小分子有机硅单体N-(3-三甲氧基硅基乙基)乙二胺的有效蓝色发光
许邹明 王玉霞 戴 鹏 孔维权
(中国科学院能量转换材料重点实验室,中国科学技术大学材料科学与工程系,合肥230026)
小分子有机硅单体N-(3-三甲氧基硅基乙基)乙二胺的有效蓝色发光
许邹明 王玉霞*戴 鹏 孔维权
(中国科学院能量转换材料重点实验室,中国科学技术大学材料科学与工程系,合肥230026)
详细研究了N-(3-三甲氧基硅基乙基)乙二胺(TMSEEDA)的电子结构、光物理性质以及热稳定性.通过密度泛函理论计算获得了基态和第一激发态的结构参数.计算结果还表明在Si-O骨架内存在离域电子,这导致了长波长的吸收.在270 nm紫外光的激发下,溶液和固态均能观察到一个宽且强的蓝色发射,其峰位于430 nm,固态的强度高出纯物质5倍左右.在乙醇溶液中,发光强度随着TMSEEDA浓度的增大而增加,并在纯物质时达到最大值.这些结果说明TMSEEDA不存在浓度淬灭效应.我们提出了在Si-O骨架内电子离域和d-p π键模型来解释长波长吸收和蓝色发射.
光致发光;密度泛函理论;d-p π键模型;淬灭;机制
1 Introduction

Scheme 1 Molecular structure of the N-(3-trimethoxysilylethyl) ethylenediamine
π-Conjugated organic materials,especially those containing heavier main group-14 elements,1-5play a key role in photonics because of their exceptional luminescence features and thermal stability,attractively with respect to their applications in organic light-emitting diodes(OLEDs),6-8organic solid-state lasers,9organic fluorescent sensor,10and organic photovoltaic cells (OPVs).11In addition to the extensive applications as well as luminescence features,it is also considerably attractive for desirable intrinsic properties of solution-processable small molecules,such as low cost,reproducible and scalable synthesis, tunable structural and electronic properties,ease of processing in device fabrication and high molecular purity.12Hence,one of the current subjects to emerge in this field is the design and development of novel small molecules that emit visible light with high efficiency at high concentration or in solid state(the state usually for device).Most molecules,however,have highly efficient emission in the dilute solution but none or only faint emission in solid state owing to the concentration quenching caused by intermolecular interactions such as excimer formation and energy transfer in the condensed state.13,14
Extensive research on the development of new organic molecules with high luminescence efficiency has been carried out with the increasing request to fabricate the organic optoelectronic devices.For example,Smith et al.15has reported that mesityl-substituted poly(p-phenylenevinylenes)(PPVs)as well as small molecule models featuring mesityl groups were highly luminescent at high concentration even in solid films.Recently,Shimizu and Hiyama14have reviewed the recent studies on highly fluorescent small organic molecules,such as N,B,C=N,or Si containing molecules and oligothiophenes derivatives, which guides the design of small molecules with efficient photoluminescence(PL)quantum yields in solid state.In many cases,Si containing chromophores not only lead to a reduced π-π*energy gap due to the enhanced σ-π interaction in comparison to their pure C analogues but often red-shift the fluorescence spectra into the visible range.16,17
N-(3-trimethoxysilylethyl)ethylenediamine(TMSEEDA),which is extensively used as the silane coupling agent,consists of ethylenediamine and trimethoxysilane units linked by an ethyl group(Scheme 1).It has recently been shown that density functional theory(DFT)is hardly expensive and can provide accurate results on systems such as organic molecules.18,19And the time-dependent DFT(TD-DFT)method is demonstrated to be an useful and reliable tool to discuss the excited states of organic molecules.20-22In this article,we use UV-Vis absorption and photoluminescence(PL)spectra in combination with DFT/ TD-DFT methods to investigate the optical properties and electronic structure characteristic of TMSEEDA,trying to elucidate the origin of absorption and PL spectra,which is expected to shed light on the design of OLED.The thermal decomposition features are also studied by thermogravimetric(TG)techniques atthetemperaturerangefromroomtemperatureto800°C.
2 Experimental
2.1 Materials
TMSEEDA was purchased from Meryer Chemical Technology Co.(99%,Shenzhen,China).All other reagents were of analytic grade and received from Shanghai Chemical Reagent Factory and used as received without purification.
2.2 Spectroscopic measurement
UV-Vis absorption spectra were recorded on UV-365 UV-Vis spectrophotometer(Shimadzu,Japan)and unless otherwise stated all spectra were recorded as a solution in anhydrous ethanol.The steady-state fluorescence spectra were measured using FluoRoLOG-3-TAU(Jobin Yvon,France)fluorescence spectrometer following excitation by a xenon lamp at 270 nm.The thermal properties of NSTMEE were detected by differential scanning calorimetry using an SDT Q600 TG analyzer(TA Instruments-Waters LLC,America),which was carried out at a heating rate of 10°C·min-1from room temperature to 800°C under nitrogen atmosphere.The nitrogen flow during the experiments was 100 mL·min-1.
3 Theoretical calculation
The ground state geometries were fully optimized without any symmetry constraint using Beckeʹs three-parameter hybrid exchange functional with the Lee-Yang-Parr correlation functional(B3LYP)method23,24along with the 6-311G(d,p)basis set on all atoms,25which were employed in consideration of both accuracy and efficiency.Harmonic frequency calculation was performed for the optimized structure to establish that the stationary point was local minimum on the corresponding potential energy surfaces.And the TD-DFT was applied to optimize the first singlet excited state(S1)with the 6-311+G(d,p) basis set and B3LYP method.The absorption and emission spectra were carried out using TD-DFT/6-311+G(d,p)method based on the optimized ground state structure and the lowest singlet excited state structure,respectively.The continuous absorption and emission spectra were simulated with the help of GaussView 5.0.8 software with the width at half-height of 2500 cm-1on the basis of the calculated vertical excited energy and their corresponding oscillator strengths.
All of these calculations were performed with the Gaussian 03 package of programs26on a personal computer.
4 Results and discussion
4.1 Geometrical and electronic structures of TMSEEDA
Simply,the TMSEEDA molecule without any mesityl groups is linear structure and composed of ethylenediamine and Si-O moieties,as shown in Scheme 1.Obviously,the silicon atom possesses a tetrahedral coordination environment and is bonded to three oxygen atoms and a carbon atom.

Fig.1 Optimized molecular structures with atom numberings of TMSEEDAin the ground state(a)and the first singlet excited state(b)obtained at the B3LYP/6-311G(d,p)and 6-311+G(d,p) levels,respectively
As we know,the optical and electronic properties of organic materials are closely related to the molecular geometrical and electronic structures.Thus,it is important and necessary to optimize the geometrical structure of isolated TMSEEDAby energy minimization under the level of DFT B3LYP,in conjunction with the 6-311G(d,p)basis set.Fig.1(a)shows the chemical and optimized structure of the title molecule at ground state (S0)with numbering of the atoms.The corresponding selected geometry parameters containing bond lengths,bond angles, and torsion angles are listed in Table 1,in accordance with the atom numbering scheme given in Fig.1.As can be seen from the Table 1,an unusual finding is that the atoms N22,C4,C1, Si33,and O8 are practically coplanar with the dihedral angles N22C4C1Si33 and C4C1Si33O8 being-176.8°and 176.9°,respectively.This better planarity can remarkably increase the conjugation degree.The Si-O skeleton,however,is non-coplanar with dihedral angle of-34.0°.In addition,the Si-O bond lengths are approximately 0.164 nm.This valve is very close to that in siloxane derivatives,27where the interaction between the lone pairs on the oxygen atom and the vacant d-type polarization function on the silicon atoms is well-known but disputed.
When going from the ground state S0to the first singlet excited state S1,there are some variations(Table 1).The structure of excited monomer of TMSEEDA by TDDFT/6-311+G(d,p)is displayed in Fig.1(b).The bonds Si-O and Si-C are elongated,whereas bonds C-O are shortened.It is obvious that the excited structure has a strong coplanar,that is,the conjugation is better in the excited structure.For Si-O skeleton,the dihedral angles are no larger than 4°in the excited state.
Immediately,It is noticeable that lots of effects,such as hyperconjugation interaction,28bond iconicity,292pπ-3pπ overlap,30and d-p π-bonding,31-33have been invoked to explain the variation of the Si-X(X=O or N)bond involved in organosilicon compounds.A cursory survey of the most relevant structural aspects prompts that a most possible dative d-p π conjugation, by the formation of the three Si—O groups involved in the TMSEEDA,could exist,which is very similar to the d-p π conjugate in PO3-
4species.Generally,it is found that d-p π conjugate effect often occurs in some cluster compounds,34which can induce a more stable system with the lone pairs of O atoms wandering into the 3d orbitals on Si atom.For the title molecule,TMSEEDA,a simple molecular orbital(MO)modeling of Si—O provides a reliable description of the possible formation process of the delocalized π bond,as illustrated in Fig.2. Silicon atom appears to be of sp3-type hybrid and bonds with the atoms C1,O7,O8,and O9 leading to four σ bonding orbitals.However,d spatial orbital in Si atom can have positive and covalent overlap with the lone pairs occupied in p orbitals provided by the three adjacent O atoms,giving rise to a delocalized π bonding orbital.Thus,π-delocalization is constructed bythe overlap between the empty Si 3d and O 2pzorbitals.The effectiveness of the covalent overlap,to some degree,brings about lower energy and more stable conformer.In addition,it is not necessary that all the four atoms of the delocalized d-p π-bonding have a two-dimensional coplanar structure due to the largeness and diffusion for silicon empty 3d orbital,which can facilitate overlap with the p orbital side by side in multi-direction.
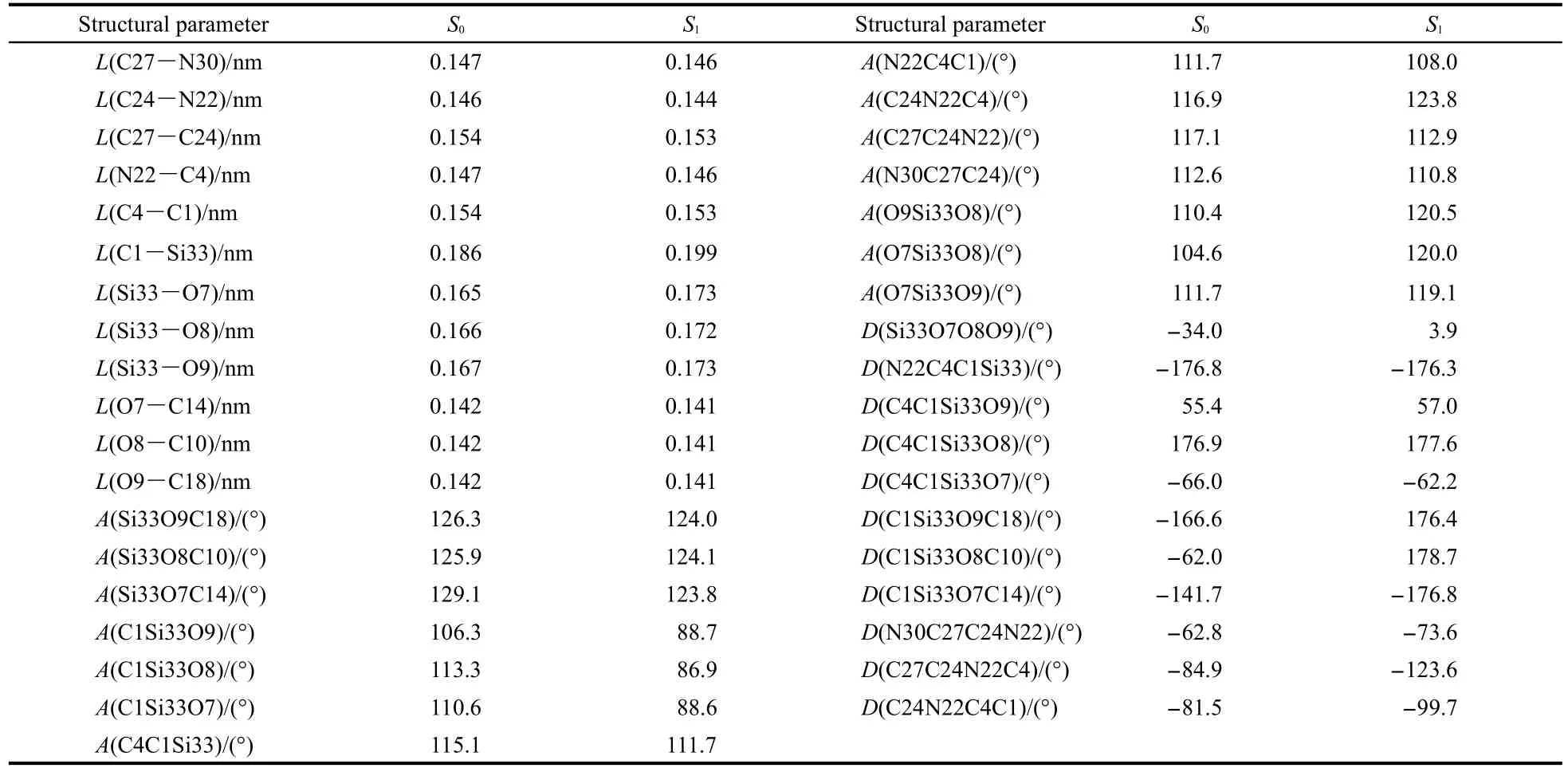
Table 1 Selected optimized geometrical parameters obtained for TMSEEDA

Fig.2 Schematic representation of the interactions between d orbital of silicon and p orbital of oxygen in Si-O skeleton
Furthermore,to clarify the bonding properties of Si-O and scrutinize the electron density distribution between orbitals,a natural bond orbital(NBO)analysis was performed using the DFT/B3LYP wave functions in combination with 6-311G(d,p) basis set.The results are shown in Table 2.As can be seen,it is found that oxygen atoms have considerable participation at the Si-O bond(always approximately 90%)and silicon atom has 2.18%,2.30%,2.28%d character in Si33-O7,Si33-O8, Si33-O9 bonds,respectively,meaning that the contribution of Si 3d orbitals to Si-O bonds cannot be ignored,which is rather consistent with character of d-p π bonding.
4.2 Electronic and photophysical properties
Fundamental photophysical properties of the title molecule are also investigated.Fig.3 shows the experimental and simulated UV-Vis absorption spectra for TMSEEDA in ethanol solution and gas state,respectively.As can be clearly seen,in solution the most intriguing feature is relatively a strong absorption band occurs at 242 nm in addition to a weak and broad absorption band at 341 nm.No doubt that the long wavelength absorp-tion band is highly related to the d-p π-bonding within Si-O skeleton.The former is assigned to spin allowed π-π*electronic transition on Si-O skeleton and the latter is assigned to n-π*transition which most probably originates not only from the promotion of one electron of lone pairs of O to the π*orbital that is delocalized in Si-O skeleton but also from the large electron donating capacity of the nitrogen-containing group,-NH or-NH2.

Table 2 Natural bond orbital analysis for bond characters of TMSEEDAcalculated with B3LYP/6-311G(d,p)level

Fig.3 Experimental(ethanol solution)and simulated(gas state) UV-Vis absorption spectra of TMSEEDAGaussian broadening with half-band width 2500 cm-1for the simulated spectrum
We compute singlet-singlet electronic transition based on the optimized geometries of the ground state using TD-DFT method at the B3LYP/6-311+G(d,p)level in order to gain a detailed insight into the nature of the UV-Vis absorption observed experimentally.The simulated absorption spectrum is superimposed in Fig.3 and adequately scaled for comparison with the experimental one.The first peak position and shape are reproduced well by the TD-DFT(B3LYP)simulation.However,the second peak with a smaller oscillator strength misses from the 300-400 nm region which is caused by the n-π*transition.This failure is presumably due to inherent limitations of the B3LYP.35Another reason may be due to the optically forbidden excited state for n-π*transition.
In addition to the absorption spectra,the emission spectra, measured at room temperature,of TMSEEDA in its original and in the solid state are displayed in Fig.4.The spectral shape and peak position(432 nm)of the emission spectra in the solid state(Fig.4(b))are almost identical to the one in the solution (Fig.4(a)),but slightly blue-shifted(4 nm).In addition,it is found that a quantitative comparison of PL intensity of the condensed state to the solution phase yields over 5 times,indicating the absence of luminescence quenching when aggregated into solid state.Similar phenomenon has also been observed in many organic compounds characteristic of“aggregation induced emission”.36The inset in Fig.4 shows the concentration dependence of PL intensity for the same peak position at 428 nm,which is carried out varying volume fraction from 10%to 100%pure TMSEEDAin ethanol solution.
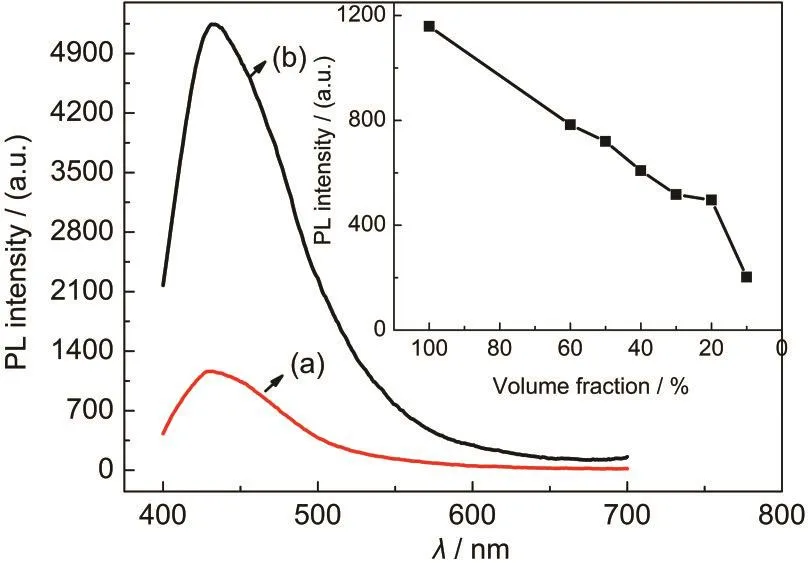
Fig.4 PLspectra of TMSEEDA(a)the original;(b)the solid state.Inset:concentration dependence of PL intensity in ethanol solutions with the varying volume fraction of TMSEEDA; excitation wavelength:270 nm
The most interesting feature of the PL spectra is that PL intensity increases as increasing content of the title molecule in ethanol solution and reaches the maximum for the pure chromophore.A pronounced difference is noted upon comparison with other organic molecules in this regard,as PL quenching will usually arise when the concentration of organic molecule in solution reaches at some point,where they may aggregate to form less emissive excimers or exciplexes resulting from interor intra-molecule interaction such as hydrogen bonding and π-π stacking.Hence,this suggests that for the TMSEEDA solution in high concentration or in condensed state,there might be lack of formation of excimer and weak intermolecular interaction of excited molecules with the surrounding molecules.It is known that various factors,such as chemical combination, steric hindrance,and weakness of interaction,may inhibit the excimer formation.37Both of the huge steric hindrance of Si-O-C skeletons and alkyl groups as well as non-planar and twisted structure functioning as“insulating”the molecule played the key role in this regard.
Fig.5 shows the normalized fluorescence spectra for the same volume fraction(10%)TMSEEDA in selected solvents: toluene,chloroform,methanol,ethylene glycol.With the increasing polarity of the medium surrounding TMSEEDA,emission maxima exhibit slightly red-shift,from 425 nm for toluene to 445 nm for ethylene glycol.This situation commonly occurs in the compounds that present π-π*transition,suggesting π-π*absorption mainly contributes to the high blue emission at about 430 nm.

Fig.5 Normalized PLspectra for 10%TMSEEDAin different solutions under 270 nm excitation at room temperature

Fig.6 Experimental(ethanol solution)and simulated(gas state) emission spectra of TMSEEDAGaussian broadening with half-band width 2500 cm-1for the simulated spectrum
In order to gain insight into the nature of the fluorescence emission,the optimized geometry in the first excited state was used as import data to calculate singlet-singlet electronic transition using TD-DFT method at the B3LYP/6-311+G(d,p)level,yielding the vertical electronic transitions energy of S1→S0. Fig.6 shows the comparison between the calculated emission spectra and the experimental one.It can be seen that the simulated emission spectrum mainly consists with the experimental fluorescence spectrum but exhibits a small(about 35 nm)red shift.And,the experimental data are detected in the ethanol solution,while the computed data are obtained in gas phase by employing a single molecule,which may bring about the bathochromic shift.
4.3 Thermal analysis
To evaluate the thermal stability of the title molecule,the TG and differential thermogravimetry(DTG)analysis curves are also studied and interpreted in Fig.7.They were measured under a nitrogen atmosphere.The degradation process results in DTG curve with several peaks,which indicates the complexity of the degradation.The temperatures at mass losses of 10%, 30%,50%,and maximum decomposition of the samples are 120,195,250,and 600°C,respectively.So,the thermal stability of TMSEEDA is practically not high.In general,the DTG curve exhibits three distinct mass loss stages.The first mass loss stage occurs from room temperature to about 135°C,most possibly resulting from moisture evaporation.38The second mass loss step,around 135-400°C,might start the decomposition of ethylenediamine,three methyl and ethyl groups as the hard segments degradation.This stage is the main degradation for TMSEEDA.The last mass loss step might be related to the formation of silica.
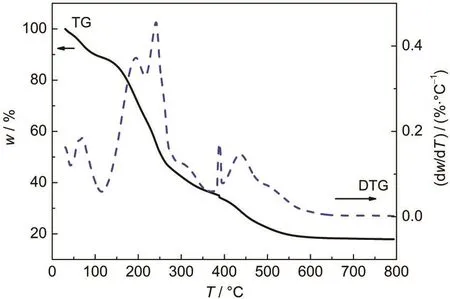
Fig.7 TG and DTG curves of TMSEEDAThe curves were carried out at a heating rate of 10°C·min-1from room temperature to 800°C under nitrogen atmosphere.
5 Conclusions
In summary,the structural,optical,and thermal characteristics of TMSEEDA have been studied using experimental methods combined with the DFT and TD-DFT calculations.Blue emission with its maximum at about 430 nm is observed with 270 nm excitation at room temperature for both the solution and condensed states.The emission spectrum calculated by TD-DFT(B3LYP)is qualitatively coincident with the experimental one.The absorption spectra and the DFT calculation results suggest the most possible presence of π delocalization electrons within Si-O skeletons.The d-p π bonding,stemming from the interaction between n orbital of the oxygen lone pair electrons and the d-type polarization functions of the silicon atoms,rationally explains the fluorescence spectra.However,the thermal stability of TMSEEDA is not very high and it started to decompose when the temperature exceeds 135°C. The method of organic-inorganic hybrid may improve the thermal stability of TMSEEDA.
(1) Ponomarenko,S.A.;Kirchmeyer,S.Conjugated Organosilicon Materials for Organic Electronics and Photonics;Springer-Verlag:Berlin,2011;pp 33-45.
(2)Chen,J.W.;Cao,Y.Macromol.Rapid Commun.2007,28,1714.
(3) Chen,Z.K.;Huang,W.;Wang,L.H.;Kang,E.T.;Chen,B.J.; Lee,C.S.;Lee,S.T.Macromolecules 2000,33,9015.
(4)Kim,H.K.;Ryu,M.K.;Kim,K.D.;Lee,S.M.;Cho,S.W.; Park,J.W.Macromolecules 1998,31,1114.
(5) Kim,K.D.;Park,J.S.;Kim,H.K.;Lee,T.B.;No,K.T. Macromolecules 1998,31,7267.
(6) Adachi,C.;Tsutsui,T.;Saito,S.Appl.Phys.Lett.1990,56,799.
(7) Friend,R.H.;Gymer,R.W.;Holmes,A.B.;Burroughes,J.H.; Marks,R.N.;Taliani,C.;Bradley,D.D.C.;Dos Santos,D.A.; Bredas,J.L.;Logdlund,M.;Salaneck,W.R.Nature 1999,397, 121.
(8) Zhao,Y.L.;Duan,L.;Qiao,J.;Zhang,D.Q.;Wang,L.D.;Qiu, Y.Acta Phys.-Chim.Sin.2010,26,531.[赵云龙,段 炼,乔 娟,张德强,王立铎,邱 勇.物理化学学报,2010,26, 531.]
(9) Samuel,I.D.W.;Turnbull,G.A.Chem.Rev.2007,107,1272.
(10) Sreejith,S.;Divya,K.P.;Ajayaghosh,A.Chem.Commun. 2008,2903.
(11)Hains,A.W.;Liang,Z.Q.;Woodhouse,M.A.;Gregg,B.A. Chem.Rev.2010,110,6689.
(12) Duan,L.A.;Hou,L.D.;Lee,T.W.;Qiao,J.A.;Zhang,D.Q.; Dong,G.F.;Wang,L.D.;Qiu,Y.J.Mater.Chem.2010,20, 6392.
(13) Jakubiak,R.;Collison,C.J.;Wan,W.C.;Rothberg,L.J.;Hsieh, B.R.J.Phys.Chem.A 1999,103,2394.
(14) Shimizu,M.;Hiyama,T.Chem.Asian J.2010,5,1516.
(15) Smith,R.C.;Gleason,L.B.;Protasiewicz,J.D.J.Mater. Chem.2006,16,2445.
(16)Yan,D.C.;Thomson,M.D.;Backer,M.;Bolte,M.;Hahn,R.; Berger,R.;Fann,W.;Roskos,H.G.;Auner,N.Chem.Eur.J. 2009,15,8625.
(17)Yamaguchi,S.;Xu,C.;Yamada,H.;Wakamiya,A. J.Organomet.Chem.2005,690,5365.
(18) Liu,J.N.;Chen,Z.R.;Yuan,S.F.Acta Phys.-Chim.Sin.2005, 21,402.[刘军娜,陈志荣,袁慎峰.物理化学学报,2005,21, 402.]
(19) Zhan,W.S.;Pan,S.;Wang,Q.;Li,H.;Zhang,Y.Acta Phys.-Chim.Sin.2012,28,78. [詹卫伸,潘 石,王 乔,李 宏,张 毅.物理化学学报,2012,28,78.]
(20) Zhao,G.J.;Han,K.L.J.Phys.Chem.A 2007,111,2469.
(21) Ren,Y.L.;Wan,J.;Liu,J.J.;Wan,H.W.Acta Phys.-Chim.Sin. 2004,20,1089.[任彦亮,万 坚,刘俊军,万洪文.物理化学学报,2004,20,1089.]
(22) Song,Z.L.;Zhang,F.S.;Chen,X.Q.;Zhao,F.Q.Acta Phys.-Chim.Sin.2003,19,130.[宋争林,张复实,陈锡侨,赵福群.物理化学学报,2003,19,130.]
(23) Becke,A.D.J.Phys.Chem.1993,98,5648.
(24) Lee,C.;Yang,W.;Parr,R.G.Phys.Rev.B 1988,37,785.
(25) Boronat,M.;Corma,A.Phys.Chem.Phys.Chem.1999,1,537.
(26) Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03, Revision B.02;Gaussian Inc.:Pittsburgh,PA,2003.
(27)Newton,M.D.;Gibbs,G.V.Phys.Chem.Miner.1980,6,221.
(28) Wetzel,D.M.;Brauman,J.I.J.Am.Chem.Soc.1988,110, 8333.
(29)Apeloig,Y.;Karni,M.J.Am.Chem.Soc.1984,106,6676.
(30) Olsson,L.;Ottosson,C.H.;Cremer,D.J.Am.Chem.Soc.1995, 117,7460.
(31) Pauling,L.J.Phys.Chem.1952,56,361.
(32) Jaffe,H.H.J.Phys.Chem.1954,58,185.
(33) Janes,N.;Oldfield,E.J.Am.Chem.Soc.1986,108,5743.
(34) Cheng,W.D.;Guo,G.C.;Huang,J.S.;Lu,J.X.Polyhedron 1995,14,3649.
(35) Furche,F.Annual Reports in Computational Chemistry; Elsevier:Amsterdam,2005;pp 19-30.
(36) Feng,W.K.;Kong,S.;Xiao,L.X.;Wang,S.F.;Gong,Q.H. Acta Phys.-Chim.Sin.2010,26,1929.[冯文科,孔 胜,肖立新,王树峰,龚旗煌.物理化学学报,2010,26,1929.]
(37) Birks,J.B.;Christophorou,L.G.Proc.R.Soc.London Ser.A 1964,277,571.
(38) Jeguirim,M.;Dorge,S.;Trouve,G.Bioresour.Technol.2010, 101,788.
October 26,2011;Revised:January 5,2012;Published on Web:January 13,2012.∗
.Email:wyxm@ustc.edu.cn;Tel:+86-551-3601695.
Efficient Blue Emission from Small-Molecule Organosilicon Monomer N-(3-Trimethoxysilylethyl)ethylenediamine
XU Zou-Ming WANG Yu-Xia*DAI Peng KONG Wei-Quan
(CAS Key Laboratory of Materials for Energy Conversion,Department of Materials Science and Engineering, University of Science and Technology of China,Hefei 230026,P.R.China)
We investigated the electronic structure,photophysical properties,and thermal stability of N-(3-trimethoxysilylethyl)ethylenediamine(TMSEEDA).The optimized structural parameters in the ground state and first excited state were obtained from density functional theory calculations.The results showed that there was probably π electron delocalization within the Si-O skeleton,which induced the long wavelength absorption.A broad and intense blue emission with a maximum at 430 nm was observed for both the solution and the solid state with 270 nm excitation at room temperature.The absorption intensity for the solid state was five-times that of the pure TMSEEDA.For the ethanol solution,the photoluminescence intensityincreasedwithincreasingconcentrationof TMSEEDA andreachedamaximum ata concentration of 100%.These results suggest there is no concentration quenching for TMSEEDA.An accepted model of electron delocalization and d-p π-bonding within the Si-O skeleton was applied to explain the long wavelength absorption and blue emission.
Photoluminescence;Density functional theory;d-p π-bonding model;Quenching; Mechanism
10.3866/PKU.WHXB201201131
O641
The project was supported by the National Natural Science Foundation of China(5067095).
国家自然科学基金(5067095)资助项目

