十字花科蔬菜中萝卜硫素含量的影响因素
周晨光,朱 毅,罗云波
(中国农业大学食品科学与营养工程学院,北京100083)
萝卜硫素(1-异硫氰酸-4-甲磺酰基丁烷,sulforaphane,SFN)作为一种异硫氰酸盐,是由十字花科植物特有的次级代谢产物——硫甙葡萄糖苷(glucosinlates,Glu)经体内黑芥子苷酶(myrosinase enzyme)酶解所得[1]。近年来,其显著的抗氧化性[2]、消炎作用[3]以及抗癌活性[4-6]受到了众多研究者的关注。目前,有关SFN抑制Ⅰ相代谢酶活性、诱导Ⅱ相代谢酶表达[8-10]、阻止DNA加合物的形成[4,11]、抑制癌细胞中促进生长的信号通路[12-13]引发癌细胞的细胞周期阻滞及诱导癌细胞凋亡[14-16]等显著的肿瘤防治机制已得到了较为深入的研究,SFN也因此被认为是蔬菜中目前所发现的抗癌效果最显著的活性物质之一[17],这使得富含SFN的十字花科蔬菜如甘蓝、西兰花、油菜、萝卜等受到了越来越多的消费者的认可与喜爱[18]。但同时,由于SFN自身较差的稳定性[19],导致十字花科蔬菜在不同的生长环境、储藏条件、加工方法下,蔬菜中SFN含量变化产生较大的差异,从而直接影响到十字花科蔬菜抑癌防癌的功效[20]。鉴于此,本文对十字花科蔬菜在不同生长环境、加工条件及贮藏环境下SFN含量变化趋势进行了阐述,旨在为提高十字花科蔬菜的营养价值提供参考。
1 萝卜硫素的形成
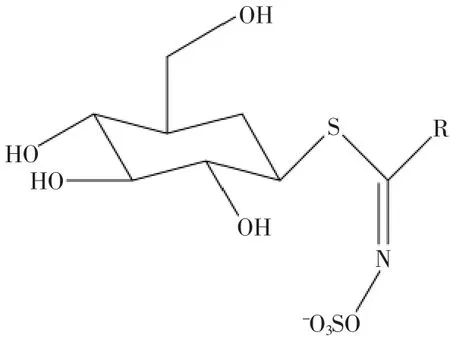
图2 硫甙葡萄糖苷结构[27]Fig.2 Structure of glucosinolates
硫甙葡萄糖苷(glucosinlates,以下简称硫苷,结构见图2)作为十字花科植物在生长过程中的次生代谢产物,广泛存在于植物的根、茎、叶和种子当中[21],目前所发现已达120余种[18]。硫苷的主要结构是由β-D-硫葡萄糖基、磺酸肟基团以及一个来源于氨基酸的侧链R组成。依据其R侧链的不同分为脂肪族、芳香族和吲哚族三大类[22]。当植株受到诸如咀嚼、切割、研磨等而引起组织破坏时,原本存在于组织液泡中的黑芥子苷酶便得以释放[23],并与一类脂肪族硫苷——Glucoraphanin相结合[24],生成葡萄糖(glucose)、萝卜硫素(sulforaphane)以及萝卜硫素腈(sulforaphane nitrile)[20,25-26],Glucoraphanin的转化过程见图3。
2 生长条件对十字花科植物中SFN含量的影响
2.1 自然生长中SFN含量的变化
研究发现,西兰花在自然生长状态下,伴随着呼吸速率的加快、茎叶的生长,在种子萌发的最初18h内SFN含量从3.5mg/g下降到0.75mg/g,而在接下来的40h内SF累积量迅速增加并在第40h达到峰值(3.38mg/g),随后在第60h又降低至0.93mg/g[28]。该结果与W illiams等[29]所得数据相似,即SFN含量在西兰花种子萌芽最初2d内呈下降趋势,随后逐渐升高,在生长至第4d时再次下降并维持在一个较低水平。类似结论在萝卜、花椰菜、卷心菜等其他十字花科植物中也得到了验证[30]。针对该实验结果,初步推断是与植物体内硫苷的分解代谢、黑芥子酶酶活变化相关[29]。Martinez等[31]在测定了多个品种的西兰花和萝卜从种子到芽苗阶段体内SFN含量后指出,从种子到7d芽苗的生长阶段内,SFN含量在西兰花和萝卜中总体呈下降趋势,且不同品种间差异较大。
2.2 光照的影响
一般来说,光照对于植物的生长和发育具有重要意义,光照条件的变化能够显著影响植物的生长特性。Pérez等[32]研究发现,光照条件能够显著影响西兰花苗中SFN的含量,与16h光照/8h黑暗条件下生长的西兰花苗相比,在完全无光条件下生长7d的西兰花苗中SFN相对含量减少33%。该结论与Rosa等[33]的实验结果相悖,其认为不同光照条件对于卷心菜幼苗的根和茎叶中SFN含量几乎毫无影响。据初步推断,以上两种截然不同的结论可能与实验所选取的蔬菜品种差异有直接关系。除此之外,鲜有文献描述关于光照条件与十字花科植物幼苗中SFN含量之间的联系,因此,有关光照与十字花科蔬菜SFN的积累量之间的联系有待进一步的深入研究。
2.3 氮、硫元素的影响
氮、硫元素作为植物生长过程中不可或缺的营养元素,对十字花科植物中SFN的合成与代谢具有较大的影响。Zhao等[34]测定了油菜中硫苷在N、S两种元素作用下的含量变化情况后得出结论,N元素通过参与色氨酸的合成来显著提高芳香族硫苷的含量,而S元素则通过参与蛋氨酸的合成来影响脂肪组和吲哚族硫苷的生成量。然而,Aires等[35]以西兰花苗为实验材料进行实验后发现,肥料中添加的N和S元素不仅无法提高西兰花苗中硫苷含量,相反抑制了SFN的前体——Glucoraphanin的合成,从而间接影响幼苗中SFN的累积量,因此建议避免在西兰花幼苗阶段施用富含N、S的肥料。Gerendás等[36]则对甘蓝施用不同比例的N、S元素后发现,肥料中两种元素的增加与SFN的积累无正相关性,且随着施用肥料中N/S的比例从2∶1增加到50∶1的过程中,SFN的含量从100μmol/kg急剧下降至10μmol/kg左右,损失率接近90%。
2.4 其他因素的影响
鉴于低浓度蔗糖溶液能够促进模式植株拟南芥中抗坏血酸、花青素等活性物质含量的积累[37-38],Guo等[39]向生长中的西兰花苗定期喷洒不同浓度的蔗糖溶液后发现,88mmol/L浓度的蔗糖溶液能够显著提高西兰花苗中SFN、抗坏血酸以及花青素的含量,初步推断是因为蔗糖在上述化合物合成过程中起到了信号转导、提高自身渗透压的缘故[40]。
Yuan等[41]在种植萝卜苗的培养基中添加了不同浓度的NaCl后发现,10mmol/L与50mmol/L的NaCl溶液能够显著降低萝卜苗中SFN的含量,而当萝卜苗受到100mmol/L的NaCl溶液处理后,5d和7d的萝卜苗中SFN含量分别升高了50%和128%,但同时,该浓度的NaCl溶液抑制萝卜种子萌发率也高达35%。依据Zapata等[42]相类似的实验结果推论,较高浓度的盐胁迫能够通过诱导西兰花中相关基因调控硫甙葡萄糖苷的表达量,从而提高SFN含量的增加。
3 加工方式对十字花科蔬菜中SFN含量的影响
十字花科类的蔬菜在加工过程中,其硫甙葡萄糖苷-黑芥子苷酶系统即遭到改变,从而导致硫甙葡萄糖苷的次级代谢产物的种类以及含量产生了较大变化[43]。这些变化主要受加工方式、持续时间以及蔬菜品种的影响[44]。
3.1 常规加工方式对SFN含量的影响
Lambrix等[45]通过研究模式植株拟南芥发现,植物体内的表皮特异硫蛋白(epithiospecifier protein,ESP)能够抑制SFN的生成,并促使SFN的前体硫苷Glucoraphanin转变为不具有抗癌活性的萝卜硫素腈(sulforaphane nitrile)。研究人员以西兰花为材料,研究了加工过程中ESP与SFN两者含量的关系后也得出同样结论,即蔬菜在加工烹饪过程中,ESP能够诱导萝卜硫素腈含量的积累,且这一过程以消耗大量SFN为代价[46]。在此基础上,Matusheski等[47]对西兰花苗加工烹饪后发现,若将加工温度控制在40~70℃范围内,则能有效抑制蔬菜中ESP的活性,大大降低了诱导萝卜硫素腈生成的几率,同时从另一方面也促进了SFN生成量的增加。
Kato等[48]在对日本石川区当地一种名为nakajimana的十字花科蔬菜进行研究后得出结论,任何热加工方式都会对蔬菜中SFN含量造成不同程度的影响,将烹饪温度尽可能地控制在50~65℃则可以在抑制ESP活性的同时,在一定程度上避免黑芥子苷酶失活,从而能降低SFN含量的损失。若想在最大程度上摄取蔬菜中的SFN,则应尽量避免蒸煮等热加工方式。Wang等[49]对比了微波、煮沸、热蒸汽三种热加工方式及作用时间对西兰花中SFN含量的影响,结果发现,与未经处理的西兰花相比,微波与煮沸两种加工方式无论作用时间的长短(0~3m in),均极大降低了SFN的含量,且最高损失量达85%,其主要原因可能是这两种加热方式在很大程度上钝化了黑芥子苷酶的活性,而相比之下,利用热蒸汽对西兰花进行处理并将加热时间控制在1~3m in则能较好地保存SFN的完整性。该结论与Jones等[50]得到的实验数据相一致,其通过测定在热蒸汽、煮沸、微波三种加热方式下西兰花苗中的温度变化后发现,煮沸和微波两种加热方式的传热速率远大于蒸汽加热,这两种加工方式在短时间内促使西兰花苗中的黑芥子苷酶遭到破坏,从而抑制了西兰花苗中SFN的生成,而蒸汽加热方式则相对温和,避免了黑芥子苷酶在短时间内失活,提高了SFN的积累量。
3.2 超高压加工技术对SFN含量的影响
超高压加工技术(high hydrostatic pressure,HHP)作为一种新型非热加工技术,在保持食物天然成分的完整性、延长食品保藏期以及节约能源、减少污染等方面具有不可比拟的优势。Koo等[51]以卷心菜为对象进行研究后证实,在60℃或更高温度的加工条件下,ESP活性的确能受到有效抑制,但促进SFN生成的黑芥子苷酶在此温度下也会遭到一定程度的破坏,而当采用超高压加工技术后发现,30℃、400Mpa的加工条件能在明显抑制卷心菜中ESP活性,同时也较完整的保留黑芥子苷酶的活性,从而进一步提高了SFN生成量。Van等[52]分别在不同温度、处理时间和压力值条件下对西兰花进行加工,随后测定了蔬菜中硫苷Glucoraphanin生成SFN的转化率,结果发现,当加热温度为20℃时,在0~500MPa范围内SFN生成量与压力值的大小成正比例关系且在500MPa、35min达到了最大转化率72%,而当加热温度上升至40℃和60℃时,SFN的转化率均有不同程度的下降,且15m in的处理时间后SFN生成率高于30m in的处理时间。而当加热温度升高至100℃时,无论作用时间长短,蔬菜中均无SFN检出。
然而,超高压非热加工技术并非适用于所有种类的十字花科蔬菜。Ghawi等[25]利用超高压加工技术处理卷心菜后发现,在35~55℃、100~400MPa条件下,卷心菜中的黑芥子苷酶对高压表现出的敏感性显著高于其他十字花科蔬菜。在高压情况下,卷心菜中的黑芥子苷酶极易失活,从而致使蔬菜中硫苷Glucoraphanin无法被酶解催化生成SFN,大大降低了卷心菜的营养价值。
4 采后贮藏条件对十字花科蔬菜中SFN含量的影响
4.1 温度对SFN含量的影响
一般来说,控制温度是保藏蔬菜自身品质、延长货架期最基本、最常用的手段之一。Galgano等[53]在6℃、相对湿度95%的贮藏条件下测定了西兰花中SFN的稳定性,结果发现,SFN损失量在7、21、35d时分别为16%、29%和39%。而当贮藏温度降低至-18℃时,在贮存至第35d和60d时西兰花中SFN损失量分别为15%和39%。由此看出,低温贮藏更有利于延缓蔬菜中SFN的流失。但是,贮藏温度也非越低越好,依据Song等[54]的实验结论,-85℃的超低温条件能够引起花椰菜中SFN的迅速流失,在贮存7d后花椰菜中SFN减少量即高达33%,其主要原因由于过低温度破坏了植物的部分细胞结构,从而影响到了硫苷水解生成异硫氰酸盐的过程。
4.2 相对湿度对SFN含量的影响
大量实验证明,在一定温度范围内,相对湿度(RH)对于蔬菜中SFN的含量也具有一定的影响。在较低的RH(60%)下,20℃、贮存5d的西兰花中SFN损失率高达80%[55],而较高的RH(98%~100%)则能够更好地维持蔬菜组织中细胞膜的通透性,保证了西兰花中诸如SFN等活性物质的稳定性[56]。但是,当贮藏温度降低到4℃时,在60%和100%RH条件下SFN损失量均显著降低且并无明显差异[57]。由此发现,在冷藏条件下(<4℃)RH的高低对于西兰花中SFN的损失影响较小,而当贮藏温度在常温(10~20℃)的范围内时,保持较高的RH值则能很好地防止SFN的流失。
4.3 气调贮藏对SFN含量的影响
目前,气调贮藏(Controlled atmosphere,CA)通过采用低温、低氧和较高的二氧化碳浓度的手段,能够明显降低果蔬呼吸作用、减缓后熟衰老过程,因而被认为是保持果蔬品质和延长贮藏寿命最有效的贮藏方式。Schouten等[58]在1.5%~21%O2浓度、0~15%CO2浓度以及5~18℃的条件下对西兰花进行了气调贮藏的条件优化组合,结果发现,在5℃、1.5%的O2浓度以及6%的CO2浓度下,13d后西兰花中SFN的含量较同温度下未经气调控制的样品高75%。气调贮藏的优越性在花椰菜、甘蓝等蔬菜中也得到了类似的验证[59]。以上结果充分显示了气调贮藏对于果蔬中SFN的积极影响。
4.4 保鲜剂处理对SFN含量的影响
1-MCP(1-甲基环丙烯)作为一种常用的乙烯受体抑制剂,具有安全无毒、低量高效的优点,它能阻断果蔬内源乙烯与受体的结合、抑制组织器官的呼吸作用,达到保质保鲜的目的[60-61]。相关研究人员通过利用10μL/L浓度的1-MCP对芥蓝熏蒸24h后发现,经1-MCP处理后的芥蓝,在20℃下贮藏7d后SFN减少量为39.5%,远小于未经处理的89.3%[60]。在本实验中,虽然1-MCP在常温下显示出对于减缓蔬菜中SFN含量流失具有较明显的效果,但其未能就低温贮藏条件下1-MCP的作用效果进行进一步探究。
低浓度乙醇作为另一种常见的果蔬保鲜剂,在果蔬贮藏中也得到了广泛的应用。由于采后的西兰花在室温条件下极易腐败变质,Xu等[62]为提高西兰花的贮藏品质,采用了不同浓度乙醇溶液进行熏蒸处理,结果发现,500μL/L的乙醇溶液不仅能够大幅度延长西兰花的货架期、减缓叶绿素降解,同时显著减少了在4d的贮藏期内SFN的损失,其主要作用机理为通过降低西兰花在贮藏过程中的呼吸强度来减少SFN前体硫苷在贮藏过程中的自降解。
5 展望
随着人们生活水平的提高,具有食疗功效的食品受到越来越多消费者的青睐。十字花科蔬菜富含抑癌抗癌效果显著的萝卜硫素,在药食领域具有广阔的应用前景。然而,由于萝卜硫素自身的不稳定性,使得十字花科蔬菜中的萝卜硫素在加工和贮藏过程中极易遭到破坏,从而降低了商品价值。因此,对于十字花科蔬菜而言,如何在生长、加工以及贮藏过程中提高萝卜硫素含量、防止其遭到破坏的技术研究就显得极为关键。目前国内外研究人员针对如何减少萝卜硫素损失的加工和贮藏方法进行了大量研究,发现采用诸如超高压非热加工、气调贮藏以及保鲜剂处理等手段均能较好的减少蔬菜中萝卜硫素的损失。然而,有关如何在蔬菜生长过程中促进萝卜硫素前体硫苷含量增加,从而提高蔬菜中萝卜硫素积累量的相关研究甚少,因此可以考虑在分子水平上,通过改造调控硫苷以及相关酶表达量的基因,优化和选育出抑癌抗癌效果更加显著的蔬菜新品种。
[1]Matusheski N V,Wallig M A,Juvik JA,et al.Preparative HPLCmethod for the purification ofsulforaphaneand sulforaphane nitrile from Brassica oleracea[J].Journal of Agricultural and Food Chemistry,2001,49(4):1867-1872.
[2]Chen H,Wu J,Zhang J,et al.Protective effects of the antioxidant sulforaphane on behavioral changes and neurotoxicity in mice after the administration of methamphetamine[J].Psychopharmacology,2012,222(1):37-45.
[3]Guo S,Qiu P,Xu G,et al.Synergistic Anti-inflammatory Effects of Nobiletin and Sulforaphane in Lipopolysaccharide-Stimulated RAW 264.7 Cells[J].Journal of Agricultural and Food Chemistry,2012,60(9):2157-2164.
[4]Kensler T,Egner P,Agyeman A,etal.Keap1-Nrf2 Signaling:A Target for Cancer Prevention by Sulforaphane[M].Springer Berlin/Heidelberg,2012:1-15.
[5]Herr I,Rausch V.Stem Cells and Cancer Stem Cells[M].Netherlands:Springer,2012:27-32.
[6]沈莲清,苏光耀,王奎武.西兰花种子中硫苷酶解产物萝卜硫素的提纯与抗肿瘤的体外实验研究[J].中国食品学报,2008,8(5):15-21.
[7]Liang H,Lai B,Yuan Q.Sulforaphane Induces Cell-Cycle Arrest and Apoptosis in Cultured Human Lung Adenocarcinoma LTEP-A2 Cells and Retards Growth of LTEP-A2 Xenografts in Vivo[J].Journal of Natural Products,2008,71(11):1911-1914.
[8]Myzak M C,Dashwood RH.Chemoprotection by sulforaphane:Keep one eye beyond Keap1[J].Cancer Letters,2006,233(2):208-218.
[9]Wagner A E,Ernst I,Iori R,et al.Sulforaphane but not ascorbigen,indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture[J].Experimental Dermatology,2010,19(2):137-144.
[10]Wang H,Khor T O,Yang Q,et al.Pharmacokinetics and Pharmacodynamics of Phase II Drug Metabolizing/Antioxidant Enzymes Gene Response by Anticancer Agent Sulforaphane in Rat Lymphocytes[J].Molecular Pharmaceutics,2012,9(10):2819-2827.
[11]Fiala JL A,Egner P A,Wiriyachan N,et al.Sulforaphane-Mediated Reduction of Aflatoxin B1-N7-Guanine in Rat Liver DNA:Impacts of Strain and Sex[J].Toxicological Sciences,2011,121(1):57-62.
[12]Hahm E-R,Singh S V.Sulforaphane Inhibits Constitutive and Interleukin-6-Induced Activation of Signal Transducer and Activator of Transcription 3 in Prostate Cancer Cells[J].Cancer Prevention Research,2010,3(4):484-494.
[13]Hutzen B,WillisW,Jones S,et al.Dietary agent,benzyl isothiocyanate inhibits signal transducer and activator of transcription 3 phosphorylation and collaborateswith sulforaphane in the growth suppression of PANC-1 cancer cells[M].BioMed Central,2009.
[14]Clarke J D,Hsu A,Yu Z,et al.Differential effects of sulforaphane on histone deacetylases,cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells[J].Molecular Nutrition&Food Research,2011,55(7):999-1009.
[15]Jiang H,Shang X,Wu H,et al.Combination Treatmentwith Resveratrol and Sulforaphane Induces Apoptosis in Human U251 Glioma Cells[J].Neurochemical Research,2010,35(1):152-161.
[16]Moon D-O,Kim M-O,Kang S-H,et al.Sulforaphane suppresses TNF-α-mediated activation of NF-κB and induces apoptosis through activation of reactive oxygen speciesdependent caspase-3[J].Cancer Letters,2009,274(1):132-142.
[17]Gamet-Payrastre L,Li P,Lumeau S,et al.Sulforaphane,a Naturally Occurring Isothiocyanate,Induces Cell Cycle Arrest and Apoptosis in HT29 Human Colon Cancer Cells[J].Cancer Research,2000,60(5):1426-1433.
[18]Fahey JW,Zalcmann A T,Talalay P.The chemical diversity and distribution of glucosinolates and isothiocyanates among plants[J].Phytochemistry,2001,56(1):5-51.
[19]Jin Y,Wang M,Rosen R T,et al.Thermal Degradation of Sulforaphane in Aqueous Solution[J].Journal of Agricultural and Food Chemistry,1999,47(8):3121-3123.
[20]王会霞,李晨,薛峰,等.加工处理方式对冲菜中硫代葡萄糖苷的影响[J].食品科学,2011,32(7):168-172.
[21]Fahey JW,Wehage SL,Holtzclaw W D,et al.Protection of Humans by Plant Glucosinolates:Efficiency of Conversion of Glucosinolates to Isothiocyanatesby theGastrointestinalMicroflora [J].Cancer Prevention Research,2012,5(4):603-611.
[22]Wittstock U,Halkier B A.Glucosinolate research in the Arabidopsisera[J].Trends in Plant Science,2002,7(6):263-270.
[23]Scholl C,Eshelman B D,Barnes D M,et al.Raphasatin Is a More Potent Inducer of the Detoxification Enzymes Than Its Degradation Products[J].Journal of Food Science,2011,76(3):504-511.
[24]O’Hare T J,Wong L S,Force L E,et al.Glucosinolate composition and anti-cancer potential of daikon and radish sprouts[C].International Horticultural Congress&Exhibition,2006,8:77-78.
[25]Ghawi SK,Methven L,Rastall R A,et al.Thermal and high hydrostatic pressure inactivation of myrosinase from green cabbage:A kinetic study[J].Food Chemistry,2012,131(4):1240-1247.
[26]杨瑛洁,李淑燕,胡国伟,等.硫代葡萄糖苷的降解途径及其产物的研究进展[J].西北植物学报,2011,31(7):1490-1496.
[27]Elfakir C,Dreux M.Simultaneous analysis of intact and desulfated glucosinolates with a porous graphitized carbon column[J].Journal of Chromatography A,1996,727(1):71-82.
[28]Gu Y,Guo Q,Zhang L,et al.Physiological and biochemical metabolism of germinating broccoli seeds and sprouts[J].Journal of Agricultural and Food Chemistry,2012,60(1):209-213.
[29]Williams D J,Critchley C,Pun S,et al.Epithiospecifier protein activity in broccoli:The link between terminal alkenyl glucosinolates and sulphoraphane nitrile[J].Phytochemistry,2008,69(16):2765-2773.
[30]West L G,Meyer K A,Balch B A,et al.Glucoraphanin and 4-Hydroxyglucobrassicin Contents in Seeds of 59 Cultivars of Broccoli,Raab,Kohlrabi,Radish,Cauliflower,Brussels Sprouts,Kale,and Cabbage[J].Journalof Agriculturaland Food Chemistry,2004,52(4):916-926.
[31]Martinez-Villaluenga C,Peñas E,Ciska E,et al.Time dependence of bioactive compounds and antioxidant capacity during germination of different cultivars of broccoli and radish seeds[J].Food Chemistry,2010,120(3):710-716.
[32]Pérez-Balibrea S,Moreno D A,García-Viguera C.Influence of light on health-promoting phytochemicals of broccoli sprouts [J].Journal of the Science of Food and Agriculture,2008,88(5):904-910.
[33]Rosa E A S,Rodrigues P M F.The effect of light and temperature on glucosinolate concentration in the leaves and roots of cabbage seedlings[J].Journal of the Science of Food and Agriculture,1998,78(2):208-212.
[34]Zhao F,Evans E J,Bilsborrow P E,et al.Influence of nitrogen and sulphur on the glucosinolate profile of rapeseed(brassica napus l)[J].Journal of the Science of Food and Agriculture,1994,64(3):295-304.
[35]Aires A,Rosa E,Carvalho R.Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts(Brassica oleracea var.italica)[J].Journal of the Science of Food and Agriculture,2006,86(10):1512-1516.
[36]Gerendaás JS,Breuning S,Stahl T,et al.Isothiocyanate Concentration in Kohlrabi(Brassica oleracea L.Var.gongylodes)Plants As Influenced by Sulfur and Nitrogen Supply[J].Journal of Agricultural and Food Chemistry,2008,56(18):8334-8342.
[37]Solfanelli C,Poggi A,Loreti E,et al.Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis [J].Plant Physiology,2006,140(2):637-646.
[38]Nishikawa F,Kato M,Hyodo H,et al.Ascorbatemetabolism in harvested broccoli[J].Journal of Experimental Botany,2003,54:2439-2448.
[39]Guo R,Yuan G,Wang Q.Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts[J].Scientia Horticulturae,2011,128(3):159-165.
[40]胡克玲,朱祝军.喷施蔗糖和葡萄糖对小白菜硫代葡萄糖苷含量的影响[J].核农学报,2010,24(4):840-845.
[41]Yuan G,Wang X,Guo R,etal.Effectofsaltstresson phenolic compounds,glucosinolates,myrosinase and antioxidant activity in radish sprouts[J].Food Chemistry,2010,121(4):1014-1019.
[42]Zapata P J,Serrano M A,Pretel M T,et al.Polyamines and ethylene changes during germination of different plant species under salinity[J].Plant Science,2004,167(4):781-788.
[43]Dekker M,Verkerk R,Jongen W M F.Predictivemodelling of health aspects in the food production chain:a case study on glucosinolates in cabbage[J].Trends in Food Science& Technology,2000,11(4-5):174-181.
[44]Oerlemans K,Barrett D M,Suades C B,et al.Thermal degradation of glucosinolates in red cabbage[J].Food Chemistry,2006,95(1):19-29.
[45]Lambrix V,Reichelt M,Mitchell-Olds T,et al.The Arabidopsis Epithiospecifier Protein Promotes the Hydrolysis of Glucosinolates to Nitrilesand Influences Trichoplusia niHerbivory [J].The Plant Cell Online,2001,13(12):2793-2807.
[46]Williams D J,Critchley C,Pun S,et al.Epithiospecifier protein activity in broccoli:The link between terminal alkenyl glucosinolatesand sulphoraphane nitrile[J].Phytochemistry,2008,69(16):2765-2773.
[47]Matusheski N V,Juvik JA,Jeffery E H.Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli[J].Phytochemistry,2004,65(9):1273-1281.
[48]Kato M,Imayoshi Y,Iwabuchi H,et al.Kinetic changes in glucosinolate-derived volatiles by heat-treatmentandmyrosinase activity in nakajimana(Brassica rapa L.cv.nakajimana)[J].Journal of Agricultural and Food Chemistry,2011,59(20):11034-11039.
[49]Wang G C,Farnham M,Jeffery E H.Impact of Thermal Processing on Sulforaphane Yield from Broccoli(Brassica oleracea L.ssp italica)[J].Journal of Agricultural and Food Chemistry,2012,60(27):6743-6748.
[50]Jones R B,Frisina C L,Winkler S,et al.Cooking method significantly effects glucosinolate content and sulforaphaneproduction in broccoli florets[J].Food Chemistry,2010,123(2):237-242.
[51]Koo S Y,Cha K H,Song D G,et al.Amplification of sulforaphane content in red cabbage by pressure and temperature treatments[J].Journal of Applied Biological Chemistry,2011,54(2):183-187.
[52]Van Eylen D,Bellostas N,Strobel BW,et al.Influence of pressure/temperature treatments on glucosinolate conversion in broccoli(Brassica oleraceae L.cv Italica)heads[J].Food Chemistry,2009,112(3):646-653.
[53]Galgano F,Favati F,Caruso M,et al.The Influence of Processing and Preservation on the Retention of Health-Promoting Compounds in Broccoli[J].Journal of Food Science,2007,72(2):S130-S135.
[54]Song L,Thornalley P J.Effect of storage,processing and cooking on glucosinolate content of Brassica vegetables[J].Food and Chemical Toxicology,2007,45(2):216-224.
[55]Rodrigues A S,Rosa E A S.Effectof post-harvest treatments on the levelofglucosinolates in broccoli[J].Journal of the Science of Food and Agriculture,1999,79(7):1028-1032.
[56]Lutz JM,Hardenburg R E,Wright R C.The commercial storage of fruits,vegetables,and florist and nursery stocks[M].Washington:USDeptof Agriculture,1968.
[57]Rangkadilok N,Tomkins B,Nicolas M E,etal.The Effect of Post-Harvest and Packaging Treatments on Glucoraphanin Concentration in Broccoli(Brassica oleracea var.italica)[J].Journal of Agricultural and Food Chemistry,2002,50(25):7386-7391.
[58]Schouten R E,Zhang X,Verkerk R,et al.Modelling the level of the major glucosinolates in broccoli as affected by controlled atmosphere and temperature[J].Postharvest Biology and Technology,2009,53(1-2):1-10.
[59]Cies'lik E,Leszczyńska T,Filipiak-Florkiewicz A,et al.Effects of some technological processes on glucosinolate contents in cruciferous vegetables[J].Food Chemistry,2007,105(3):976-981.
[60]Golding JB,Shearer D,Wyllie SG,et al.Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit[J].Postharvest Biology and Technology,1998,14(1):87-98.
[61]Bo Sun,Huizhuan Yan,Na Liu JW,等.1-MCP处理对芥蓝采后品质、抗氧化物及芥子油甙含量的影响[J].保鲜与加工,2012,12(2):55.
[62]Xu F,Chen X,Jin P,et al.Effect of ethanol treatment on quality and antioxidant activity in postharvest broccoli florets[J].European Food Research and Technology,2012,235:1-8.

