吉西他滨联合铂类治疗卵巢癌的研究进展
由甲申 贾晓辉
吉西他滨化学名称为 2’-脱氧-2’,2’-盐酸二氟脱氧胞苷(β-异构体),是细胞周期特异性抗代谢类药物,主要作用于DNA合成期(S期)的肿瘤细胞。卵巢癌是女性生殖器官常见的肿瘤之一,发病率仅次于子宫颈癌和子宫体癌而列居第3位,死亡率却占各类妇科肿瘤的首位。目前以铂类为主的一线放化疗存在易复发转移、肿瘤耐药等问题。吉西他滨可以逆转卵巢癌癌肿瘤细胞对铂类的抵抗,与铂类具有协同抗肿瘤作用。二者联用是治疗复发转移间期≥6个月的复发卵巢癌的标准方案之一。另有临床研究显示,吉西他滨与铂类联用可作为有用的卵巢癌一线治疗方案。本文将GEM联合铂类治疗卵巢癌的研究进展综述如下。
1 GEM与卡铂(CBP)联合
2006年Pfisterer的一项Ⅲ期临床研究中,应用含GEM的方案治疗了356例经铂类一线治疗后6个月以上复发的晚期卵巢癌患者。患者随机给予GEM 1000 mg/m2,第1天,第8天,每3周1次,CBP AUC4,第1天,每3周1次;对照组CBP AUC5,第1天,每3周1次,主要研究终点是疾病无进展生存期(PFS)。结果表明GEM+CBP对比CBP单药,中位PFS分别为8.6个月对比 5.8个月(P=0.0031),PFS得到延长。另外ORR分别为47.2%和30.9%(P=0.0016),中位OS分别为18.0个月对比17.3个月[1]。Bookman发表在2006和2009年的Ⅲ期临床研究显示,五种方案的PFS或OS无统计学上的显著差异。这些方案包括GEM/CBP/紫杉醇(PTX)/聚乙二醇脂质体阿霉素(PLD)和拓扑替康(VP-16)。总体研究的中位 PFS为 16.0个月,中位 OS为 44.1个月[2,3]。
Papadimitriou和Kose的Ⅱ期临床研究表明:GEM联合CBP治疗铂类耐药的复发卵巢癌有效率(ORR)为40.5% ~62.5%,中位疾病进展时间(TTP)为9~9.6个月,OS为24.5个月,骨髓抑制、呕吐、神经疾病是与使用GEM联合CBP联合方案的最常见的毒性反应[4,5]。
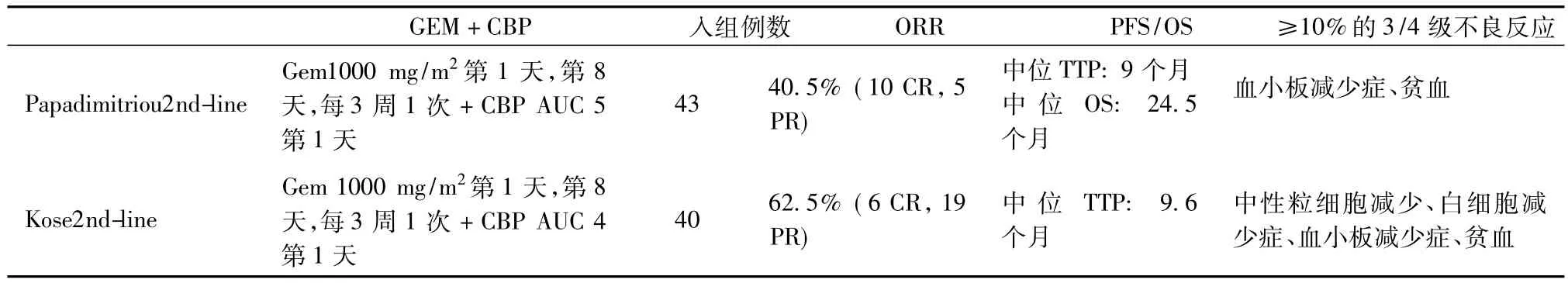
表1 已发表的GEM联合CBP治疗卵巢癌的Ⅱ期临床试验
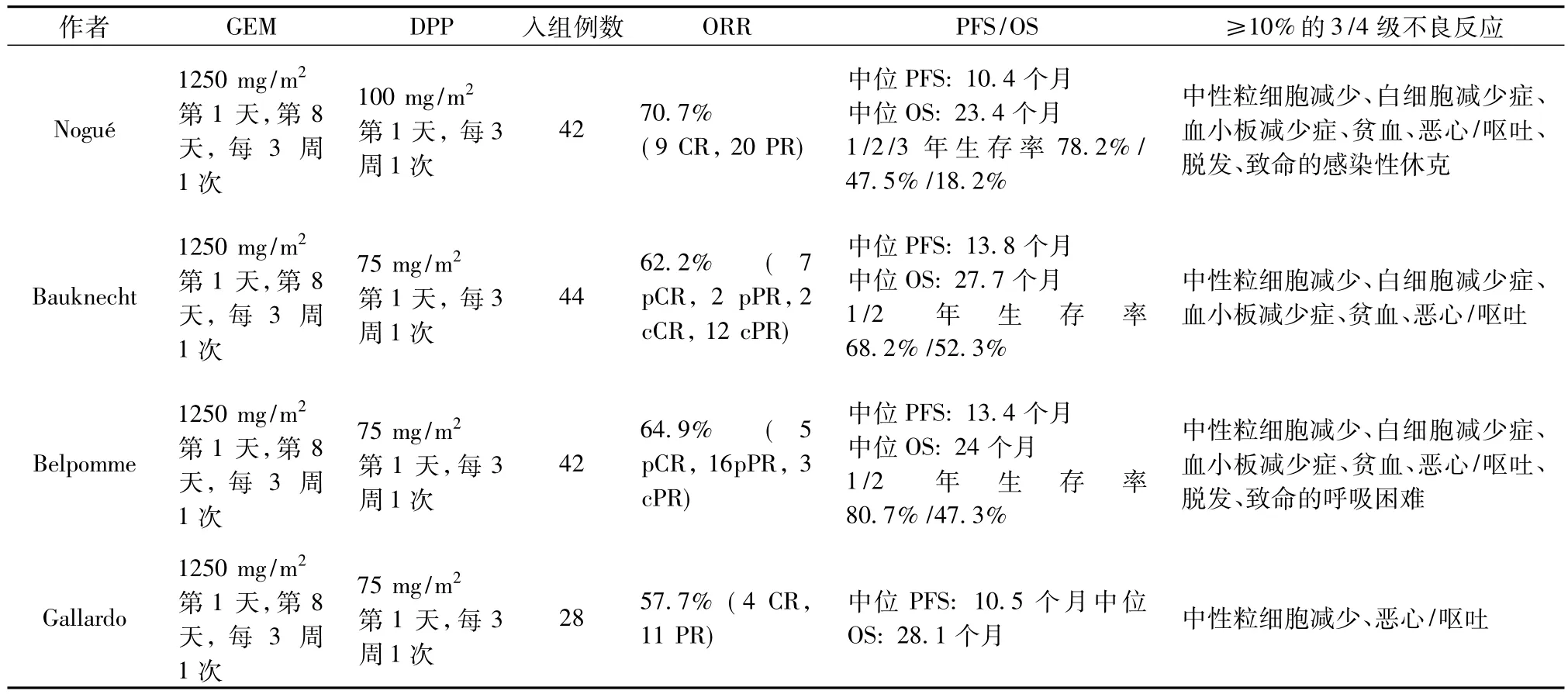
表2 已发表的GEM联合DPP一线治疗卵巢癌的Ⅱ期临床试验
2 GEM与顺铂(DDP)联合
临床研究及资料显示GEM与DDP有协同作用。几项Ⅱ期临床研究显示GEM+DDP一线治疗晚期卵巢癌ORR为57.7% ~70.7%,PFS为10.4~13.8个月,OS为23.4~28.1个月,并且GEM+DDP联合方案≥10%的3/4级不良反应主要为中性粒细胞减少、白细胞减少症、血小板减少症、贫血、恶心/呕吐、脱发[6-9]。
三项Ⅱ期临床试验研究显示GEM+DDP二线治疗晚期卵巢癌ORR为16% ~70%,PFS为4.9~5.97个月,OS为13.2~20.2个月,中性粒细胞减少、白细胞减少症、血小板减少症、贫血、恶心/呕吐为GEM+DDP联合方案≥10%的3/4级不良反应[10-12]。

表3 已发表的GEM联合DPP二线治疗卵巢癌的Ⅱ期临床试验
3 GEM与奥沙利铂(OXA)联合
OXA与DDP或CBP无交叉耐药,更适用于对CBP或DDP耐药的患者。几项Ⅱ期临床研究显示GEM联合OXA二线治疗卵巢癌的反应率为18.7% 到37%,中位TTP为6.1~7.8个月,中位OS为10~17.8个月,骨髓抑制、呕吐、虚弱和神经毒性是GEM联合OXA治疗卵巢癌最常见的毒性反应[13-17]。
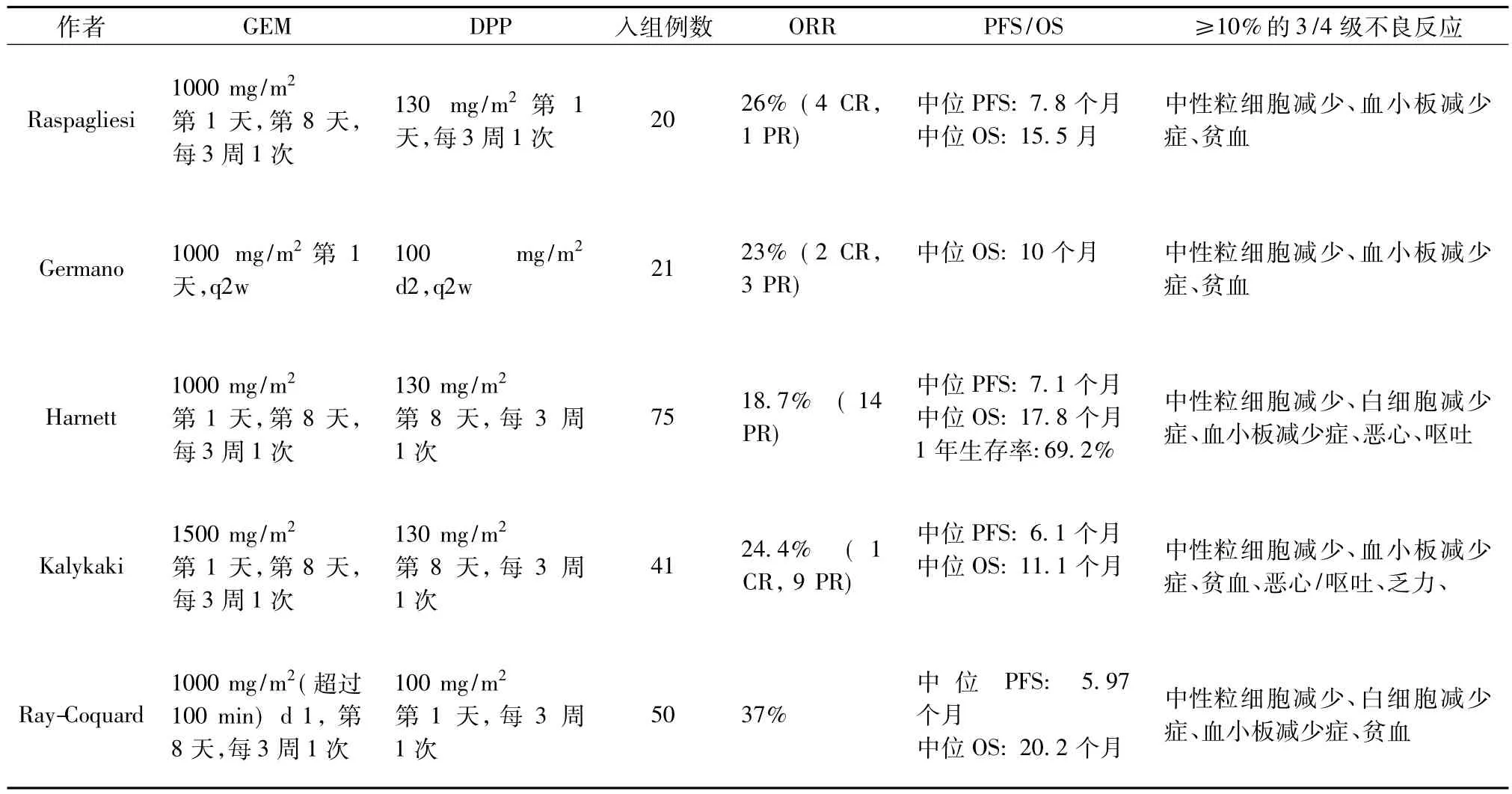
表4 已发表的GEM联合OXA二线治疗卵巢癌的Ⅱ期临床试验
4 小结
GEM联合铂类治疗对铂类敏感或耐药的卵巢癌均有效;GEM+CBP联合疗效最好,生存期最长,可能成为治疗卵巢癌的标准方案;GEM+DDP或OXA具有一定的有效率,可以根据患者不同的情况进行选择。
[1]Pfisterer J,Plante M,Vergote I,et al.Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer:an intergroup trial of the AGO-OVAR,the NCIC CTG,and the EORTC GCG.J Clin Oncol,2006,24(29):4699-4707.
[2]Bookman MA.GOG0182-ICON5:5-arm phase III randomized trial of paclitaxel(P)and carboplatin(C)vs combinations with gemcitabine(G),PEG-liposomal doxorubicin(D),ortopotecan(T)in patients(pts)with advanced-stage epithelial ovarian(EOC)or primary peritoneal(PPC)carcinoma.J Clin Oncol,2006,24(18S):A5002.
[3]Bookman MA,Brady MF,McGuire WP,et al.Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer:a phase III trial of the Gynecologic Cancer Intergroup.J Clin Oncol,2009,27(9):1419-1425.
[4]Papadimitriou CA,Fountzilas G,Aravantinos G,et al.Secondline chemotherapy with gemcitabine and carboplatin in paclitaxelpretreated,platinum-sensitive ovarian cancer patients:a Hellenic Cooperative Oncology Group Study.Gynecol Oncol,2004,92(1):152-159.
[5]Kose MF,Sufliarsky J,Beslija S,et al.A phase II study of gemcitabine plus carboplatin inplatinum-sensitive,recurrent ovarian carcinoma.Gynecol Oncol,2005,96(2):374-380.
[6]Nogué M,Cirera L,Arcusa A,et al.Phase II study of gemcitabine and cisplatin in chemonaive patients with advanced epithelial ovarian cancer.Anticancer Drugs,2002,13(8):839-845.
[7]Bauknecht T,Hefti A,Morack G,et al.Gemcitabine combined with cisplatin as first-linetreatment in patients 60 years or older with epithelial ovarian cancer:a phase II study.Int J Gynecol Cancer,2003,13(2):130-137.
[8]Belpomme D,Krakowski I,Beauduin M,et al.Gemcitabine combined with cisplatin as first-linetreatment in patients with epithelial ovarian cancer:a phase II study.Gynecol Oncol,2003,91(1):32-38.
[9]Gallardo D,Calderillo G,Serrano A,et al.A phase II study of gemcitabine plus cisplatin inpreviously untreated advanced ovarian cancer.Anticancer Res,2006,26(4B):3137-3141.
[10]Nagourney RA,Brewer CA,Radecki S,et al.Phase II trial of gemcitabine plus cisplatin repeating doublet therapy in previously treated,relapsed ovarian cancer patients.Gynecol Oncol,2003,88(1):35-39.
[11]Brewer CA,Blessing JA,Nagourney RA,et al.Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer:a phase II study of the Gynecologic Oncology Group.
[12]Bozas G,Bamias A,Koutsoukou V,et al.Biweekly gemcitabine and cisplatin in platinumresistant/refractory,paclitaxel-pretreated,ovarian and peritoneal carcinoma.Gynecol Oncol,2007,104(3):580-585.Gynecol Oncol,2006,103(2):446-450.
[13]Raspagliesi F,Zanaboni F,Vecchione F,et al.Gemcitabine combined with oxaliplatin(GEMOX)as second-line chemotherapy in patients with advanced ovarian cancer refractory or resistant to platinum and taxane.Oncology,2004,67(5-6):376-381.
[14]Germano D,Bilancia D,Di Nota A,et al.Bi-weekly oxaliplatin(oxa)and gemcitabine(gem)incisplatin pretreated patients with relapsed ovarian cancer(roc):preliminary data of a phase II trial.J Clin Oncol,2004,22(14S):A5104.
[15]Harnett P,Buck M,Beale P,et al.Phase II study of gemcitabine and oxaliplatin in patients withrecurrent ovarian cancer:an Australian and New Zealand Gynaecological Oncology Groupstudy.Int J Gynecol Cancer,2007,17(2):359-366.
[16]Kalykaki A,Papakotoulas P,Tsousis S,et al.Gemcitabine plus oxaliplatin(GEMOX)in pretreated patients with advanced ovarian cancer:a multicenter phase II study of the Hellenic Oncology Research Group(HORG).Anticancer Res,2008,28(1B):495-500.
[17]Ray-Coquard I,Weber B,Cretin J,et al.Gemcitabine-oxaliplatin combination for ovarian cancer resistant to taxane-platinum treatment:a phase II study from the GINECO group.Br J Cancer,2009,100(4):601-607.

