expressed in term of mg GAE per 100 g FW.
2.3.9.Ferric reducing capacity
In brief,2 mL anthocyanins sample with different concentrations was added into 1 mL phosphate buffer solution (pH 6.6)and 1 mL 1% K3Fe(CN)6.The reaction mixture was allowed to stand in a water-bath at 50°C for 20 min.After incubation,1 mL 10% (m/V) trichloroacetic acid was added into the mixture.The solution was soon placed in a centrifuge at a speed of 3000 r/min for 10 min.Afterwards,2 mL suspensions was mixed with 3 mL deionized water and 0.2 mL 0.1%(m/V)ferric chloride.Eventually,the absorbance was measured at 700 nm.A standard curve was obtained by using GA solution with concentrations ranging from 0.02 to 0.10 mmol/L(additive volume of 2 mL) and the formula wasY(absorbance)=45.915X(GA concentration,mg/mL)+0.0229(r=0.9996).We examined the additive amounts of sample and GA from the obtained formula when absorbance was 0.500.As mentioned previously,the results were expressed in term of mg GAE per 100 g FW.
2.4.Statistical analysis
All samples were measured in triplicate and the results were expressed as M±SD.Statistical analysis system SPSS 16.0 was applied to analyze the data.
3.Results and discussion
3.1.Anthocyanins identification
Fig.1(a)represents the HPLCchromatogram of anthocyanins from Yunnan edible rose.As shown,3 anthocyanins were revealed with peak 1 representing about 94.9%of the total peak area according to normalization method.Anthocyanins identifi cation was mainly conducted by comparingtRand elution order of sample with those of standards as well as mixed standards under the same HPLC conditions.Peak 1 and Peak 2 hadtRof 12.823 and 15.651 min,respectively,which was in coincidence with standard 1(Cy-3,5-diglu)and standard 2(Cy-3-glu)as revealed in Fig.1(a),(b)and(c).In addition,the mixed standards(Fig.1(d))showed exactly the sametRand elution order with individuals.It is thus clear that the mixing behavior has no effect on the HPLC characteristics of each standard.Now it was reasonable to infer that Peak 1 and Peak 2 were Cy-3,5-diglu and Cy-3-glu,respectively[21].
Based on the test results of HPLC-PAD,Peak 3(tR=16.405 min,Fig.1(a))accounted for only a very small part(2.5%)of the total anthocyanins content.As a result of the low content or not easy for ionization,no fragments were detected in total ion chromatography(TIC)when tested through mass spectrometer(Fig.2(b)).Finally,due to lack of detailed information and standard compounds for comparing,the exact structure of Peak 3 could not be identifie yet.However,Wu and Prior[22]once suggested that the hydroxyl and sugar moiety in molecular would increase the anthocyanin polarity and make it elutes early in reverse C18column.Deducing from this principle,Peak 3 was theoretically an anthocyanins of relatively lower polarity than Cy-3-glu.Meanwhile,according to previous reports[17],anthocyanins rarely exist without sugar moiety in nature.So Peak 3 was most likely to be anthocyanins with only one glycoside and have fewer hydroxyls compared with Cy-3-glu.
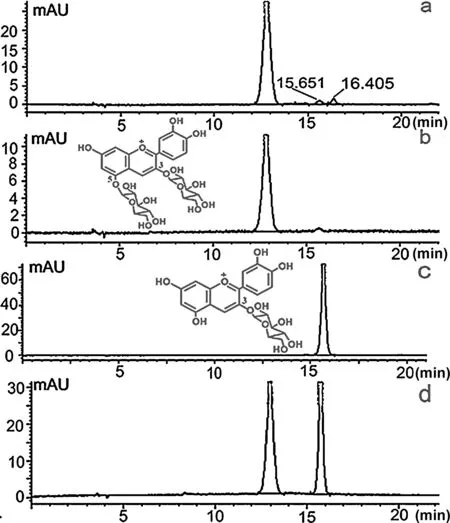
Fig.1.Reverse-phase HPLC chromatogram of sample (a),Cy-3,5-diglu standard(b),Cy-3-glu standard(c)and mixed standard(d)detected at 520 nm.
The definit chemical structure of major anthocyanins in the sample was further confirme by the comparison of UV-vis absorption maxima and mass spectral analysis with data that reported in previous studies[22].Fig.2(a)is the HPLC profil of HPLC-PAD-ESI/MSand a dominant peak(tR=4.29 min)was detected.As can be seen from the UV-vis spectra of detected anthocyanins (Fig.3),no UV absorption was detected in the 320 nm region,which indicated no acylation with aromatic acids[23].
Taking the MS data([M+H]+=m/z611;MS/MS=m/z449;MS/MS=m/z287) into consideration (Fig.4),we speculated that two fragment ions were resulted from the neutral loss of a glucose moiety(m/z162)as graphically demonstrated in Fig.5.Moreover,according to the investigation of 147 rose individuals by Stolker [24],the distinctive glycosylation pattern in roses results in anthocyanidin 3,5-O-diglucoside being the firs stable anthocyanin.From the UV-vis information,it was possible to identify the anthocyanin as bioside because theA440nm/Aλmaxratio was 21% [25].Combining the above information,we sufficientl confirme that peak 1 was Cy-3,5-diglu,which was well consistent with the conclusions of Lee et al.[26].

Fig.2.HPLC chromatogram(a)and TIC(b)of HPLC-PAD-ESI/MS.
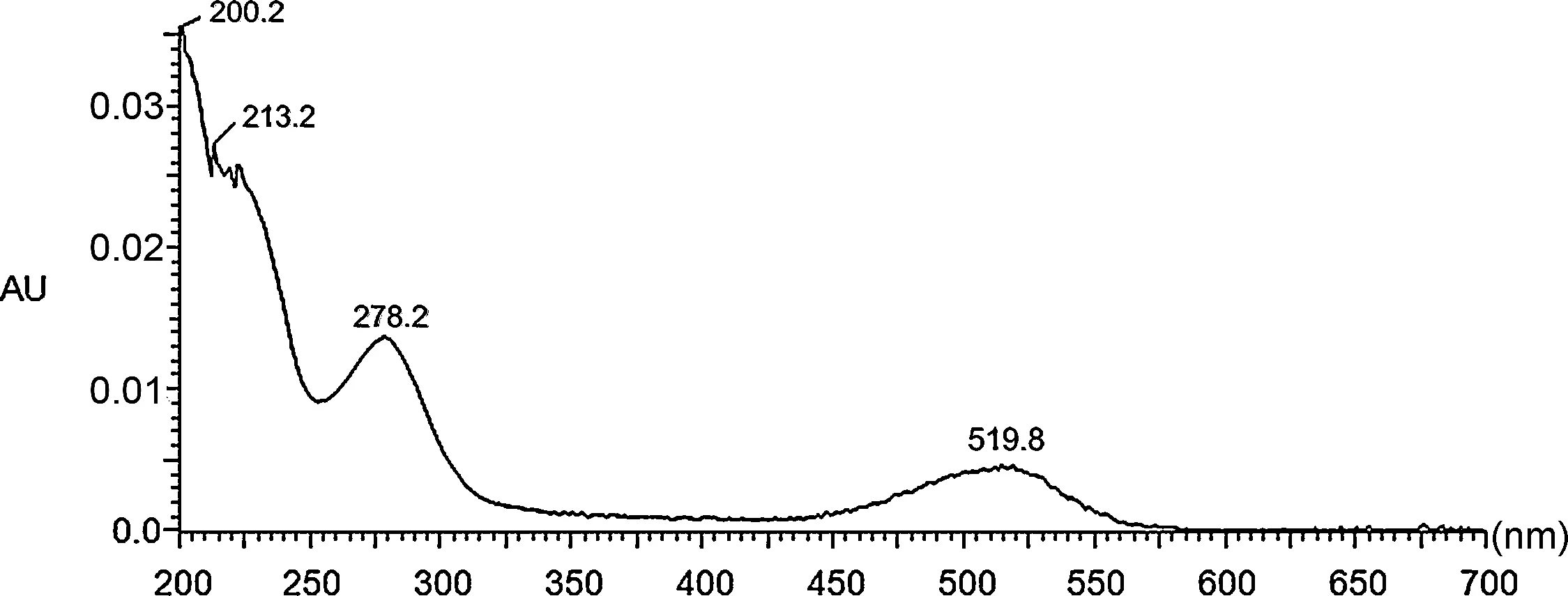
Fig.3.UV-vis spectra of the major anthocyanins obtained with PAD.

Fig.4.Mass Spectrum of the major anthocyanins from Yunnan edible rose.
3.2.Anthocyanins and total phenolic content
The purifie solution was diluted 5 times and then the absorbance was measured at 520 nm.The result was 0.288±0.002,equivalently the anthocyanins content in petals of Yunnan edible rose was (353.56±2.50)mg Cy-3,5-diglu per 100 g FW,which is comparable to that previously reported(375 mg/100 g)[26].
As to total phenolic content,the extracted solution was diluted 15 times and the absorbance was 0.498±0.004 at 765 nm.Accordingly,total phenolic content in petals of Yunnan edible roses was (2087.43±17.37)mg GAE/100 g FW.This level is approximately 5 times higher than the wildLycium ruthenicumfrom Qinghai-Tibet Plateau [17].The high total phenolic content to a certain extent indicated the huge potential of antioxidant activity[27].
3.3.Antioxidant activities
Free radical scavenging is an extensive mechanism of antioxidant.Anthocyanins can donate hydrogen which is able to form the reduced state with free radicals.DPPH radical scavenging activity of sample is shown in Fig.6(a).It could be easily observed that the anthocyanins exhibited notable DPPH radical scavenging activity which ranged from 8.9% to 87.5% when the concentrations varied from 0.003 to 0.064 mg/mL.There was positive linear correlation between DPPH radical scavenging rate and the concentration of sample at first while the trend slowed down as the concentration increased further and an overall parabolic trend was finall observed.The DPPH radical scavenging activity value was 2089 mg GAE/100 g FW.

Fig.5.Demonstration of fragment ions formation in ESI/MS system.

Fig.6.Antioxidant activities of isolated anthocyanins determined by DPPH(a),ABTS(b)and ferric reducing capacity assays(c).
The ABTS radical scavenging activity presented a similar trend with DPPH as could be detected in Fig.6(b) and the value was 639 mg GAE/100 g FW.Furthermore,a positive linear correlation was noticed between DPPH and ABTS radical scavenging activity according to the analysis results of SPSS 16.0 software(r=0.9948,P=0.000).In both assays,the Yunnan edible rose exhibited higher antioxidant activity than the mentioned wildLycium ruthenicum(DPPH:(308.37±31.54)mg GAE/100 g FW;ABTS:(459.00±25.92)mg GAE/100 g FW)[17].
The reducing power of a compound is widely applied to evaluate its ability to donate electrons and may serve as a significant indicator of its potential antioxidant activity.The ferric ion reducing capacity of anthocyanins is shown in Fig.6 (c).Different from Fig.6(a) and (b),a positive linear correlation was observed between the reducing power and concentration of sample (r=0.9969).The value of reducing capacity was 1400 mg GAE/100 g FW,which was almost 4 times higher than Nasturtium fl wers(406 mg GAE/100 g FW)that reported by Garzón and Wrolstad [28].All of these results suggested that Yunnan edible rose could be used as good source of natural pigment to replace the artificia food colorants.
In summary,the present study indicated that the anthocyanins of Yunnan edible rose played an important role as antioxidants.It also implied at the same time the potential utilization of edible roses in the improvement of nutritional value of foods and their preservation.
4.Conclusions
In view of the results of both HPLC-PAD and HPLC-PADESI/MS analysis,the major anthocyanins in Yunnan edible rose was Cy-3,5-diglu that accounted for nearly 94.9% of the total anthocyanins.The total phenol and anthocyanins content of petals were (2087.43±17.37)mg GA equivalents and(353.56±2.50)mg Cy-3,5-diglu equivalents per 100 g FW,respectively.The methanol extract from roses showed excellent reducing capacity and free radical scavenging activity against both DPPH and ABTS.
Therefore,anthocyanins as a type of natural edible pigment,safe non-toxic,rich in resources and with a certain biological activity,have great application potential in food,cosmetic as well as pharmaceutical fields However,the physiological properties of Yunnan edible rosesin vivostill remain to be further studied.
- 食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Anti-inflammator and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats
- Health risk from fluorid exposure of a population in selected areas of Tamil Nadu South India
- Effect of pH,temperature and heating time on the formation of furan in sugar-glycine model systems
- Radical-scavenging activity,ACE-inhibiting capability and identificatio of rapeseed albumin hydrolysate
- Red onion extract(Allium cepa L.)supplementation improves redox balance in oxidatively stressed rats

