Reproductive traits of polycystic ovary syndrome in female rhesus monkeys
TANG Xiang-Hui, CAO Yue-Ling, YANG Ze-Xing, ZHAO Fu-Xian
(1. Kunming Institute of Zoology, theChinese Academy of Sciences, Kunming 650223, China; 2. The Fourth Affiliated Hospital of Kunming Medical College, Kunming 650021, China)
Reproductive traits of polycystic ovary syndrome in female rhesus monkeys
TANG Xiang-Hui1,*, CAO Yue-Ling2, YANG Ze-Xing2, ZHAO Fu-Xian2
(1.Kunming Institute of Zoology,theChinese Academy of Sciences,Kunming650223,China; 2.The Fourth Affiliated Hospital of Kunming Medical College,Kunming650021,China)
The objective of this study was to set up a rhesus monkey model of polycystic ovary syndrome (PCOS), which is globally prevalent among reproductive-aged human women, and to understand the reproductive traits of PCOS female monkeys. Six adult female rhesus monkeys aged 6-10 a, were divided into a PCOS group and a control group. The PCOS group were given two cycles of subcutaneous injections of propionic acid testosterone (PAT), 3.5 mg/kg body weight, on day 1, day 3, and day 5 of the menstrual cycle, respectively, and then given muscle injections of human chorionic gonadotropin (HCG), 350 IU/kg body wtight, on day 7, day 9, and day 11, respectively. Results showed that high levels of serum LH and T [(5.35±0.17) IU/L and (7.58±0.14) ng/mL, respectively], and a high ratio value of LH/FSH (5.35/1.30=4.12) were observed in the PCOS group. No significant differences were found in serum FSH, E2, and P in the PCOS group compared with those of the control. Polycystic ovaries in the PCOS monkeys were recorded by live ultrasound. The blastocysts rates of the PCOS vs. the control were 23.53% vs. 66.67%, and there was a significant difference between the two groups. This study shows that PAT coupled with HCG can induce PCOS in rhesus monkeys in the short term. The reproductive features of PCOS monkeys were similar to those of PCOS patients.
PCOS; Rhesus monkey; Reproduction; Controlled ovary stimulation
Polycystic ovary syndrome (PCOS) is a heterogeneous syndrome in women characterized by luteinizing hormone (LH) hypersecretion, ovarian hyperandrogenism, hyperinsulinemia from insulin resistance, and reduced fecundity. Given the 6.6% estimated prevalence of PCOS in reproductive-aged women in the United States (i.e., at least 4 million affected women), the annual economic burden of PCOS in the United States is at least $4.4 billion (Dumesic et al, 2007). In China, studies have shown that the prevalence of PCOS in reproductive-aged women in Jinan city is 6.46% (Chen et al, 2005), and different types of menstruation have been recorded among 2 100 cases of the so-called ‘Rotterdam criteria’ PCOS patients, that is, amenorrhea (31.2%), menstrual thin (63.8%), and regular menstrual cycle (5%) (Xu et al, 2009). In addition, PCOS is prevalent among puberty aged females worldwide. Previous research on the clinical features of puberty PCOS between the Uygur ethnic and Han ethnic peoples in Xinjiang, China, showed that compared with normal women, there was no difference in the menarche in puberty aged PCOS patients, but abnormal menstruation was prevalent among the latter (Lin & Ding, 2008).
The etiology and pathophysiology of PCOS remains unclear. Thus, the treatments only scratch the surface of the problem and drugs are needed to ease the symptoms. As human reproduction is concerned, several morphological findings in PCOS patients implicate increased recruitment of growing follicles from the primordial follicle pool with the development of the polycystic ovaries. In PCOS, the growth of follicles is impaired at the 6-8 mm size when granulosa cells normally begin to express aromatase and convert androgens produced by LH-stimulated theca cells to estradiol (E2) in the presence of FSH (Gougeon, 1996; Jakimiuk et al, 1998). Unfortunately, experimental constraints on the use of human tissue for biomedical research limit our knowledge of PCOS origins and its developmental effects on human reproduction. Given the limitations in human studies, establishing a generally accepted PCOS animal model to help investigate the etiology, pathophysiology, and treatment of PCOS is needed.
Rhesus monkey provides unique insight into the pathophysiology of PCOS. Not only do female macaques closely resemble women in terms of genome (Blekhman et al, 2008), reproductive biology (Abbott et al, 2004; Jimenez et al, 2005; Tarantal, 1992; Tarantala & Gargosky 1995; Tarantal et al, 1997), metabolic physiology (Wagner et al, 2006), and aging (Lee et al, 1995;Wu et al, 2005), they also exhibit PCOS-like traits spontaneously (Arifin et al, 2008) as well as following experimentally-induced androgen excess during early or late gestation (Abbott et al, 1998; 2005) or after acute exposure to androgen excess in adulthood (Vendola et al, 1998). Such spontaneous and experimentally-induced PCOS-like traits are unparalleled to date in other species, providing an important model for human disease (Abbott et al, 2006; Rosenfield, 2007).
Rhesus models of PCOS are beneficial for observation and evaluation and are especially suitable for research of reproductive dysfunction. Abbott et al (1998) found that female rhesus monkeys exposed in utero to levels of testosterone equivalent to those found in fetal males show many clinical and biochemical features of PCOS. However, the cost and long breeding cycle for producing the prenatally androgenized PCOS rhesus monkeys are not conducive to large-scale research. To establish a female rhesus monkey PCOS model in China, we focused on a short term procedure to produce a PCOS rhesus monkey and understand the reproductive traits of PCOS female monkey.
1 Materials and Methods
1.1 Hormones and chemicals
Unless stated otherwise, all chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
1.2 Animals and hormone treatment
Six adult female monkeys (Macaca mulatta), 6-10 a, were used in this study and were maintained at the Kunming Primate Research Center, Kunming Institute of Zoology (KIZ) according to standard protocols (SCXK2008-0001). Three animals were placed in the PCOS group and the other three for control group. Rhesus macaques in all experiments were given standard primate chow with occasional supplementation of fresh fruit. The monkey chow formulation provides 75% of calories as carbohydrate, 10% as fat, and 15% as protein. Each female rhesus monkey (body weight 4-6 kg) was maintained in a cage (1 100 cm × 800 cm × 900 cm), which had a tap to provide filtered fresh waterad libitum. All animals were kept in a room with light control (12 h light/12 h dark) and a temperature of 23-25 °C with a relative humidity of 40%-55%. The animal care and use committee of KIZ approved all experiments and animal protocols.
The PCOS monkeys were developed as follows: Animals were given subcutaneous injections of 3.5 mg/kg body weight propionic acid testosterone (PAT) on day 1, day 3, and day 5 of the menstrual cycle, respectively, and then given muscle injections of 350 IU/kg body weight human chorionic gonadotropin (HCG) on day 7, day 9, and day 11, respectively. All PCOS rhesus monkeys were treated in this way for two consecutive menstrual cycles. The control animals were administered with normal saline each time.
1.3 Morphometry
At the end of the dosing periods, ultrasound and laparoscopy were performed on the monkeys under ketamine anesthesia to obtain a live picture of ovaries for morphometric analysis. Follicle size was determined by measurement of the largest cross-sectional diameter on the screen of the ultrasound. Ovaries were imaged abdominally using a Diasus ultrasound system (Dynamic Imaging Ltd., Livingston, Scotland, UK), equipped with a 10-22 MHz linear-array transducer.
1.4 Determination of serum hormones
Serum was collected for hormone measurement at the time of the subcutaneous injections of PAT and at the end of the dosing periods. Serum FSH, LH, estradiol, testosterone, and progesterone levels were measured by RIA according to the kit manual.
1.5 Controlled ovary stimulation (COS)
Treatment with recombinant human FSH (rhFSH; Gonal-F; Laboratories Serono SA, Aubonne, Switzerland) was initiated on day 3 of the menstrual for both PCOS and control monkeys. Vaginal bleeding was monitored daily to detect the onset of menses. Both groups received intramuscular (im) treatments of 18 IU rhFSH, twice daily, 10-12 h apart for eight consecutive days for controlled ovary stimulation, as described previously (Yang et al, 2009). Monkeys were then given 1 000 IU HCG (Serono Laboratories, SA) intramuscularly on day 11 of the menstrual cycle. Oocytes retrieval was performed by laparoscopic follicular aspiration 32 to 35 h after hCG administration. Follicular contents were placed into HEPES-buffered TALP (modifed Tyrode solution with albumin, lactate, and pyruvate) medium containing 0.3% bovine serum albumin (BSA) at 37 °C. Oocytes were stripped of cumulus cells by mechanical pipetting after brief exposure (<1 min) to hyaluronidase (0.5 mg/mL) to allow classifcation of nuclear maturity as metaphase I (MI; no germinal vesicle, no polar body) and metaphase II (MII; one polar body). Mature oocytes (MII) were placed in hamster embryo culture medium-10 (HECM-10) at 37 °C, 5% CO2humidifed air until IVF.
1.6 IVF and embryo culture
To assess developmental competence of retrieved oocytes, freshly collected mature oocytes were inseminated as described previously (Yang et al, 2009). Briefy, hyperactivated spermatozoa and mature oocytes (MII) were co-incubated for 12-16 h at 37 °C in a humidifed atmosphere of 5% CO2. Fertilized oocytes exhibiting two pronuclei were cultured for embryonic development in 50 μL drops of HECM-10 containing 10% fetal bovine serum, covered with mineral oil, for up to 7 days at 37 °C in humidifed 5% CO2in air, with changes of culture medium every second day. Progress of embryo growth was monitored daily using Nomarski optics (at ×200-×400 magnifcation) on a Nikon (Japan) Diaphot TMD microscope.
1.7 Statistical analysis
Results obtained were presented as the Mean±SD(unless stated otherwise). Statistical evaluations were performed with SPSS software (version 13.0; SPSS Inc., Chicago, IL). Values withP<0.05 were considered significantly different.
2 Results
2.1 Hormones change of PCOS monkeys
Serum hormones of PCOS monkeys are shown in Tab.1. Five serum hormones were measured in both the PCOS and control groups. After androgen administration, the PCOS monkeys showed different serum LH and testosterone (T) profiles compared with those of the control group and were characterized by high levels of serum LH and T (5.35±0.17) IU/L and (7.58±0.14) ng/mL, respectively. A high ratio value of LH/FSH (5.35/1.30=4.12) was observed in the PCOS group. There were no significant differences in the serum FSH, E2, and P in the PCOS group compared with those of the control, although serum levels of these hormones were lower than that of the counterparts.
2.2 Ovary changes in PCOS monkeys
After establishment of the PCOS animal model, based primarily on the LH/FSH ratio value (LH/FSH>2), ultrasounds were performed for each animal for live observation of ovary status. A typical ovary image in the PCOS monkeys is shown in Fig. 1, in which more than five cysts were recorded under the condition of nontreatment. These cysts looked like stimulated follicles during the course of superovulation.
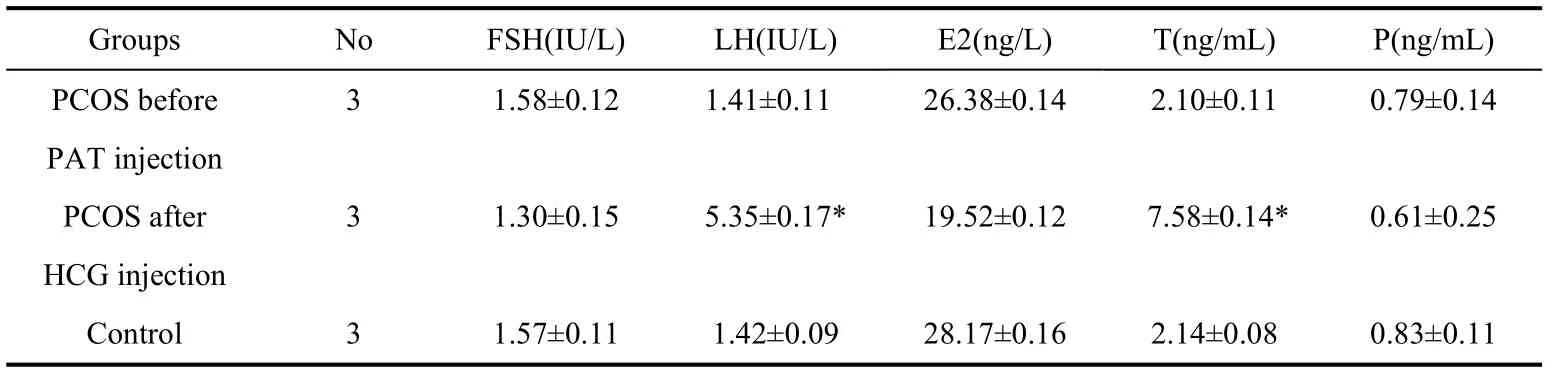
Tab.1 Serum hormones of the PCOS and control monkeys

Fig.1 Typical polycystic ovary image in the PCOS monkey
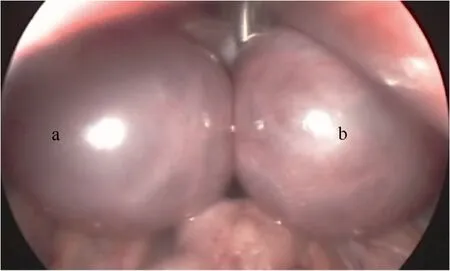
Fig.2 Laparoscopic image of PCOS monkey ovaries overstimulated after treatment of COS
2.3 Response of PCOS monkeys to COS
Ovaries of PCOS monkeys showed a much stronger response to the COS compared with those of the control. Fig. 2 show a typical laparoscopic image of PCOS monkey ovaries over-stimulated after treatment of COS. However, fewer oocytes were retrieved from PCOS monkeys than the number of follicles recorded by ultrasound. This inconformity was not observed in the control animals where oocytes retrieved was in accordance with follicle numbers recorded by ultrasound on the day before oocytes retrieval (Tab. 2).
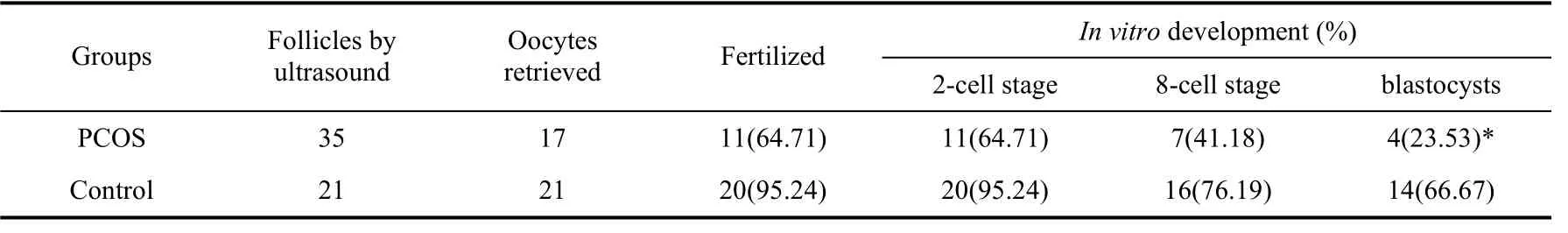
Tab. 2 Oocytes number retrieved from PCOS and their potential in vitro development
2.4 Developmental potential of oocytes from PCOS monkeys
Embryos derived from PCOS oocytes were compromised when they were culturedin vitrofor blastocyst development. The blastocyst rates of PCOS vs. control were 23.53% vs. 66.67%, respectively, and there was significant difference between two groups (Tab. 2).
3 Discussion
This study shows that PAT coupled with HCG induced PCOS in rhesus monkeys in the short term, which recapitulated reproductive features of human PCOS, including PCO morphology, elevated serum androgen levels, high ratio value of LH/FSH (>2) and enlarged ovary size. The PCOS monkeys showed a strong response to controlled ovary stimulation, a characteristic in PCOS women that result in ovarian hyperstimulation (OHS) under the treatment of controlled ovary stimulation.
A neuroendocrine hallmark of PCOS women is enhanced LH hypersecretion from enhanced gonadotropin-releasing hormone (GnRH) pulsatility. Consequently, serum immuno- and bioactive LH levels are increased in about 70% of PCOS patients (Lobo, 1991), with elevated LH pulse amplitude and increased LH pulse frequency causing a two-three fold elevation in circulating LH versus FSH levels (Waldstreicher et al, 1988). The high ratio value of LH/FSH (5.35/1.30=4.12) observed in the PCOS monkey was consistent with findings in PCOS humans. Moreover, elevated serum testosterone levels (7.58±0.14) ng/mL in PCOS monkeys plus polycystic ovaries scanned by ultrasound were fully compliant with the definition of PCOS, which is defined by the Rotterdam criteria as any two of the following three findings: clinical/biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovaries (The Rotterdam ESHRE/ASRM- sponsored PCOS consensus workshop group, 2004). Many PCOS patients undergoingin vitrofertilization (IVF) achieve a clinical pregnancy rate that is not comparable to that of similarlytreated normal women. These PCOS patients also have increased risk of implantation failure and pregnancy loss (Ludwig et al, 1999) as well as impaired oocyte fertilization unrelated to gross chromosomal abnormalities or nuclear maturation (Heijnen et al, 2006; Hwang et al, 2005; Kodama et al, 1995; Lu et al, 2006; Ludwig et al, 1999; Sengoku et al, 1997). Moreover, obese PCOS patients experience low oocyte fertilization and failure of embryos to implant in their own uterus or those of their surrogates (Cano et al, 1997), implicating impaired oocyte developmental competence. In this study, oocytes retrieved from the PCOS monkeys were obviously less than the number of follicles, indicating that some follicles of PCOS monkeys detected by ultrasound were, in fact, cysts in which there were no oocytes at all. Embryos derived from oocytes of PCOS monkeys also showed impaired competence inin vitrodevelopment (Tab. 2).
Developing an animal model for PCOS studies has been studied for many years. To date, however, no universally accepted animal model has been established. The rat PCOS model has been widely used for research. However, recent studies in rats showed that the infertility of PCOS-like rats was mostly due to exogenous androgen and only ovulation and ovarian polycystic change were similar to humans, with serum hormone levels, ovarian weight, size and response to treatment of COS totally different from PCOS human patients (Mannerås et al, 2007). Consequently, PCOS rats are considered unsuitable as reproductive studies for PCOS humans.
Rhesus monkeys are the closest animal to human reproductive physiology and have an ovarian and menstrual cycle similar to those of humans. These monkeys are particularly suitable as animal models that focus on human reproductive dysfunction studies. This and other studies demonstrate that rhesus monkeys are the most suitable animal for research on polycystic ovary syndrome in humans.
In summary, all reproductive traits mentioned above demonstrated that PCOS monkeys can be developed by administration of PAT coupled with HCG in the short term. The reproductive features of PCOS monkeys were similar to those of PCOS patients. Further study is needed using this animal model to explore the etiology and pathophysiology, especially in the reproductive dysfunction of PCOS humans.
Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. 1998. Insights into the development of PCOS from studies of prenatally androgenized female rhesus monkeys [J].Trends Endocrinol Metab,9(2): 62-67.
Abbott DH, Foong SC, Barnett DK, Dumesic DA. 2004. Nonhuman primates contribute unique understanding to anovulatory infertility in women [J].ILAR J,45(2): 116-131.
Abbott DH, Barnett DK, Bruns CM, Schramm RD, Dumesic DA. 2005. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome [J].Hum Reprod Update,11(4): 357-374.
Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. 2006. Animal models and fetal programming of PCOS [M] // Azziz R, Nestler JE, Dewailly D. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. 2nded. Totowa, NJ: Humana Press Inc., 259-272.
Arifin E, Shively CA, Register TC, Cline JM. 2008. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey(Macaca fascicularis) [J].Vet Pathol,45(4): 512-515.
Blekhman R, Man O, Herrmann L, Boyko AR, Indap A, Kosiol C, Bustamante CD, Teshima KM, Przeworski M. 2008. Natural selection on genes that underlie human disease susceptibility [J].Curr Biol,18(12): 883-889.
Cano F, García-Velasco JA, Millet A, Remohí J, Simón C, Pellicer A. 1997. Oocyte quality in polycystic ovaries revisited: identification of a particular subgroup of women [J].J Assist Reprod Genet,14(5): 254-260.
Chen ZJ, Zhao JL, Zhou FR, Li Y, Zhao LX, Ma ZX. 2005. The primary investigation of prevalence of the polycystic ovary syndrome in general Han ethnic women in Jinan city [J].Prog Obstet Gyneol,14(6): 442-444. (in Chinese)
Dumesic DA, Abbott DH, Padmanabhan V. 2007. Polycystic ovary syndrome and its developmental origins [J].Rev Endocr Metab Disord,8(2): 127-141.
Gougeon A. 1996. Regulation of ovarian follicular development in primates: facts and hypothesis [J].Endocr Rev,17(2): 121-155.
Heijnen E, Eijkemans M, Hughes E, Laven J, Macklon N, Fauser B. 2006. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome [J].Human Reprod Update,12(1): 13-21.
Hwang JL, Seow KM, Lin YH, Hsieh BC, Huang LW, Chen HJ, Huang SC, Chen CY, Chen PH, Tzeng CR. 2005. IVF versus ICSI in sibling oocytes from patients with polycystic ovarian syndrome: a randomized controlled trial [J].Hum Reprod,20(5): 1261-1265.
Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA. 1998. Aromatase mRNA expression in individual follicles from polycystic ovaries [J].Mol Hum Reprod,4(1): 1-8.
Jimenez DF, Leapley AC, Lee CI, Ultsch MN, Tarantal AF. 2005. Fetal CD34+ cells in the maternal circulation and long-term microchimerism in rhesus monkeys(Macaca mulatta) [J].Transplantation,79(2): 142-146.
Kodama H, Fukuda J, Karube H, Matsui T, Shimizu Y, Tanaka T. 1995. High incidence of embryo transfer cancellations in patients with polycystic ovarian syndrome [J].Hum Reprod,10(8): 1962-1967. Lee CI, Fletcher MD, Tarantal AF. 1995. Effect of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+and hematopoietic progenitor cells [J].Pediatr Res,58(2): 315-322.
Lin L, Ding Y. 2008. Approach the characteristics and treatments of the puberty polycystic ovary syndrome of the Uygurs and Hans in Xinjiang [J].Prog Obstet Gynecol,17(4): 281-285. (in Chinese)
Lobo RA. 1991. The syndrome of hyperandrogenic chronic anovulation [M]// Mishell DR Jr, Davajan V, Lobo RA. Infertility, Contraception and Reproductive Endocrinology. 3rded. Cambridge: Blackwell Scientific Publications. 447-487.
Lu XE, Yang XF, Li MG, Zhou FZ, Zhu YM, Huang HF. 2006. Outcome ofin vitrofertilization-embryo transfer in treatment of polycystic ovarian syndrome [J].J Zhejiang Univ:Med Sci,35(3): 319-322. (in Chinese)
Ludwig M, Finas DF, Al-Hasani S, Diedrich K, Ortmann O. 1999. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients [J].Hum Reprod,14(2): 354-358.
Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. 2007. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome [J].Endocrinology,148(8): 3781-3791.
Rosenfield RL. 2007. Identifying children at risk for polycystic ovary syndrome [J].J Clin Endocrinol Metab,92(3): 787-796.
Sengoku K, Tamate K, Takuma N, Yoshida T, Goishi K, Ishikawa M. 1997. The chromosomal normality of unfertilized oocytes from patients with polycystic ovarian syndrome [J].HumReprod,12(3): 474-477.
Tarantal AF. 1992. Sonographic assessment of nongravid female macaques (Macaca mulatta and Macaca fascicularis) [J].J Med Primatol,21(6): 308-315.
Tarantal AF, Gargosky SE. 1995. Characterization of the insulin-like growth factor (IGF) axis in the serum of maternal and fetal macaques (Macaca mulattaandMacaca fascicularis): A crosssectional study [J].Growth Reg,5(4): 190-198.
Tarantal AF, Laughlin LS, Dieter J, Tieu J, Hendrickx AG, Overstreet JW, Lasley BL. 1997. Pregnancy detection by ultrasound and chorionic gonadotropin during the peri-implantation period in the macaque (Macaca fascicularis) [J].Early Pregnancy,3(4): 281-290.
The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) [J].Hum Reprod,19(1): 41-47.
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. 1998. Androgens stimulate early stages of follicular growth in the primate ovary [J].J Clin Invest,101(2): 2622-2629.
Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ Jr, Kaplan JR. 2006. Old world nonhuman primate models of type 2 diabetes mellitus [J].ILAR J,47(3): 259-271.
Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF Jr. 1998. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization [J].J Clin Endocrinol Metab,66(1): 165–172.
Wu JM, Zelinski MB, Ingram DK, Ottinger MA. 2005. Ovarian aging and menopause: current theories, hypotheses, and research models [J].Exp Biol Med,230(11): 818-828.
Xu XH, Tan YC, Shi YH, Wang B, Ju XQ, Zheng QM, Hao CF, Chen ZJ. 2009. Different types of menstrual cycle and their significance in Chinese women diagnosed with polycystic ovary syndrome according to the Rotterdam consensus criteria [J].Natl Med J Chn,89(37): 2604-2606. (in Chinese)
Yang S, He X, NiuY, Hildebrandt TB, Jewgenowc K, Goeritz F, Tang X, Chang Y, Zhou Q, Ji W. 2009. Ovarian response to gonadotropin stimulation in juvenile rhesus monkeys [J].Theriogenology,72(2): 243-250.
多囊卵巢综合征猕猴的生殖特性
唐向辉1,*,曹跃龄2,杨泽星2,赵富鲜2
(1. 中国科学院昆明动物研究所,云南 昆明 650223; 2. 昆明医学院第四附属医院,云南 昆明 650021)
多囊卵巢综合征(PCOS)是临床上常见的一种内分泌失调性疾病,也是造成无排卵性不孕的重要原因。对多囊卵巢综合征动物模型的研究有 10余年,迄今尚未建立起较为理想的模型动物。该研究的目的在于构建猕猴多囊卵巢综合征动物模型,并分析该模型动物的一些主要生殖特性。将6只成年雌性猕猴(6~10 a)平均分成2组:PCOS模型组和对照组。模型组动物的建立方法是:在月经周期的第1、3、5天,分别皮下注射丙酸睾丸酮一次,剂量为 3.5 mg/kg体重;接着在第 7、9、11天,分别肌注人绒毛膜促性腺激素一次,剂量是 350 IU/kg体重;连续注射2个月经周期。对照组动物注射生理盐水。结果显示:PCOS模型组动物呈现出高血清LH和 T,分别为(5.35±0.17) IU/L和(7.58±0.14) ng/mL,而且血清 LH/FSH值(5.35/1.30=4.12);模型组动物血清FSH、 E2和P的含量与对照组无显著差异。腹部B超扫描结果提示,模型组动物卵巢多囊化。对两组动物进行超排处理后,模型组动物卵巢呈明显的过刺激现象,模型组动物胚胎体外培养的囊胚率为 23.53% ,显著低于对照组(66.67%)(P<0.05),即用丙酸睾丸酮联合人绒毛膜促性腺激素,能够建立PCOS猕猴模型,该模型动物的一些主要生殖特性与人类PCOS相似。
多囊卵巢综合征;猕猴;生殖;控制性卵巢刺激
Q418; Q492.5; Q954.592
A
0254-5853-(2012)01-0037-06
2011-10-24;接受日期:2011-12-10
唐向辉(1960-),男,副研究员,硕士,研究方向:生殖生物学和生物技术
10.3724/SP.J.1141.2012.01037
date: 2011-10-24; Accepted date: 2011-12-10
This work was supported by Yunnan Key Laboratory of Animal Reproductive Biology(2010-03)*
(通信作者), E-mail: tangxh@mail.kiz.ac.cn

