Characterization of spontaneous breast tumor in tree shrews (Tupaia belangeri chinenesis)
XIA Hou-Jun, WANG Chun-Yan, ZHANG Hai-Lin, HE Bao-Li,JIAO Jian-Lin,*, CHEN Ce-Shi,*
(1.Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology,the Chinese Academy of Sciences, Kunming, 650223, China; 2. Graduate School of the Chinese Academy of Sciences, Beijing100039, China; 3. Kunming Medical University, Kunming, 650031, China)
Characterization of spontaneous breast tumor in tree shrews (Tupaia belangeri chinenesis)
XIA Hou-Jun1,†, WANG Chun-Yan1,2,3,†, ZHANG Hai-Lin1, HE Bao-Li3,JIAO Jian-Lin3,*, CHEN Ce-Shi1,*
(1.Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology,the Chinese Academy of Sciences, Kunming,650223,China; 2.Graduate School of the Chinese Academy of Sciences, Beijing
100039,China; 3.Kunming Medical University, Kunming,650031,China)
Breast cancer is a common malignant tumor. It is essential to develop suitable animal models for discovering novel preventive and therapeutic approaches. Tree shrews (Tupaia belangeri chinensis) have a closer evolutionary relationship with humans than do rodents, which have been widely used in laboratory research. Spontaneous breast tumors were identified in tree shrews in 1960s; however, no detailed studies about tree shrew breast tumors have been conducted to date. Here, we characterized a spontaneous breast tumor from tree shrews by Haematoxylin Eosin(H&E) staining. This tumor was identified as a papillary tumor. Immunohistochemical staining (IHC) for progesterone receptor (PR), Ki-67 and cleaved caspase-3 showed that tumor cells were positive for PR, highly proliferative, and less apoptotic compared to normal breast epithelial cells. Thus, the spontaneous tumor of tree shrew is very close to human papillary tumors in terms of morphology and pathology and we concluded that tree shrew may be a suitable animal model for breast cancer research.
Spontaneous breast cancer; Tree shrew; Immunohistochemistry
Breast cancer initiates from mammary epithelial cells located in the lobules or ducts of the breast (Sariego,2010). Despite a relatively lower incidence of breast cancer in Asian countries compared to Western countries(Jemal et al, 2006), the incidence of breast cancer has dramatically increased over the past 30 years in Chinese urban women (Fan et al, 2009). The primary risk factors for breast cancer include sex and age (Thomas &Leonard, 2009), genetic background such as gene mutations ofBRCA1/2(Diamond et al, 2009), lack of childbearing or breastfeeding (Collaborative Group on Hormonal Factors in Breast Cancer, 2002), levels of hormone such as estrogen (Yager & Davidson, 2006),and race, economic status, and environment (Hulka &Stark, 1995). Breast cancer is usually divided into four subtypes according to the molecular profile: luminal A(ER+ and/or PR+, HER2-), luminal B (ER+ and/or PR+,HER2+), HER2+ (ER-, PR-, HER2+), and basal-like(ER-, PR-, HER2-, also known as triple-negative) (Carey et al, 2006). The traditional treatments for breast cancer patients include surgery, chemotherapy, radiotherapy,hormone therapy, and biologically targeted therapy.However, the five-year survival ratio for breast cancer patients with metastasis is about 27%. Early diagnosis and effective targeted therapy are promising approaches to save breast cancer patients. It is extremely important to establish appropriate animal models to study the mechanism and pathogenesis of breast cancer and to develop novel preventive and therapeutic approaches.
Rodents are widely used for cancer research and have several advantages, such as small size, rapid propagation, low cost, the availability of a variety of inbred strains, and matured transgenic techniques.However, rodent species are not close to humans in regards to genetic evolution, anatomy, physiology, and organ systems. Non-human primates are very similar to humans in terms of genetic evolution and should be,therefore, more ideal models for studying the mechanisms of breast cancer and testing potential therapeutic drugs. Tree shrews (Tupaia belangeri chinensis) are considered as primate-like animals and are more similar to humans compared to rodents. Tree shrews are classified as a primitive prosimian and belong to a separate family ofScandentia. Recently, molecular phylogenetic studies demonstrated that tree shrews should be given the same rank as the primates, and form the Euarchonta group together with the orders Primates and Dermoptera (Janecka et al, 2007). Tree shrews are small mammals native to the tropical forests of Southeast Asia, including southwest China, Burma, Indonesia, and the Philippines. Tree shrews are easy to maintain, small in size, rapid in propagation, and low in cost compared to larger non-human primates. Therefore, tree shrews might be a superior model for cancer research compared to rodents and non-human primates.
Spontaneous tumors have been previously identified in tree shrews. In 1966, the first spontaneous breast cancer was observed in tree shrews (Elliot et al, 1966).After that, several different types of spontaneous cancers such as hepatocellular carcinoma (Hofmann et al, 1981),epidermoid carcinoma (Darai et a1, 1982), Leydig cell tumor (Brack, 1988), and pulmonary tumor (Brack et al,1996) have been reported. However, there is no detailed characterization of breast tumors from tree shrews to date. Here, we characterize a breast tumor from a female tree shrew. The information may be valuable for establishing breast cancer models in tree shrews.
1 Materials and Methods
1.1 Animals
Tree shrews were obtained from Kunming Institute of Zoology, the Chinese Academy of Science or Kunming Medical University. Normal breast tissue and tumors were isolated after animals were euthanized. All tissue samples were immediately frozen by liquid nitrogen and stored at −80 °C. Animal care and experimental protocols were approved by the Animal Ethics Committee of the Kunming Institute of Zoology,the Chinese Academy of Science, China.
1.2 H&E staining
Surgical specimens were obtained and fixed in 10%neutral-buffered formalin and embedded in paraffin.Standard hematoxylin and eosin staining was performed.
1.3 IHC staining
The antibodies used in this study were anti-Ki67(Maixin, China), anti-cleaved caspase 3 (Cell signaling,USA), anti-PR (Maixin), and anti-HER2 (Maixin). The 4 μm thick tissue sections were first deparaffinized in xylene and rehydrated in graded ethanol. Following that,antigen retrieval was completed by immersing the slides in 10 mmol/L citrate buffer, pH 6.0, at 120 ℃ for 8 mins using an autoclave. Endogenous peroxidase was blocked with 45 mL of methyl alcohol and 5 mL of 30%hydrogen peroxide. After three washes with phosphatebuffered saline (PBS), PBS with 2% BSA was used to block the non-specific reaction for 1h at room temperature. After three washes with PBS, the slides were incubated with diluted primary antibodies (anti-Ki-67 (1:100), anti-cleaved-caspase 3 (1:200), anti-PR(1:100), or anti-HER-2 (1:100)) in 4% goat serum in 1×PBS with 0.2% Triton-X 100 overnight at 4 ℃. The slides were washed three times with PBS and subsequently incubated with biotinylated, goat antirabbit secondary antibody at a dilution of 1:200 for 20 mins, followed by three washes with PBS. We used 3,3’-diaminobenzidine (DAB) as the chromogen to detect the signal. The slides were counterstained with hematoxylin buffer for 3 mins at room temperature and washed with running water. The slides were stained with 10−20 drops of ammonium hydroxide and washed with running water. Finally, the slides were cleaned in xylene and mounted with permount.
2 Results
2.1 Normal tree shrew mammary glands
Mammary glands from a virgin tree shrew was isolated and examined by H&E staining. Normal mammary gland duct from the tree shrew showed two layers of epithelial cells (Fig. 1). The inner layer was glandular epidermis and the outer layer was myoepithelium. The structure of terminal end buds was also observed.

Fig. 1 Structure of normal breast mammary glands from a virgin tree shrew
2.2 A mammary gland tumor from the tree shrew
Tree shrew (#0709) was caught from the wild and experienced artificial domestication. The age of this tree shrew was estimated to be 1−2 a. This animal showed a history of mammary swelling over a 3-week period. The mammary tumor was formed spontaneously and grew very rapidly. When the tumor size increased to 3.8×2.6 cm (length×width), the animal was euthanized because the tumor was scraped by the cage. The tumor was isolated and observed anatomically. The tumor had an intact capsule and was well confined by a membrane.The surface of the tumor was grayish red, solid, and crisp(Fig. 2).
2.3 Histopathological analysis of the tumor
The tumor from the tree shrew #0709 was diagnosed as intraductal papilloma in which epithelium cells grow papillarily with intact basal membrane. The papilloma consisted of branching fronds of stroma supporting an epithelium layer composed of epithelial and myoepithelial cells (Fig. 3)
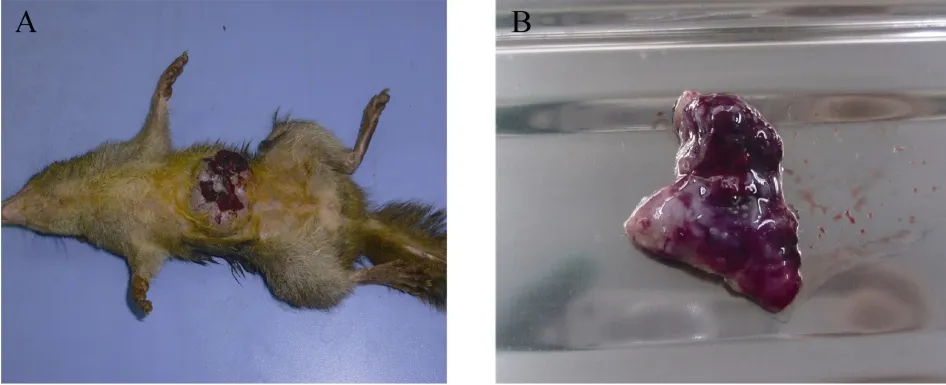
Fig. 2 Gross anatomy of tree shrew #0709 mammary neoplasia
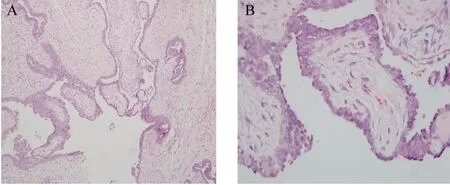
Fig. 3 Morphology and structure of mammary intraductal papilloma from tree shrew #0709
To better compare the structure of intraductal papillomas from the tree shrew and humans, we performed H&E staining for a human intraductal papilloma (Fig. 4). The basic microscopic structure of human intraductal papilloma consists of the proliferation of ductal epithelium supported by frond forming fibrovascular stroma. The most orderly form of papilloma consists of branching fronds of stroma supporting an epithelium layer composed of epithelial and myoepithelial cells. A similar microscopic morphology and structure was observed in the tree shrew intraductal papilloma.
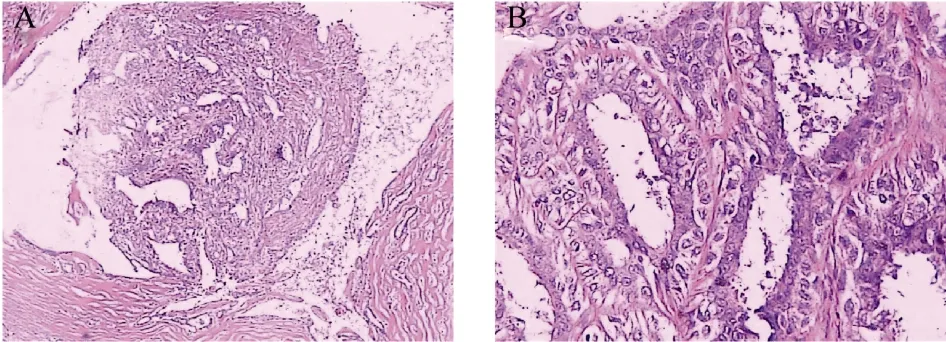
Fig. 4 Structure of a human intraductal papilloma
2.4 Immunohistochemistry analysis of the tumor
The Ki-67 protein is a biomarker for cell proliferation. The percentage of Ki-67 positive tumor cells is often positively correlated with the clinical outcome of cancer patients. In addition, cleaved caspase-3 is widely used to assess the extent of apoptosis. The ER, PR, and HER2 were used for breast cancer diagnosis.We performed IHC for Ki-67 and cleaved-caspase 3 to analyze the tumor proliferation/apoptosis index. The percentage of Ki-67 positive cells in the tumor was about 40% while the percentage of cleaved-caspase 3 positive cells in the tumor was about 1%−3%. In addition, ER,PR, and HER2 were stained to estimate the type of spontaneous tumor following human standards.Unfortunately, two different anti-human ER antibodies did not work (data not shown). The tree shrew tumor showed positive for PR and negative for HER2 (Fig 5).

Fig. 5 Immunohistochemistry staining of the tree shrew mammary tumor
To compare differences between the breast tumor and normal breast in tree shrew, mammary glands from a lactating tree shrew were also examined by IHC. The percentage of Ki-67 positive cells was ~5%, which is much less than that in the tumor (~40%). In addition, the mammary gland epithelial cells from the tree shrew in the lactation stage showed positive PR and Cl-C3, but negative HER2 staining (Fig. 6).
3 Discussion
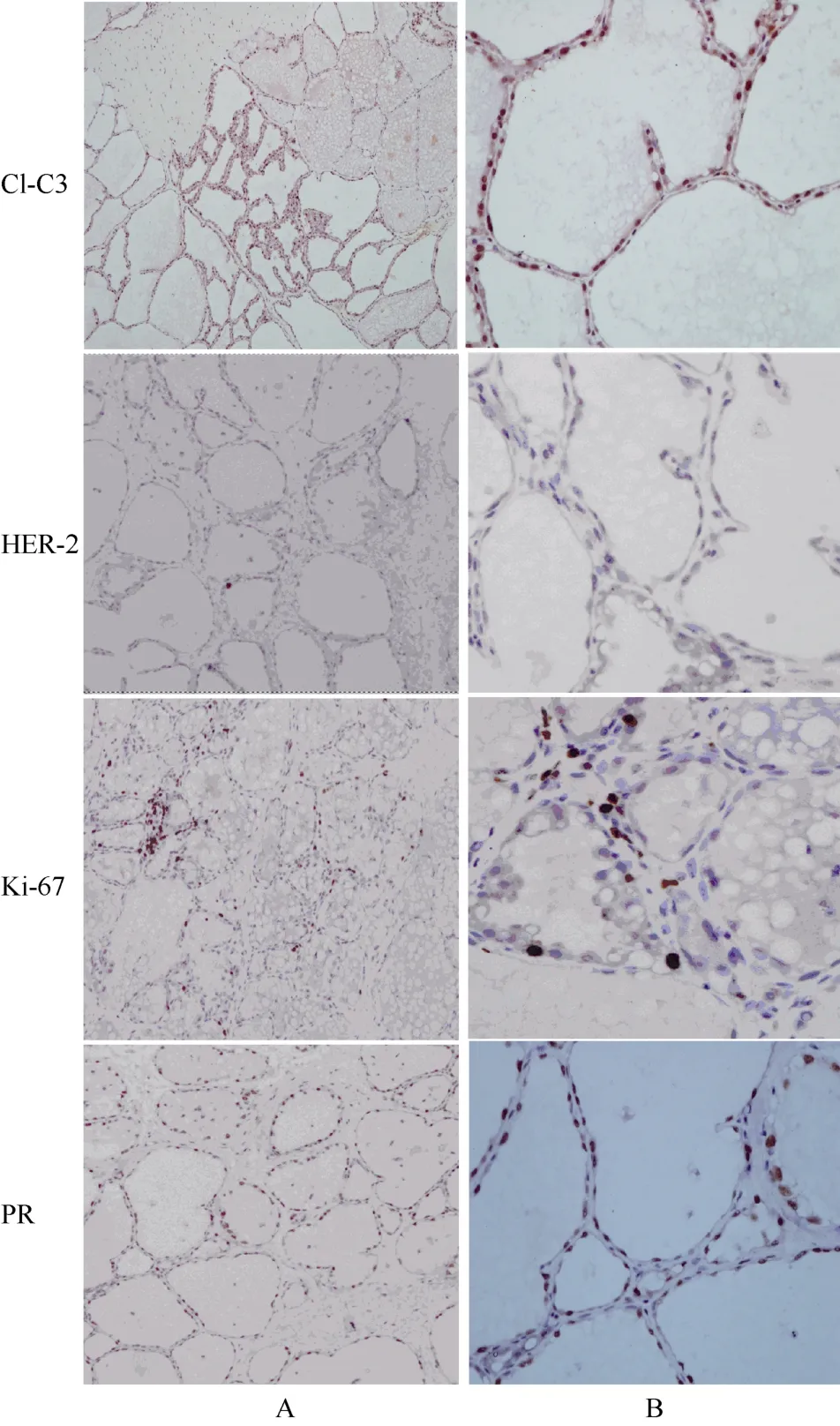
Fig. 6 Immunohistochemistry staining of the tree shrew in the lactation stage
Despite spontaneous tree shrew breast cancers being reported in 1966 (Elliot et al), there have been no following studies since then. This may be because the incidence of spontaneous breast cancer is rare in tree shrews. In this paper, we analyzed a spontaneous breast tumor from a wild tree shrew and described its morphological,pathological, and immunohistochemical features.
Based on H&E staining, the tumor from tree shrew#0709 was an intraductal papilloma, which is a type of benign tumor that grows in ducts of the breast. In humans, the incidence of intraductal papilloma is less than 10% of benign tumors (Cilotti et al, 1991) and they are characterized by painless bloody nipple discharge and a palpable breast mass (Wei et al, 2009). The intraductal papilloma may progress to papillary carcinoma when mammary gland epidermis infiltrate into the stroma, with or without invasion (Pal et al, 2010).The breast tumor reported by Elliot et al (1966) was adenocarcinoma, which is an epithelium cancer that originates in glandular tissue.
It is well known that the balance between cell proliferation and apoptosis determines tumor growth and progression. The Ki-67 protein is a proliferative biomarker detected during all active phases of the cell cycle, but not in resting cells (Lopez et al, 1991). The spontaneous intraductal papilloma from the tree shrew showed a very high percentage of Ki-67 positive cells, suggesting that the tumor cells were highly proliferative. The ratio of positive Ki-67 cells was very close to that of the human Ki-67 labeling index in breast cancers (Fasanella et al,2011). In contrast, apoptosis is the most effective approach to eliminate transformed cells. The key mediators of apoptosis are caspases. Caspase 3 is a common effector caspase activated by upstream initiating caspases. Here we used cleaved caspase 3 (Cl-C3) to detect the index of apoptosis. A low percentage of positive Cl-C3 was found in the spontaneous intraductal papilloma. The high proliferative/apoptotic index in this spontaneous intraductal papilloma explains the rapid tumor growth.
To further determine if the tree shrew is an ideal animal model for studying human breast cancer, more spontaneous breast tumors from tree shrews need to be characterized. However, there are some limitations for studying spontaneous tumors. Firstly, the incidence of tree shrew breast tumors is sporadic and rare. Secondly,spontaneous tumors are identified accidentally and it is difficult to trace the carcinogens. Therefore, it will be necessary to develop experimental breast tumor models of the tree shrew in the future.
In conclusion, we characterized a spontaneous breast tumor from a tree shrew. Based on H&E staining and pathological diagnosis, the tumor was determined to be an intraductal papilloma. The tumor showed positive PR and negative HER2 staining. The tumor was highly proliferative and less apoptotic compared to the normal breast based on Ki-67 and cleaved caspase-3 IHC results.These findings suggest that the tree shrew is a promising animal model for studying breast cancer.
Brack M. 1988. Malignant Leydig cell tumour in aTupaia belangeri:case report and literature review of male genital tumours in nonhuman primates[J].Lab Anim,22(2): 131-134.
Brack M, Schwartz P, Heinrichs T, Schultz M, Fuchs E. 1996. Tumors of the respiratory tract observed at the German Primate Center,1978-1994[J].J Med Primatol,25(6): 424-434.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K,Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MCU, Nielsen TO, Moorman PG, Earp HS, Millikan RC. 2006. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study[J].JAMA,295(21): 2492-2502.
Cilotti A, Bagnolesi P, Napoli V, Lencioni R, Bartolozzi C. 1991.Solitary intraductal papilloma of the breast. An echographic study of 12 cases[J].Radiol Med,82(5): 617-620.
Collaborative Group on Hormonal Factors in Breast Cancer. 2002.Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries,including 50302 women with breast cancer and 96973 women without the disease[J].Lancet,360(9328): 187-195.
Darai G, Zöller L, Hofmann W, Möller P, Schwaier A, Flügel RM. 1982.Spontaneous malignomas inTupaia(tree shrew)[J].Amer J of Primtol,2(2): 177-189.
Diamond JR, Borges VF, Eckhardt SG, Jimeno A. 2009. BRCA in breast cancer: from risk assessment to therapeutic prediction[J].Drug News Perspect,22(10): 603-608.
Elliot OS, Elliot MW, Lisco H. 1966. Breast cancer in a tree shrew(Tupaia glis)[J].Nature,211(5053): 1105.
Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu JS, Shen KW, Shen ZZ,Shao ZM. 2009. Breast cancer in a transitional society over 18 years: trends and present status in Shanghai, China[J].Breast Cancer Res Treat,117(2): 409-416.
Fasanella S, Leonardi E, Cantaloni C, Eccher C, Bazzanella I, Aldovini D, Bragantini E, Morelli L, Cuorvo LV, Ferro A, Gasperetti F,Berlanda G, Dalla Palma P, Barbareschi M. 2011. Proliferative activity in human breast cancer: Ki-67 automated evaluation and the influence of different Ki-67 equivalent antibodies[J].Diagn Pathol,6(S1): S1-S7.
Hofmann W, Möller P, Schwaier A, Flügel RM, Zöller L, Darai G. 1981.Malignant tumours inTupaia(tree shrew)[J].J Med Primatol,10(2-3): 155-163.
Hulka BS, Stark AT. 1995. Breast cancer: cause and prevention[J].Lancet,346(8979): 883-887.
Janecka JE, Miller W, Pringle TH, Wiens F, Zitzmann A, Helgen KM,Springer MS, Murphy WJ. 2007. Molecular and genomic data identify the closest living relative of primates[J].Science,318(5851): 792-794.
Jemal A, Siegel R, Ward E, Murray T, Xu JQ, Smigal C, Thun MJ.2006. Cancer statistics[J].CA Cancer J Clin,56(2): 106-130.
Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P,Boisseau MR. 1991. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes[J].Cytometry,12(1): 42-49.
Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, Gupta RK, Paz B,Vora L, Guzman E, Artinyan A, Somlo G.. 2010. Papillary carcinoma of the breast: an overview[J].Breast Cancer Res Treat,122(3): 637-645.
Sariego J. 2010. Breast cancer in the young patient[J].Am Surg,76(12):1397-1400. 17
Thomas GA, Leonard RCF. 2009. How age affects the biology of breast cancer[J].Clin Oncol (R Coll Radiol),21(2): 81-85.
Wei H, Jiayi F, Qinping Z, Junyi S, Yuan S, Li L, Dongwei S, Liying Q.2009. Ultrasound-guided vacuum-assisted breast biopsy system for diagnosis and minimally invasive excision of intraductal papilloma without nipple discharge[J].World J Surg,33(12):2579-2581.
Yager JD, Davidson NE. 2006. Estrogen carcinogenesis in breast cancer[J].N Engl J Med,354(3): 270-282.
自发性树鼩乳腺肿瘤的特性
夏厚军1,†, 王春艳1,2,3,†, 张海林1, 何保丽3, 角建林3,*, 陈策实1,*
(1. 中国科学院和云南省动物模型与人类疾病机理重点实验室, 中国科学院昆明动物研究所, 云南 昆明 650223;2. 中国科学院研究生院, 北京 100039; 3. 昆明医学院, 云南 昆明 650031)
乳腺癌是严重危害女性健康的常见恶性肿瘤, 建立合适的乳腺癌动物模型对于研究人类乳腺癌的生物学机制及发展新的防治方法至关重要。相对于常用的啮齿类动物, 树鼩(Tupaia belangeri chinensis,tree shrew)因在进化层次上更接近于人类而可用于建立更适合的乳腺癌模型。该文详细了介绍一例树鼩自发性乳头状良性乳腺肿瘤。免疫组化结果显示该例肿瘤孕激素受体阳性且Ki-67阳性细胞比例显著增加; 而活化的Caspase 3阳性细胞比例较低; 且肿瘤的形态和病理与人导管内乳头状肿瘤非常接近。提示利用树鼩建立乳腺肿瘤模型的可行性。
自发性乳腺肿瘤; 树鼩; 免疫组化
Q492; Q539.91
A
0254-5853-(2012)01-0055-05
2011-12-02;接受日期:2012-01-05
10.3724/SP.J.1141.2012.01055
date: 2011-12-02; Accepted date: 2012-01-05
s: Yunnan Province High-Profile Talent Project (2010CI114); the Chinese Academy of Sciences, Basic Frontier Project (KSCX2-EW
J-23)
*Corresponding authors (通信作者), E-mail: chenc@mail.kiz.ac.cn; jiaojianlin66@163.com†These authors contributed equally to this work
——李振声

