Clogging Process Caused by Organic Particle Accumulation and Biofilm Growth in Subsurface Wastewater Infiltration System
PAN Jing(潘 晶),YU Long(于 龙),WANG Yan(王 艳)
1 College of Chemistry and Life Science,Shenyang Normal University,Shenyang 110034,China
2 Experimental Centre,Shenyang Normal University,Shenyang 110034,China
Introduction
Subsurface wastewater infiltration system (SWIS) is a process suitable for decentralized domestic wastewater treatment in villages,small towns or scattered residential areas.It is an effective way to treat wastewater according to integrated mechanisms of chemical,physical,and biological reactions as it passes through the unsaturated soil in infiltration system[1,2].Compared to theconventionalactivated sludge and biofilm processes,this system has better performance in organic substance and phosphorus removal,lower construction and operational costs,and easier management and maintenance[2-4].
In recent years,there has been increased interest in the SWIS.Many studies have been carried out about the infiltration system,such as construction styles of the SWIS[5],filled soil[6],hydraulic loading rate,nitrogen and phosphorus removal[1,6],substrate clogging[7-9],and eco-environmental response assessment[10].
In infiltration system,clogging would critically obstruct the oxygen transport and the consequences resulted in a significant decline ofthe system's ability to treatwastewater[11,12].Clogging is a complex process and the mechanisms of clogging are not completely clear.At present,the total accumulated matter within infiltration systems which occupy pore volume is considered to be one of the major factors related to the clogging[13,14].Nguyen found that there was a general reduction trend of infiltration rate with the increase of accumulated organic matter(OM)[15].A similar conclusion was also drawn from the study of Caselles-Osorio et al.[16]On the other hand,Platzer and Mauch found there was little association between substrate OM content and infiltration rate yet[17].
Since the accumulated OM in substrate pores is mainly composed ofbiofilm and organic particles withoutbeing biodegraded timely,the respective influence of them on clogging should be paid more attention to.The great contribution of accumulated organic particles to clogging was indubitable,whereas the viewpoint of effects of biofilm on clogging was controversial.Vandevivere and Baveye emphasized the influence of the microorganism accumulation on the surface sealing[18].However,others believed that the biofilm growth had a minor effect on clogging[19-22].As biofilm is essential for SWIS to biodegrade the organic pollutants in wastewater, the understanding of the influence of biofilm on clogging is fairly significant.
In order to get further understanding of the clogging mechanism,the developing process of clogging separately caused by biofilm growth and organic particles accumulation must be investigated.The purposes in this study were:(1)to investigate the different development of clogging processes caused by organic particle accumulation and biofilm growth,(2)to analyze the cause of the different clogging process.
1 Materials and Methods
1.1 Pilot system set up and operation
A schematic representation of the six columns used for the experiment is shown in Fig.1.The column was made from clear plexiglass(120 cm in length and 50 cm internal diameter).Sampling ports were installed at 50 cm(upper layer),80 cm(middle layer),and 110 cm(bottom layer)from the top of the SWIS to test effective porosity,OM content and the biomass of substrate.The 10 cm of deep gravel(10-20 mm,diameter)was prepared at the bottom to support infiltration system and evenly distribute the treated water.Synthetic wastewater was continuously fed into each SWIS from a feed tank through a rubber hose with flow rate control valves and the synthetic influent was daily prepared.The feed tank was placed in a higher position so that influentcould flow automatically into the SWIS.Synthetic wastewater inlet was installed in advance at the depth of 50 cm below the surface.The treated water was collected through collecting pipe at the bottom of the column near the outlet.Each infiltration system filled with the same substrate made of 50% brown soil and cinder at a weight percentage of 50%.Cinder is an inorganic waste produced in the process of anthracite combustion,mainly composed of SiO2and Al2O3.
The brown soil used in this study was collected from the top 20 cm from Shenyang Ecological Station in Liaoning Province of China.The brown soil consisted of total organic carbon(TOC),1.78%;total nitrogen(TN),0.098%;total phosphorus(TP),0.049%;total potassium(TK)0.604%;sand,27.7%;silt,36.4%;clay,33.2%;and pH 6.72.The particle size distribution was investigated by high-resolution X-ray diffraction and shown in Table 1.The soil was air-dried,passed through a 5 mm sieve,and stored at 4℃ before further experiments.

Fig.1 Schematic diagram of the SWIS

Table 1 Particle size distribution of brown soil
The indoor temperature was over 15℃during the period of the experiments from May 2010 to October 2010.Two types of organic synthetic influents prepared by tap water except organic carbon source were used in this study.One organic carbon source was glucose and the other was starch.Major nutrients were supplied by adding NH4Cl and K2HPO4to the influents.Glucose was used to simulate the presence of soluble OM and investigate the clogging process caused by the biofilm growth in three columns.Starch,as a kind oforganic particulate substrate,must undergo cell external hydrolysis before it is available for biodegradation[23].Therefore,the other three starch-fed SWISes were used to investigate the development process of clogging synthetically caused by biofilm growth and particle accumulation,especially for the latter[9].
Organic loading is an important factor causing clogging of infiltration systems[9,19].In this study,the hydraulic loading was kept constant at 0.4 m3/(m2·d)and a flooding period of 24 h.A drying period of 24 h was applied to each SWIS.In order to shorten the time required for clogging,low,middle,and high OM concentrations were constructed in this study.The water quality of the synthetic wastewater is listed in Table 2.
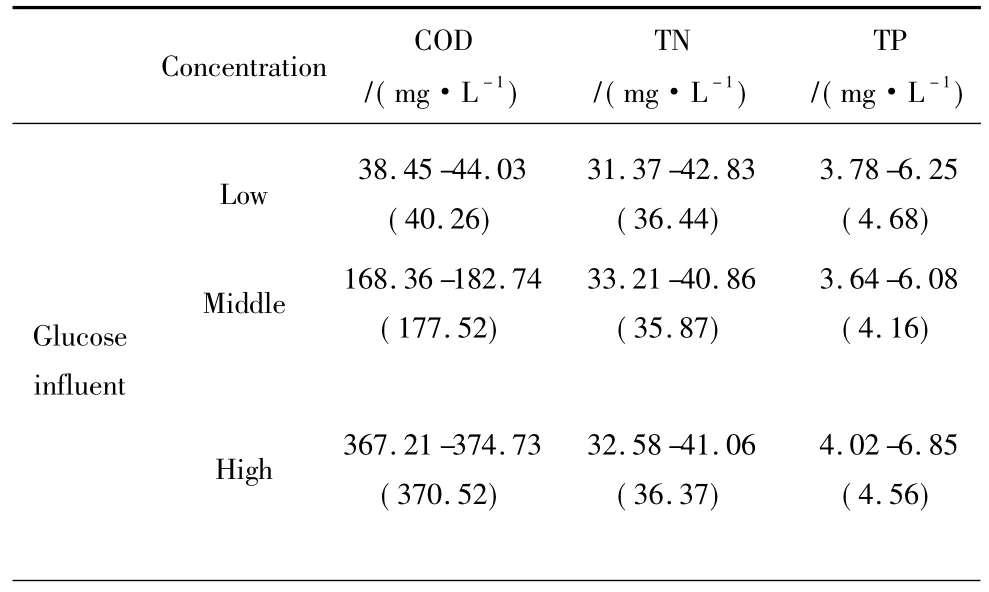
Table 2 Water quality of the synthetic organic influent

(Table 2 continued)
1.2 Sampling and analyzing
1.2.1 Effective porosity measurements
The effective porosity was measured every 15 d and tested through the balance of the water saturation and venting volume of the substrate.It can be expressed as a ratio of the values of drainage volume divided by the total volume and each layer volume of the filter before filling with substrate[24].
1.2.2 Infiltration rate measurements
The infiltration ratewasmeasured every 15 d.The measurement was based on the variable water head method of Standard for Soil Test Method(GB/T50123-1999)[25]with TST-55 infiltrometer(Nanjing Ningxin Soil Instrument Factory,China).The value of infiltration rate K(cm/s)could be calculated through Eq.(1).

where,a(cm2)is the surface area of variable water head pipe;t(s)is the change in time during the head drop;L(cm)is the length of the filter;h1(cm)is the initial water head;h2(cm)is the final water head and A is surface area of the substrate.In this study,a is 1 cm2,L is 4 cm,and A is 30 cm2.
1.2.3 OM content accumulated in substrate
The loss on ignition (LOI) method[26]was used to calculate the OM content accumulated in substrate at the end of experiments.Samples of each layer were taken with sampler and mixed evenly.Some of the above mixed substrates were dried to constant weight in a forced air oven at 80℃and then burnt at 550℃ for 4 h.The weight difference before and after burning was estimated as OM content of substrate.
1.2.4 The amount of bacterial biomass in substrate
Total bacterial biomass of substrate has previously been used to study biological clogging[27,28].The biofilm microbial biomass was evaluated using a fatty acid methyl ester method[29]at the end of experiments.This method provides an unbiased view of the complex microbial communities and viable biomass found in environmental samples such as soils[30]and sediments[31].The fatty acids of the bacteria present in the sample were extracted,converted to fatty acid methyl esters,and directly correlated to dry weight of bacterial biomass and total cell numbers[32].The method was tested by adding a known amount of bacterial biomass to different amounts of substrate.The recovery rates of fatty acids were then converted and correlated to bacterial biomass concentration.
1.2.5 Analysis methods for water quality
Influentsamples were taken everyday and analyzed immediately for TN,TP and chemical oxygen demand(COD)using Chinese EPA standard methods[33].
2 Results and Discussion
2.1 Clogging process of systems with different organic influent
Figure 2 shows the changes of infiltration rate with different OM concentration over time in starch-fed or glucose-fed systems.At the beginning the infiltration rate does not change significantly and the rapid decline of the infiltration rate is observed during the operation time of around 15 d in glucose-fed systems.However,the infiltration rate of starch-fed systems decreases significantly at the incipientstage ofoperation.In previousstudy,Pan concluded the lowest limit infiltration rate(KL)was around 0.3 m/d and clogging would occur in SWIS when infiltration rate was lower than KL[34].The infiltration rates of all starch-fed systems with different COD concentrations decreased to KLwithin 75 d.

OM concentration showed some effects on the clogging process.The infiltration rates were lower for the systems fed with higher concentration influents(Fig.2).For the starch-fed system with COD concentration of 372.86 mg/L,infiltration rate decreased to KLafter running for 45 d while the reaching time was prolonged to 75 d when the organic concentration was 41.47 mg/L.However,none of the infiltration rate of the glucose-fed system dropped to KLafter running for 180 d.Suliman et al.considered that the effect of biological growth on infiltration rate was negligible[22].Zhao et al.suggested that except for organic loading,suspended solids content in the influent should also be paid attention to in clogging,especially for the wastewater containing a large amount of particles[9].
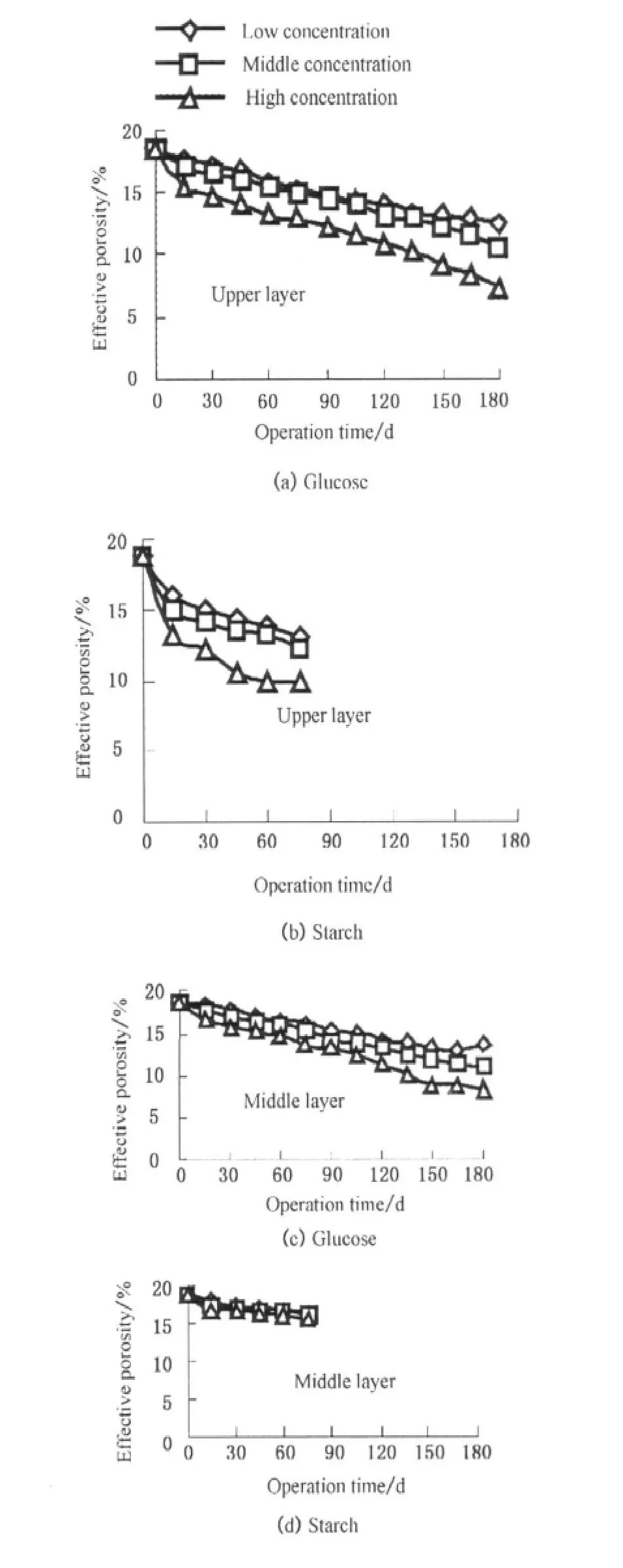
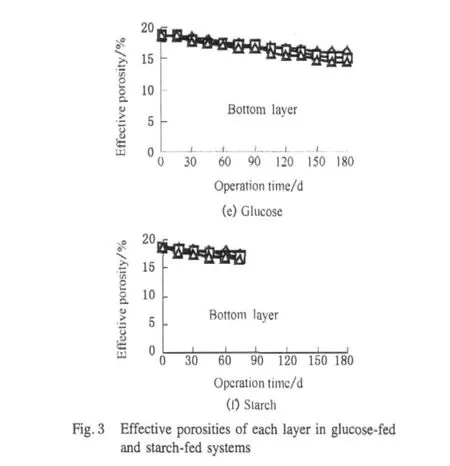
Both biofilm growth and suspended solid accumulation could occupy the pore space and then reduce the pore size of the SWIS.Figure 3 shows the changes of effective porosity for each layer in glucose-fed and starch-fed systems.It could be found the reductions of effective porosity of the upper and middle layers was significant and that of the bottom was slight in glucose-fed systems.This result can be explained that the nutrient and dissolved oxygen levels are higher in upper and middle layers which is in favor of the bacterial cells adhere or grow there.The COD concentration had a significant influence on the effective porosity of glucose-fed systems.For instance,the effective porosity of upper layer decreased from 12.6%to 7.2%when the COD concentration increased from 40.26 to 370.52 mg/L at the end of experiments.The reason was that biofilm could grow anywhere the organic influent flow across and then reduced the effective porosity there[9].For the starch-fed systems,the effective porosity only dropped rapidly in the upper layers,and it did not decrease basically with time in the middle and bottom layers.The reason was a large number of starch particles in influents adhered to substrate in upper layers,forming deposits and then accumulating in the pores there by reducing the effective porosity.Starch particles in influents could hardly flow into middle and bottom layers with the occurrence of clogging.
2.2 Clogging characteristics within the systems
Figure 4 shows the distribution of infiltration rates of each layer at the end of experiments.In general,the infiltration rates were lower in the upper layer.Especially for the starch-fed systems,the values of the infiltration rate of the upper layer all declined to KL.Considering the distribution of OM content along the depth of columns(Fig.5),the infiltration rate was negatively related to the accumulated OM content,which confirmed that the accumulation of OM within substrate was one of the main reasons to the substrate clogging.This result was in accord with the research conclusions by Zhan et al.[35]The main composition of accumulated OM in substrate was biofilm for the glucose-fed systems and detained starch particles for the upper layer of starchfed systems.Compared with the infiltration rates of glucose-fed system under the condition of similar OM content,the reduction of clogged layer in starch-fed system was greater.It could conclude that the influence of biofilm growth on infiltration rate was not as significant as that of starch particles,which was consistent with previous research by Suliman et al.[22]


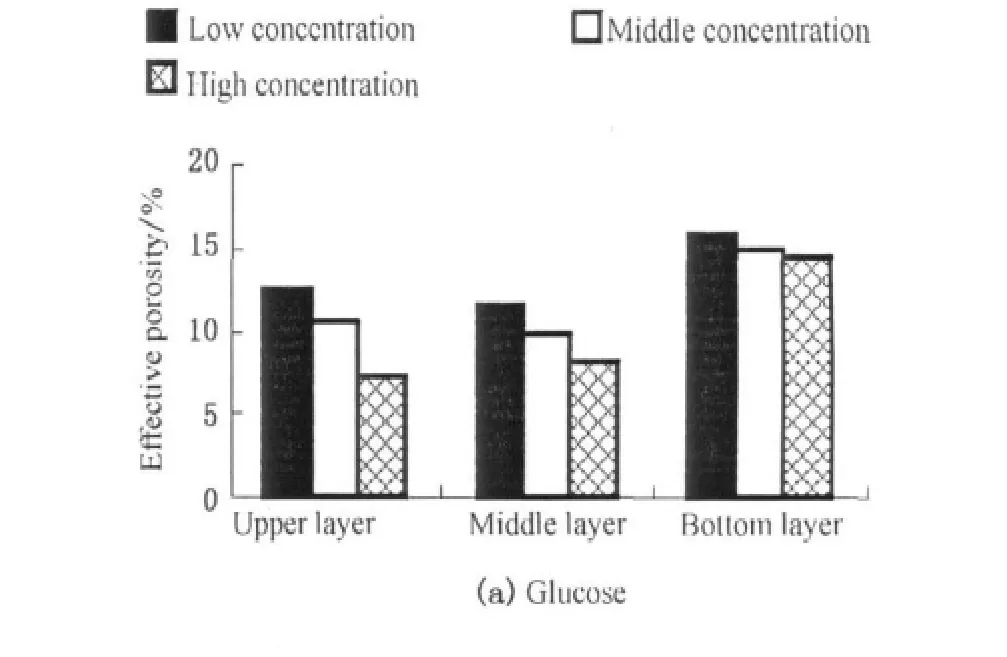
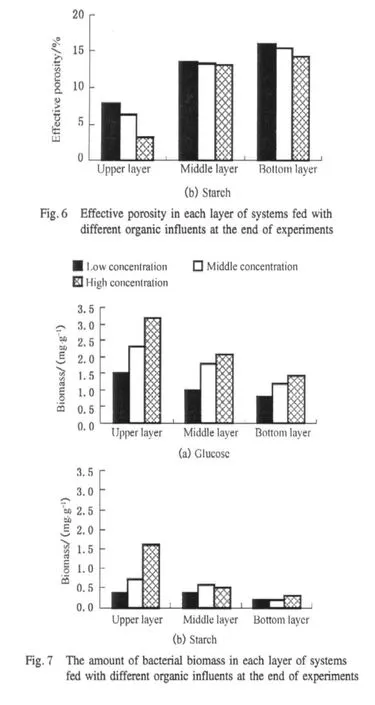
In this study,the reduction of effective porosity was caused by biofilm growth in glucose-fed systems and by organic particle accumulation and biofilm growth in starch-fed systems.Comparing the effective porosity of glucose-fed and starch-fed systems at the end of experiments in Fig.6,the effective porosity of unclogged layer in starch-fed systems was higher than that of glucose-fed systems.The biofilm growth could pronouncedly reduce the effective porosity of substrate in glucose-fed systems,especially with high organic influent.However,the biomass of upper layer was less in starch-fed systems than that of glucose-fed systems,which was not in accordance with larger drop in the effective porosities(Fig.7).The reduction of effective porosities in upper(clogged)layers of starch-fed systems was primarily caused by organic particle accumulation.Thullner et al.[36]and Liu et al.[28]thought biological growth formed continuous and uniform biofilm which covered the wall of each pore leading to reduction in the pore diameter,and Caselles-Osorio et al.considered the particles interstitially accumulated in the pores[16].According to these previous studies,it can infer that the accumulated solids could reduce the pore spaces more rapidly than the biofilm growth could under the experimental conditions.The important contribution of the biofilm growth to clogging in practice is accelerating the occurrence of clogging.
3 Conclusions
Lab-scale SWISes were used to predict the developing process of clogging separately caused by biofilm growth and organic particles accumulation.The most important conclusions resulting from this study are as follows.
OM concentration showed some effects on the clogging process.The infiltration rates were lower for the systems fed with higher concentration influents.
Both biofilm growth and particle accumulation in substrate could reduce the effective porosity and infiltration rate of the SWIS.The contribution of the accumulated organic particles to the process of clogging was greater than that of biomass growth and the clogging mainly occurred in the upper layer in starch-fed systems.
The biofilm growth surely remarkably reduced the effective porosity,especially for the high concentration organic wastewater in glucose-fed systems,but they were not clogged till the end of experiments.
[1]Zhang J,Huang X,Liu C X,et al.Nitrogen Removal Enhanced by Intermittent Operation in a Subsurface Wastewater Infiltration System[J].Ecological Engineering,2005,25(4):419-428.
[2]USEPA.Onsite Wastewater Treatment Systems Manual[M].Washington D C,USA:EPA/625/R-00/008,Environmental Protection Agency,2002:56-89.
[3]Yamaguchi T,Moldrup P,Rolston S I,et al.Nitrification in Porous Media during Rapid Unsaturated Water Flow[J].Water Research,1996,30(3):531-540.
[4]Sun T H,He Y W,Ou Z Q,et al.Treatment of Domestic Wastewater by an Underground Capillary Seepage System[J].Ecological Engineering,1998,11(1/2/3/4):111-119.
[5]Crites R W,Middlebrooks E J,Reed S C,et al.Natural Wastewater Treatment Systems[M].Boca Raton,FL:CRC Press,2006:101-119.
[6]Zhang J,Huang X,Shao C F,et al.Influence of Packing Media on Nitrogen Removal in a Subsurface Infiltration System[J].Journal of Environmental Sciences,2004,16(1):153-156.
[7]Baveye P,Vandevivere P,Hoyle B L,et al.Environmental Impact and Mechanisms of the Biological Clogging of Saturated Soils and Aquifer Materials[J].Critical Reviews in Environment Science and Technology,1998,28(2):123-191.
[8]Magesan G N,Williamson J C,Yeates G W,et al.Wastewater C∶N Ratio Effects on Soil Hydraulic Conductivity and Potential Mechanisms for Recovery[J].Bioresource Technology,2000,71(1):21-27.
[9]Zhao L F,Zhu W,Tong W.Clogging Processes Caused by Biofilm Growth and Organic Particle Accumulation in Lab-Scale Vertical Flow Constructed Wetlands[J].Journal of Environmental Sciences,2009,21(6):750-757.
[10]Sun T H,Li X F.The Techniques of Wastewater Ecological Treatment and Renovation of Urban Wastewater[M].Beijing:Chemical Industry Press,2006:29-36.(in Chinese)
[11]Langergraber G,Haberl R,Laber J,et al.Evaluation of Substrate Clogging Processes in Vertical Flow Constructed Wetlands[J].Water Science and Technology,2003,48(5):25-34.
[12]Kayser K,Kunst S.Processes in Vertical-Flow Reed Beds:Nitrification,Oxygen Transfer and Soil Clogging[J].Water Science and Technology,2005,51(9):177-184.
[13]Blazejewski R,Murat-Blazejewska S.Soil Clogging Phenomena in Constructed Wetlands with Subsurface Flow[J].Water Science and Technology,1997,35(5):183-188.
[14]Molle P,Lienard A,Boutin C,et al.How to Treat Raw Sewage with Constructed Wetlands:an Overview of the French Systems[J].Water Science and Technology,2005,51(9):11-21.
[15]Nguyen L M.Organic Matter Composition,Microbial Biomass and Microbial Activity in Gravel-Bed Constructed Wetlands Treating Farm Dairy Wastewaters[J].Ecological Engineering,2000,16(2):199-221.
[16]Caselles-Osorio A,Puigagut J,Segú E,et al.Solids Accumulation in Six Full-Scale Subsurface Flow Constructed Wetlands[J].Water Research,2007,41(6):1388-1398.
[17]Platzer C,Mauch K.Soil Clogging in Vertical Flow Reed Beds Mechanisms,Parameters,Consequences and Solutions[J].Water Science and Technology,1997,35(5):175-181.
[18]Vandevivere P,Baveye P.Effect of Bacterial Extracellular Polymers on the Saturated Hydraulic Conductivity of Sand Columns[J].Applied and Environmental Microbiology,1992,58(5):1690-1698.
[19]Healy M G,Rodgers M,Mulqueen J.Treatment of Dairy Wastewater Using Constructed Wetlands and Intermittent Sand Filters[J].Bioresource Technology,2007,98(12):2268-2281.
[20]Zhao Y Q,Sun G,Allen S J.Anti-sized Reed Bed System for Animal Wastewater Treatment:a Comparative Study[J].Water Research,2007,38(12):2907-2917.
[21]Rodgers M,Mulqueen J,Healy M G.Surface Clogging in an Intermittent Stratified Sand Filter[J].Soil Science Society of America Journal,2004,68(6):1827-1832.
[22]Suliman F,French H K,Haugen L E,et al.Change in Flow and Transport Patterns in HorizontalSubsurfaceFlow Constructed Wetlands as a Result of Biological Growth[J].Ecological Engineering,2006,27(2):124-133.
[23]Caselles-Osorio A,Garcia J.Performance ofExperimental HorizontalSubsurface Flow Constructed Wetlands Fed with Dissolved or Particulate Organic Matter[J].Water Research,2006,40(19):3603-3611.
[24]Hua G F,Zhua W,Zhao L F,et al.Clogging Pattern in Vertical-Flow Constructed Wetlands:Insight from a Laboratory Study[J].Journal of Hazardous Materials,2010,180(1/2/3):668-674.
[25]The Professional Standards Compilation Group of People's Republic of China.Standard for Soil Test Method(GB/T50123-1999)[M].Beijing:China Planning Press,1999:68-74.(in Chinese)
[26]Tanner C C,Sukias J P.Accumulation of Organic Solids in Gravel-Bed Constructed Wetlands[J].Water Science and Technology,1995,32(3):229-239.
[27]Stéphanie R P,Santo R,Pascale S,et al.Interrelationships between Biological,Chemical,and Physical Processes as an Analogy to Clogging in Aquifer Storage and Recovery Wells[J].Water Research,2000,34(7):2110-2118.
[28]Liu Q,Mancl K,Tuovinen O H.Biomass Accumulation and Carbon Utilization in Layered Sand Filter Biofilm Systems Receiving Milk Fat and Detergent Mixtures[J].Bioresource Technology,2003,89(3):275-279.
[29]MIS OperatingManual.MIDIInc.[M].USA,Newark:Delaware,1991.
[30]Ratcliff A W,Busse M D,Shestak C J.Changes in Microbial Community Structure Following Herbicide Additions to Forest Soils[J].Applied Soil Ecology,2006,34(2/3):114-124.
[31]Ringelber D B,Sutton S,White D C.Biomass,Bioactivity and Biodiversity:Microbial Ecology of the Deep Subsurface:Analysis of Ester-Linked Phospholipids Fatty Acids[J].FEMS Microbiology Reviews,1997,20(3/4):371-377.
[32]Haack S K,Garshow H,Odelson D A,et al.Accuracy,Reproducibility,and Interpretation of Fatty Acid Methyl Ester Profiles ofModelBacterialCommunities[J].Applied and Environmental Microbiology,1994,60(7):2483-2493.
[33]EPA.Chinese.Methods for Water and Wastewater Analysis[M].4th ed.Beijing:Environmental Science Publishing House of China,2002:68-89.(in Chinese)
[34]Pan J.Studies on Microbiology Character and Intensified Nitrogen RemovalTechnics in Subsurface Infiltration System[D].Shenyang:Northeastern University,1991:45-48.(in Chinese)
[35]Zhan D H,Wu Z B,Xu G L.Organic Matter Accumulation and Substrate Clogging in Integrated Vertical Flow Constructed Wetland[J].China Environmental Science,2006,23(5):457-461.(in Chinese)
[36]Thullner M,Zeyer J,Kinzelbach W.Influence of Microbial Growth on Hydraulic Properties of Pore Network[J].Transport in Porous Media,2002,49(1):99-122.
 Journal of Donghua University(English Edition)2012年2期
Journal of Donghua University(English Edition)2012年2期
- Journal of Donghua University(English Edition)的其它文章
- Isothermal Crystallization Behavior of Poly(ethylene terephthalate)/Carbon Black Masterbatch
- Reverse Solution and Parametric Design of the Conjugate Cam Weft Insertion Mechanism Based on VB.NET and UG
- A Stochastic Study on the Wicking Phenomena
- The Usability of Polyoxyethylene Stearate as Lubricant for Sizing Cotton Warp Yarns
- A New Design Method for Variable Digital Filter Based on Field Programmable Gate Array(FPGA)
