葡萄糖加氢用Ru/活性炭催化剂:改性方法对活性炭表面性能的影响
徐三魁 李利民 郭楠楠 苏运来 张 朋
(1郑州大学化学系,郑州 450001; 2河南工业大学材料科学与工程学院,郑州450001)
葡萄糖加氢用Ru/活性炭催化剂:改性方法对活性炭表面性能的影响
徐三魁1,2李利民1,*郭楠楠1苏运来1张 朋1
(1郑州大学化学系,郑州 450001;2河南工业大学材料科学与工程学院,郑州450001)
分别采用超临界甲醇流体、浓硝酸氧化、浓硝酸结合超临界甲醇流体等不同手段对椰壳活性炭进行了表面处理,用N2物理吸附、Boehm滴定、X光电子能谱仪(XPS)、电感耦合等离子原子发射光谱分析(ICP)、透射电镜(TEM)等手段研究了处理方法对活性炭表面孔结构及表面基团的影响;并以活性炭为载体,三氯化钌为活性前驱体,采用等容水浸渍法制备了钌炭催化剂,以葡萄糖加氢生产山梨醇为模型反应对制备的钌基催化剂的催化活性进行了评价.结果表明:各种处理方法对活性炭的比表面、孔径等孔结构性能影响不大;但超临界甲醇处理活性炭可明显减少活性炭表面含氧酸性基团的含量,尤其是羧基等不稳定基团的含量;而硝酸处理活性炭则可大幅度提高活性炭表面含氧酸性基团的含量,尤其是羧基等不稳定基团的含量增加更大.ICP分析结果表明:超临界甲醇处理活性炭并不改变活性炭样品对钌的吸附量,但硝酸氧化处理活性炭却能明显提高样品对钌的吸附能力.活性炭表面的这些含氧基团虽然有利于钌离子的吸附,但却不利于钌在活性炭表面的分散.由于超临界甲醇流体处理活性炭时的表面反应及萃取作用,可有效清除活性炭表面的不稳定含氧酸性基团,避免还原过程中钌的迁移聚集,使负载钌的分散度提高,有利于增强钌与活性炭间的相互作用,使钌部分缺失电子,钌的结合能升高;可明显提高负载钌炭催化剂葡萄糖催化加氢的活性.
表面改性;活性炭;钌基催化剂;表面含氧基团;氢化
1 Introduction
Because of high surface area,relative inertness,and high thermal stability,activated carbon(AC)has proved to be highly effective as a catalyst support.1-5It is known that catalyst support properties are determined by both their texture and surface chemistry.1,3-5Despite the abundance of literature has been published on it,the effect of AC texture and surface oxygen containing groups on the metal dispersion and activity is still under controversy.It has been well established in the literature that the surface oxygen containing groups,which are anchoring sites for metallic precursors as well as for metals,dominantly determine the properties of AC as a catalyst support material.1,3-5The acidic groups on the surface decrease the hydrophobicity of the carbon,leading to the accessibility of the surface to aqueous metal precursors,while the less acidic groups increase the interaction of the metal precursor or the metal particle with the support and,as a consequence,minimize the sintering propensity of metal on carbon.1,4,5Coloma et al.6reported that the dispersion of metal was highly dependent on the degree of carbon support oxidation,and the degree of dispersion is lower for AC containing higher amount of surface acidic complexes.The nature and density of surface functional groups can be modified by suitable thermal or chemical post-treatments such as gas oxidation,liquid phase oxidation with HNO3or other oxidants,ultrasonic treatment,and microwave treatment.7-15Recently,the supercritical fluid(SCF)technology has been developed rapidly due to its unusual physical properties,which can be tunable by simply changing the pressure and temperature.The SCFs have been explored as potential replacement for conventional solvents in various extractive processes,chemical reaction,and the preparation of new materials owing to its high diffusivity,good solubility,better penetration,and wetting properties.16-18However,there is no report on the method forAC treated by SCFs to our knowledge.
The hydrogenation of D-glucose to D-sorbitol is of great industrial importance because D-sorbitol is a valuable additive in foods,drugs,and cosmetics.Moreover,D-sorbitol is an intermediate in vitamin C production.Generally,Raney Ni is used as catalyst for this process due to its excellent settling properties and lower cost.However,the dissolution of nickel occurs during the hydrogenation of D-glucose,resulting in the contamination of products which limits the applications of method. The maximum allowable concentration of Ni is 2 mg·kg-1for food industry application.19As a consequence,purification of sorbitol is necessary,which renders this process economically less attractive.In addition,the alkali leaching is employed during the catalyst preparation which will bring environmental problem.Thus,great attempts have been made to develop new efficient and environmentally friendly catalysts for this process.20Ruthenium-based catalysts as efficient and stable catalysts for the hydrogenation of glucose have received much attention in recent years.19-22
In this work,we report a Ru catalyst supported with novel modified AC,which was proved to be more effective than the catalysts supported with the original AC.Our work aims at using scCH3OH,HNO3oxidation,and the HNO3oxidation combined with scCH3OH treatment to modify AC,and prepare Ru-based catalysts with them as support.The modified ACs and catalysts were characterized by various analytical techniques.The activity of catalysts was investigated through the hydrogenation of D-glucose to D-sorbitol.Correlation between the surface properties of AC and the activity of catalysts has been established.
2 Experimental
2.1 Chemicals
Coconut activated carbon was purchased from Fuzhou Shaowu Xinshen Carbon Material Company,China.D-glucose (AR),nitric acid(AR),and methanol(AR)were supplied by Tianjin Damao Fine Chemical Incorporated Company,China. RuCl3(37%(w))was provided by Guiyan Rare Metal Material Company,China.H2with purity of 99.99%was provided by Zhengzhou Gas Company.
2.2 Modification of AC
The typical procedure for the treatment of the original AC (size 0.25-0.38 mm)was as follows:
(i)For scCH3OH treated sample(marked as AC-S):Firstly, 56 mL CH3OH was added into a stainless steel autoclave of 150 mL.Secondly,2.0 g AC was placed in a stainless-steel cage fixed at the upper part of the autoclave.The autoclave temperature was adjusted up to 573 K and maintained for 8.0 h.Then,the autoclave was cooled to below 353 K and methanol gas was vented.Finally,the activated carbon was removed and dried at 373 K for 6.0 h.
(ii)For HNO3oxidized sample(marked as AC-H):4.0 g AC sample was oxidized in 30%(w)HNO3solution at 333 K for 4.0 h and washed with distilled water till the pH value of the rinsed solution reached 7.0.The AC-H sample was then dried overnight at 383 K.
(iii)For the sample which was treated by HNO3oxidation and further by scCH3OH(marked as AC-H-S):2.0 g of AC-H sample was modified with the method described in(i).
2.3 Preparation of Ru/AC catalysts
Ru based catalysts were prepared by wet impregnation method.In a typical experiment,0.6 g AC sample was added to 6.0 mL RuCl3solution.The loading amount of Ru is 5%(w)based on metal.After impregnation for 6.0 h,the mixture was then dried at 393 K for 8.0 h.The solid products were reduced using pure H2at 473 K for 2.0 h,and at 573 K for another 1.0 h.
2.4 Characterizations
The surface acidic oxygen containing groups of activated carbon were titrated by the Boehm method.23The surface areas, total pore volume,and pore diameter of AC samples were measured by nitrogen adsorption at 77 K after degassing at 373 K for 2.0 h using a Quantachrome NOVA 1000e analyzer (Quantachrome Instrument Corporation,America).Surface electronic states and surface atom amount of activated carbon were analyzed by X-ray photoelectron spectroscopy(XPS)on a Perkin-Elmer PHI 5300 instrument(Perkin Elmer Corporation, America).Binding energy values were calibrated using the value of contaminant carbon(C 1s,284.6 eV)as a reference.The adsorption capacity of Ru3+on AC was analyzed by inductively coupled plasma(ICP)on an ICAT6000SERIES instrument (Heme Electron Corporation,America).The surface morphology and the particle size of Ru catalysts were determined by transmission electron micrograph(TEM)on a JEM-2100 microscope(Japan Electronics Corportion).
2.5 Activity test
The activity test was performed in a 150 mL stainless autoclave containing 0.3 g of catalyst and 50.0 mL of 50%(w)glucose aqueous solution.The initial H2pressure was 4.0 MPa with a reaction temperature of 393 K and a stirring rate of 1000 r·min-1.The specific activity of the hydrogenation was obtained by recording the decrease of H2pressure with time, which was then converted to the hydrogen uptake rate per gram of Ru(mmol·min-1·g-1)according to the ideal gas equation.In addition,the stirring effect was preliminarily investigated and our experiment proved that the rate was sufficient to eliminate the diffusion limit.
3 Results and discussion
3.1 Effect of treatment methods on surface area and pore structure of AC
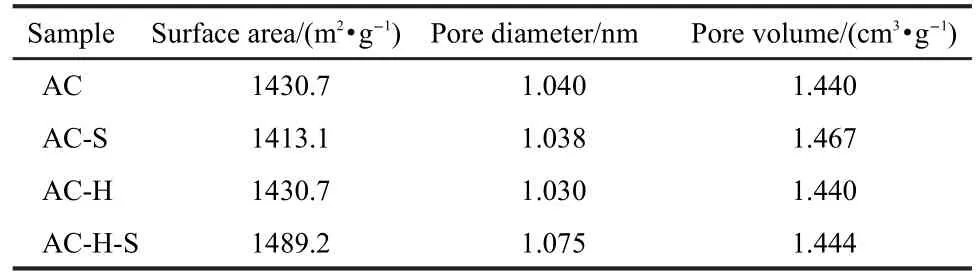
Table 1 Physical parameters of different activated carbons
The pore volume,total surface area,and mean pore diameter of samples are given in Table 1.For AC-S,the surface area dropped while the pore volume increased.It implied that some small pores disappeared while some new large pores formed. The pore volume,total surface area,and mean pore diameter of AC-H is almost unchanged compared with AC.However, these parameters increase for AC-H-S which was treated with HNO3and scCH3OH.The increase in pore volume after scCH3OH treatment can be explained that scCH3OH has a powerful extraction ability and good chemical reaction property. Because the experimental material was coconut AC,AC surely contains some organic ingredients.Thus the extraction process must take place in scCH3OH.Some organic ingredients on the outer-layers ofAC were leached.
3.2 Surface acid sites
The surface of AC showing acidic properties is due to the presence of carboxyl groups(also in the form of their cyclic anhydrides),lactones or lactols,and phenolic hydroxyl groups. These groups differ in their acidities and can be differentiated by neutralization with solutions of NaHCO3,Na2CO3,and NaOH,respectively.Table 2 shows the amount of the surface acidic groups of each sample.It can be seen that scCH3OH treatment dramatically decreases the amount of surface acidic groups on AC due to scCH3OH extraction and chemical reaction with the surface acidic oxygen containing groups.To further prove the scCH3OH extraction and reaction,the chemical composition of methanol mother solution was analyzed by GC-MS 7890A5975C(AgiLent America).The analytic results showed that the methanol mother solution contained certain amount of organic chemical compositions such as dimethyl ether and methyl propionate compared to pure methanol.
Nitric acid oxidation treatment significantly increased the content of acidic surface groups,as reported in literature.9For Table 2,it can be seen that the total amount of acid sites and the amount of carboxylic group increase evidently.It is proposed that liquid oxidation by HNO3leads to the formation ofoxygen containing groups.

Table 2 Amount of surface acidic groups onAC samples
The sample AC-H-S,which was treated by HNO3oxidation and further by scCH3OH,has much lower acidic surface group content compared to AC-H sample.The results further prove that the scCH3OH treatment would result in a decrease in acidic surface group content ofAC.
3.3 XPS characterization of activated carbon
XPS is a surface technique which can provide information of the chemical composition of the few uppermost layers of the material.Table 3 gives the XPS results of the four samples.It can be seen that the AC-S has the lowest oxygen content,while AC-H shows an increase in the oxygen content.
In order to further investigate the nature of the functional groups on the surface,deconvolution of the O 1s peak was performed using a sum of Lorentzian-Gaussian functions.2Fig.1 and Table 4 give the results obtained by the XPS deconvolution of the O 1s peak.The deconvolution of the O 1s peak gives additional information of the nature of the surface oxygen containing groups.The functionalities shown in Fig.1 is attributed to carbonyl group(peak a,531.1 eV),phenol(peak b, 532.3 eV),pyrone(peak c,533.4 eV),and carboxylic group(peak d,534.2 eV).24-26The amount of surface oxygen containing groups was calculated from the corresponding peak areas. As shown in Table 4,scCH3OH treatment can decrease the content of all the oxygen-containing acidic groups,especially the carbonyl and phenol groups.The nitric acid oxidized sample (AC-H)has the highest surface oxygen containing groups,especially carboxylic group.The XPS results are consistent with the Bohem titration results discussed above.

Table 3 XPS results of activated carbon
3.4 Adsorptive capacity test
In order to study the effects of the oxygen containing groups on the adsorption capacity of AC samples,the adsorptive capacities of Ru3+on the AC support samples were examined(Table 5).The results showed that AC-H sample had the highest adsorptive capacity.The previous experiment(XPS and Bohem titration)indicated that nitric acidic oxidization greatly increased the oxygen containing acidic groups on AC.The increase of the surface oxygen containing groups,which are anchoring sites for metallic precursors,could enhance the adsorption of Ru3+.Although the AC-S sample had lower surface oxygen containing groups than original AC sample,the two sam-ples had almost the same adsorptive capacity.Coloma et al.6reported that the decomposition-reduction of the oxygen containing surface groups formed active centers.It is possible that the scCH3OH extraction and chemical reaction with the surface acidic oxygen containing groups could lead to generate new active centers which are anchoring sites for metallic precursors.

Table 4 O 1s peak deconvolution analysis results of the activated carbons

Fig.1 O 1s XPS spectra and deconvolution results

Table 5 Ru3+adsorptive capacity onAC samples
3.5 TEM characterization of Ru catalysts
In order to investigate the effect of the oxygen containing groups of AC on the particle size distribution of ruthenium on AC supports,the morphology of these supported Ru catalysts was characterized by TEM.It can be seen from Fig.2 that the particle size of Ru varies in the order of AC-H>AC-H-S>AC>AC-S.The AC-S sample exhibits uniformly dispersed metal particles with a particle size of 1.2-3.7 nm.And the AC-H sample has the largest particle size(2.6-25.8 nm)with the broadest particle size distribution.The surface oxygen containing groups are the anchoring centers for the ruthenium precursor.It is reasonable that scCH3OH treatment increases ruthenium dispersion because some surface acidic oxygen containing groups of activated carbon are extracted or reacted,which reduces the gathering chance of ruthenium.On the contrary, HNO3oxidization greatly increases the oxygen containing acidic groups,and enhances the adsorption of Ru3+.However,some of those groups have a limited thermal stability.The decomposition of the less stable oxygen containing surface groups could result in metal gathering on the surface of supporter;thus the final dispersion of the metal phase drops.Similar results have also reported in literature,6which reported that metal dispersion was highly dependent on the degree of carbon support oxidation,being lower for the catalyst support containing higher amount of surface acidic groups.
3.6 XPS characterization of catalysts
Fig.3 presents the XPS spectra of the selected Ru/AC and Ru/AC-S catalysts.Three binding peaks(binding energy)contribute with peaks located at Ru 3d5/2(280.6 eV),Ru 3d3/2(284.4 eV),27and Ru 3p3/2(462.8 eV)in two samples.Two samples had higher Ru binding energy than pure Ru.27It is proposed that there is strong electron interaction between Ru and activated carbon supports.Furthermore,sample AC-S has higher Ru binding energy than sample AC.The result proves that the supercritical methanol treatment increases the electron interaction.This electron interaction between Ru and AC support results in Ru lacking electron,and this helps catalyst to chemisorb hydrogen and increase the activity which will be proved latter.
3.7 Catalytic activity test

Fig.2 TEM images of the Ru based catalysts

Fig.3 Ru XPS profiles of the Ru/AC and Ru/AC-S catalysts

Fig.4 Activity of commercial Ru and Ru catalysts on differentAC supports
The catalytic behaviors for the liquid phase hydrogenation of D-glucose are summarized in Fig.4.It can be seen that the activity of Ru catalysts follows the order of Ru/AC-S>Ru/AC>Ru/AC-H-S>Ru/AC-H,which is same as the order of the dispersion of Ru.The Ru/AC-S catalyst shows the highest activity,which is 1.56 times as large as that of Ru/AC catalyst.And the activity of the commercial Ru/C(Engelhand)catalyst has also been tested.The activity of Ru/C catalysts was compared between the import commercial Ru/C and home-made catalysts as shown in Fig.4.The Ru/AC-S catalyst has higher activity.
The activity result shows that scCH3OH treatment can increase the activity,whereas nitric acidic oxidization greatly decreases the activity.It has been proposed that the extraction and some chemical reaction may take place on the surface of AC in scCH3OH,and therefore result in the leaching of some oxygen containing groups.This is confirmed by previous Bohem titration,GC-MS,and XPS results.Coloma et al.6reported that the oxygen surface groups on the support had a high influence during the catalyst preparation step,acting as anchoring centers for the metal precursor.However,some of oxygen containing groups has low thermal stability at the reduction of the metal precursor.The decomposition of the less stable surface groups could result in the gathering of metal on the surface of supporter.The treatment by scCH3OH can decrease the density of surface oxygen containing groups of activated carbon,decrease metal gathering and finally increase the dispersion of ruthenium as confirmed by TEM.The higher dispersion of metal could increase the electron interaction between ruthenium composition and AC support as confirmed by XPS. And the electron interaction results in the lack of electron on Ru,benefiting the chemisorption of hydrogen which increases the activity of catalysts.Nitric acid oxidation treatment increases the density of surface oxygen containing acidic groups of activated carbon,but some of the oxygen containing groups have a limited thermal stability at the reduction of the metal precursor,which results in the gathering of metal and the decrease of activity.It can be concluded that the surface acidic oxygen containing groups of AC is not beneficial for Ru/AC catalysts in our experimental conditions.This is consistent with reference28which reported that HNO3treated activated carbon could increase the content surface acidic oxygen containing groups of activated carbon,lead to lower Ru dispersion and decrease the activity of Ru/AC in ammonia synthesis.
4 Conclusions
The activated carbon was modified by scCH3OH treatment, HNO3oxidation,and HNO3oxidation combined with scCH3OH treatment,respectively.The effect of modification methods on the structure parameters of AC including surface area,pore size,and pore volume is not obvious.Modification by scCH3OH could greatly decrease the density of surface acidic groups,especially carboxylic group,owing to scCH3OH extraction and chemical reaction.HNO3oxidation leads to the formation of surface oxygen containing groups.The surface oxygen containing groups are beneficial for the adsorption of Ru3+. But surface oxygen containing groups will also results in the gathering of metal on the surface of AC.Modification by scCH3OH leads to a better dispersion of Ru,enhances interaction force between activated carbon and Ru.Thus the activity of the catalyst is obviously increased.The maximum reaction rate for hydrogenation of glucose is 94.23 mmol·min-1·g-1and 1.56 times as large as the original AC.The Ru/AC-S catalyst has higher activity than the commercial Ru/C catalyst.
(1) Rodriguez,R.F.Carbon 1998,36,159.
(2) Figueiredo,J.L.;Pereira,M.F.R.;Freitas,M.M.A.;Orfao,J. J.M.Carbon 1999,37,1379.
(3) Radovic,L.R.;Rodriguez,R.F.Carbon Materials in Catalysis. In Chemistry and Physics of Carbon,Vol.25;Thrower,P.A. Ed.;Dekker:New York,1997;pp 243-358.
(4) Auer,E.;Freund,A.;Pietsch,J.;Tacke,T.Appl.Catal.A 1998, 173,259.
(5) Hagen,S.;Barfod,R.;Fehrmann,R.;Jacobsen,C.J.H.; Teunissen,H.T.;Chorkendorff,I.B.J.Catal.2003,214,327.
(6) Coloma,F.;Sepulveda-Escribano,A.;Fierro,J.L.G.; Rodriguez-Reinoso,F.Appl.Catal.A 1997,150,165.
(7)Aksoylu,A.E.;Madalena,M.;Freitas,M.A.;Fernando,M.; Pereira,R.;Figueiredo,J.L.Carbon 2001,39,175.
(8)Aksoylu,A.E.;Freitas,M.A.;Figueiredo,J.L.Appl.Catal.A 2000,192,29.
(9) Song,W.;Li,Y.;Guo,X.H.;Li,J.;Huang,X.M.;Shen,W.J. J.Mol.Catal.A 2010,328,53.
(10) Zheng,X.L.;Zhang,S.;Xu,J.X.;Wei,K.M.Carbon 2002,40, 2597.
(11) Rasheed,A.;Howe,J.Y.;Dadmun,M.D.;Britt,P.F.Carbon 2007,45,1072.
(12) Kowalczyk,Z.;Sentek,J.;Jodzis,S.;Mizera,E.;Goralski,J.; Paryjczak,T.;Diduszko,R.Catal.Lett.1997,45,65.
(13) Han,W.F.;Liu,H.Z.;Zhu,H.Catal.Commun.2007,8,351.
(14) Takaoka,M.;Yokokawa,H.;Takeda,N.Appl.Catal.B 2007, 74,179.
(15) Ding,L.X.;Wang,S.R.;Zheng,X.L.;Chen,Y.;Lu,T.H.; Cao,D.X.;Tang,Y.W.Acta Phys.-Chim.Sin.2010,26,1311. [丁良鑫,王士瑞,郑小龙,陈 煜,陆天虹,曹殿学,唐亚文.物理化学学报,2010,26,1311.]
(16) Erkey,C.J.Supercritical Fluids 2009,47,517.
(17) Jiao,J.X.;Xu,Q.;Li,L.M.J.Colloid Interface Sci.2007,316, 596.
(18)Kim,J.;Kelly,M.J.;Lamb,H.H.;Roberts,G.W.;Kiserow,D. J.J.Phys.Chem.C 2008,112,10446.
(19) Hoffer,B.W.;Crezee,E.;Mooijman,P.R.M.;Langeveld,A. D.;Kapteijn,F.;Moulijn,J.A.Catal.Today 2003,79-80,35.
(20) Gorp,K.V.;Boerman,E.;Cavenaghi,C.V.;Berben,P.H. Catal.Today 1999,52,349.
(21) Pierre,G.;Nathalie,N.;Flèche,G.;Fuertes,P.;Perrard,A. J.Catal.1998,180,51.
(22) Kusserow,B.;Schimpf,S.;Claus,P.Adv.Synth.Catal.2003, 345,289.
(23) Boehm,H.P.Carbon 1994,32,759.
(24) Mangun,C.L.;Benak,K.R.;Economy,J.;Foster,K.L.Carbon 2001,39,1809.
(25) Boehm,H.P.Carbon 2002,40,145.
(26) Lucas,A.D.;Valverde,J.L.;Canizares,P.;Rodriguez,L.Appl. Catal.A 1998,172,165.
(27)Sun,Z.Y.;Liu,Z.M.;Han,B.X.;Miao,S.H.;Miao,Z.J.;An, G.M.J.Colloid Interface Sci.2006,304,323.
(28) Han,W.F.;Zhao,B.;Huo,C.;Liu,H.Z.Chin.J.Catal.2004, 25,194.[韩文锋,赵 波,霍 超,刘化章.催化学报,2004, 25,194.]
September 14,2011;Revised:November 15,2011;Published on Web:November 18,2011.
Hydrogenation of Glucose Using Ru/Activated Carbon Catalysts: Effects of Modification Methods on Surface Properties of Activated Carbon
XU San-Kui1,2LI Li-Min1,*GUO Nan-Nan1SU Yun-Lai1ZHANGPeng1
(1Department of Chemistry,Zhengzhou University,Zhengzhou 450001,P.R.China;2College of Material Science and Engineering,Henan University of Technology,Zhengzhou 450001,P.R.China)
Activated carbon(AC)was modified by supercritical methanol(scCH3OH)treatment,HNO3oxidation,and HNO3oxidation in combination with scCH3OH treatment.The pristine and modified AC samples were characterized by N2physisorption,Boehm titration,X-ray photoelectron spectroscopy(XPS), inductivelycoupled plasmaatomicemissionspectroscopy(ICP-AES),andtransmissionelectron microscopy(TEM).These modifications did not significantly change the surface area and the pore size distribution of the AC.ScCH3OH treatment decreased the density of surface acidic groups,especially carboxylic groups.However,HNO3oxidation increased the density of surface acidic groups.ICP analysis revealed that the scCH3OH modified sample had a similar adsorptive capacity for ruthenium as the original AC,while the AC oxidized with HNO3had the highest adsorptive capacity of all samples tested.Ru/AC catalysts were prepared with RuCl3solution impregnation on the four aforementioned AC supports.The as-prepared catalysts were characterized by TEM,XPS and examined for their effectiveness in D-glucose hydrogenation as well.The modifications drastically affected the properties of the activated carbons and the catalysts loaded on them.The dispersion of ruthenium after impregnation was highly dependent on the density of surface acidic groups.The AC sample treated by scCH3OH,which contained a lower amount of surface acidic complexes,showed the highest dispersion of ruthenium.The XPS results showed that the scCH3OH modification enhanced the interaction between AC and Ru.The Ru/AC-scCH3OH catalyst showed the highest activity for hydrogenation of D-glucose;producing a reaction rate 1.56 times higher than that produced by Ru/AC.
Surface modification;Activated carbon;Ruthenium catalyst;Surface oxygen containing group;Hydrogenation
10.3866/PKU.WHXB201111181www.whxb.pku.edu.cn
*Corresponding author.Email:lilm@zzu.edu.cn;Tel:+86-371-67781064.
The project was supported by the National Natural Science Foundation of China(50955010).
国家自然科学基金(50955010)资助项目
O643

