Synthesis of a Novel Thiadiazine Derivative and Electrochemical Properties for Pb2+Transfer across Water/1,2-Dichloroethane Interface
BASLAK Canan BINGOL Haluk COSKUN Ahmet ATALAY Tevfik
(Department of Chemistry,Selcuk University,42099 Meram/Konya,Turkey)
1 Introduction
The electrochemical study of the facilitated transfer of heavy metal ions upon organic extractants such as phenanthrolinophanes,thiosemicarbazones,triazines,thiadiazines at polarized interfaces between two immiscible electrolyte solutions (ITIES)has been the subject of research interest by some authors.1-5This methodology has been followed in order to develop an alternating electrochemical transfer process to the traditional procedures used for complex behaviors.4-11In addition to making use of a theoretical method for the determination of values such as the stoichiometry of complexes,5-10,12the obtaining of the useful equations to evaluate the Gibbs transfer energy of very hydrophilic ions such as heavy metal ions13,14is a very important development in this field.
Thiadiazines are heterocyclic compounds catching attention due to their unique chemical structure.4The broad biological and pharmacological activities of various thiadiazines fused with an s-triazole ring have been extensively studied.15Several compounds containing thiadiazine derivatives have antimicrobial properties,antifungal activities,and anti-inflammatory.16-19An ionophore including a derivative thiadiazine has been used as a silver ion-selective electrode.20Shaaban and Fuchigami4investigated the electrochemical behaviors of a thiadiazine and its derivatives by using cyclic voltammetry.
In this study,we reported the transfer of Pb2+ion across water/1,2-dicholoroethane(1,2-DCE)interface facilitated by a novelthiadiazine derivative,5-(4-phenoxyphenyl)-6H-1,3, 4-thiadiazin-2-amine(PPTA).It is the first study relating the ion transfer across ITIES facilitated by using a thiadiazine derivative.The stoichiometry and the association constant of the complex between Pb2+ion and PPTA in 1,2-DCE were determined from the voltammetric data,and the results were compared with those reported in literature using different ligand derivatives for Pb2+.We also discussed the transfer mechanism of Pb2+ion facilitated by PPTAin organic solvent.
2 Experimental
2.1 Chemicals and Apparatus
Purified water from Milli-Q system(Millipore)with 18.2 MΩ·cm resistivity was used in order to prepare aqueous solutions.The supporting electrolyte used for the organic phase and aqueous phase were bis(triphenylphosphoranylidene)ammonium tetrakis-(4-chlorophenyl)borate(BTPPATPBCl)and LiCl(≥98.0%,Fluka,Steinheim),respectively.BTPPATPBCl was obtained from the mixture of equimolar BTPPACl(≥98.0%(AT),Fluka,Steinheim)and KTPBCl(≥98.0%(AT), Fluka,Steinheim).We purchased 1,2-DCE from Merck(≥99.5%,G.C.Grade,Merck,Darmstadt)as the organic solvent for the electrochemical experiments.Other chemicals were of the analytic grade.
The electrochemical measurements were performed by using a four-electrode potentiostat(Princeton Applied Research (PAR),263/A2,USA)with ohmic drop compensation in order to empower the cell.Melting point was recorded on an EZ Melt-MPA120 capillary apparatus and it was uncorrected.Infrared measurements were performed by Perkin Elmer 100 apparatus.1H NMR and13C NMR spectra were obtanied from Varian 400.
2.2 Synthesis of PPTA
The ligand, 5-(4-phenoxyphenyl)-6H-1,3,4-thiadiazin-2-amine,represented in Fig.1,was firstly synthesized according to the procedure described elsewhere.21
A solution was prepared by using 2-chloro-1-(4-phenoxyphenyl)ethanone(340 mg,1.38 mmol)and methanol.The prepared solution and thiosemicarbazide hydrochloride(175 mg, 1.38 mmol,1 equiv.)(≥99.0%,Fluka,Steinheim)were mixed and the reaction mixing was refluxed for 1 h.Afterwards,the solvent was removed and the residue was purified by flash chromatography in order to yield 5-(4-phenoxyphenyl)-6H-1,3, 4-thiadiazin-2-amine as a colorless solid,317 mg,81%yield. Rf 0.65 ethyl acetate/methanol(50:50,volume ratio).M.P. 127-128°C.1H and13C NMR spectra of PPTA are shown in Fig.2.
2.3 Electrochemical measurements
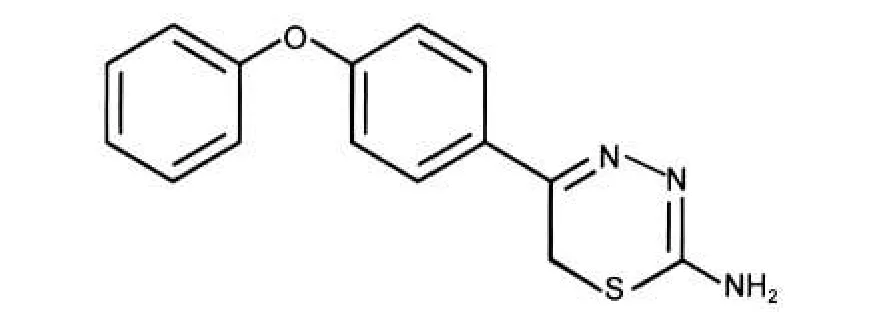
Fig.1 Structure of PPTA

Fig.2 1H NMR(a)and13C NMR(b)spectra of PPTA1H NMR(400 MHz,CDCl3,25°C),δ:8.75(s,2H,NH2),7.62-7.24(m,9H, Ar-H),2.26(s,2H,CH2S);13C NMR(100 MHz,CDCl3,25°C),δ:179.38,147.60, 139.93,135.31,133.96,132.80,129.70,129.48,128.27,127.17,77.21,13.71
The electrochemical cell was prepared by borosilicate glass with the geometrical area for water/1,2-DCE interface of 0.27 cm2.It has two Luggin capillaries in order to minimize the resistance across two reference electrodes.22,23A Faraday cage was used to minimize the background noise.We used two Ag|AgCl electrodes as the reference electrodes.The counter electrodes were two platinum wires.All experiments were carried out at room temperature((23±1)°C).To investigate the metal transfer the electrochemical cell can be represented by the following Cell:

where the polarized water/1,2-DCE interface is indicated by double line,x and y represent the concentrations of PPTA and Pb2+ion,respectively.The applied potential difference between the two reference electrodes is related to the Galvani potential difference between aqueous and organic phase.Tetrapropylammonium(TPrA+)ion was used as the internal reference electrolyte.24,25
3 Results and discussion
The adjustment of pH was not required to investigate the ion transfer across water/1,2-DCE interface facilitated by PPTA due to lack of any peak indicating the assisted proton transfer which was observed for pH of the aqueous phase in Cell 1.The transfer of Pb2+across water/1,2-DCE interface facilitated by PPTA,that was present in the 1,2-DCE phase,was studied by means of CV.Fig.3 and Fig.4 display the obtained cyclic voltammograms of the facilitated Pb2+ion transfer across water/ 1,2-DCE interface at different metal concentrations and scan rates,respectively.The initial concentration of PPTA was much higher than the concentration of metal ion in the bulk of water phase.
We obtained that the facilitated transfer potential of Pb2+ionwas independent of the Pb2+concentration and the scan rate.Moreover,the peak separations between forward and back peaks()were found close to the theoretical value of a reversible process of a divalent ion((32±2)mV).26The half-wave transfer potential value was determined as(0.296± 0.009)V according to Eq.(1)when x=1×10-2mol·L-1.

As shown by the plot in Fig.3 inset,the forward peak current,Ifp,corrected for the base current,was proportional to the bulk concentration of Pb2+ion in the range of 20 to 300 μmol· L-1.Ifpwas also proportional to the square root of scan rate(v1/2)in the v range of 25 to 400 mV·s-1as can be seen in Fig.4(the inset).These results clearly show that the transfer of Pb2+ion facilitated by PPTA is a reversible process.3Therefore,the concentration of PPTA is much higher than the concentration of metal ion(x>>y),and the facilitated ion transfer of Pb2+has reversible nature.The facilitated transfer by PPTA across water/1, 2-DCE interface is controlled by the diffusion of Pb2+in the aqueous phase according to Randles-Sevcik Equation:10

Fig.3 Cyclic voltammograms for facilitated transfer of Pb2+ ion by PPTAacross water/1,2-DCE interface at different metal concentrationsx=1×10-2mol·L-1>>y=(20−300)μmol·L-1,v=50 mV·s-1; The inset shows dependence of the forward peak current(Ifp)of the facilitated transfer of Pb2+by PPTAon the metal concentration.

The diffusion coefficient of Pb2+in the aqueous phase was calculated as(8.32±0.18)×10-6cm2·s-1by using the slope of the plot in Fig.3 inset.

Fig.4 Cyclic voltammograms for the transfer of Pb2+ion facilitated by PPTAacross water/1,2-DCE interface at different scan ratesx=1×10-2mol·L-1>>y=5×10-5mol·L-1,v=25−400 mV·s-1;The inset shows dependence of the forward peak current(Ifp)on the square root of scan rate.

Fig.5 Change of the Galvani potential of facilitated transfer of Pb2+ion on the PPTAconcentration across water/1,2-DCE interface at y=5×10-5mol·L-1and v=50 mV·s-1The inset is the dependence of the half-wave potential of facilitated transfer of Pb2+ion on the PPTAconcentration at the water/1,2-DCE interface at the experimental conditions.
The change of the Galvani potential of facilitated transfer of Pb2+ion and the dependence of the transfer potential of facilitated transfer of Pb2+ion on the PPTA concentration across water/ 1,2-DCE interface were given in Fig.5 and its inset,respectively.Whereas,the peak currents of the facilitated transfer were independent of PPTA concentration between 5×10-3and 4×10-2mol·L-1at y=5×10-5mol·L-1,where the transfer potentials shifted to less positive value.
PPTA can be soluble only in organic phase because of the standard partition coefficient(logP1,2-DCE)of PPTA between 1,2-DCE and water calculated as approximately 2.76 by theoretical approaches.27,28Hence,the facilitated transfer was occurred by the interfacial complexation and decomplexation mechanism29(TIC/TID mechanism)and can be shown as Eq.(3):

where s is the stoichiometry number of the complex.Due to the high hydrophobicity of the PPTA,the interfacial half-wave potential of complex is presented by the following equation:26

The electrochemical studies of the facilitated transfer of Zn2+,Co2+,Ni2+,Cd2+,Hg2+,and Cu2+were performed by varying ligand concentrations from 5×10-3to 4×10-2mol·L-1and the metal ion concentration from 5×10-5to 1.5×10-4mol·L-1. No peak could be visible under the described experimental conditions in this study when Co2+,Ni2+,or Zn2+ions were used.Although some voltammetric peaks were also obtained for the transfer of Cd2+ion,it was determined that the peaks gave unsatisfactory results in this system.Transfer processes of Hg2+and Cu2+ions were irreversible in good agreement with literature rdsults.3As can be seen in Fig.6,two-step voltammograms were reported in the electrochemical system where the presence of complexes with ligand(1×10-2mol·L-1)and those heavy metal ions(1×10-4mol·L-1).

Table 1 Thermodynamic parameters for the transfer of Pb2+ facilitated by PPTAacross water/1,2-DCE

Fig.6 Cyclic voltammograms of the background and the facilitated transfer of Hg2+and Cu2+ions across water/1,2-DCE interfacex=1×10-2mol·L-1,y=1×10-4mol·L−1,v=50 mV·s-1
Therefore,the facilitated transfers of Hg2+and Cu2+could not be efficiently evaluated by analyzing the cyclic voltammetry measurements.
4 Conclusions
In this work,we presented the electrochemical behavior of Pb2+ion transfer across macro water/1,2-DCE interface facilitated by 5-(4-phenoxyphenyl)-6H-1,3,4-thiadiazin-2-amine(PPTA).The facilitated transfer of Pb2+ion was determined to be electrochemically reversible.The stoichiometry,the association constant,and Gibbs(Galvani)transfer energy of complex were determined by analyzing the voltammetric results arised from the diagrams.Furthermore,the diagrams were provided an effective way to clarify the Pb2+ion transfer mechanism(TIC/TID mechanism).Consequently,the dependence of the half-wave potential on the ligand concentration displayed that the stoichiometry and association constant is 2:1(PPTA-Pb2+),lgβ2=17.1± 0.2 for Pb(PPTA)22+complex,respectively.
(1) Ferreira,E.S.;Garau,A.;Lippolis,V.;Pereira,C.M.;Silva,F. J.Electroanal.Chem.2006,587,155.
(2)Katano,H.;Kuboyama,H.;Senda,M.J.Electroanal.Chem. 2000,483,117.
(3) Bingol,H.;Akgemci,E.G.;Ersoz,M.;Atalay,T. Electroanalysis 2007,19,1327.
(4) Shaaban,M.R.;Fuchigami,T.Tetrahedron Lett.2002,43,273.
(5) Zhang,M.;Sun,P.;Chen,Y.;Li,F.;Gao,Z.;Shao,Y.Chin.Sci. Bull.2003,48,1234.
(6)Tomaszewski,L.;Reymond,F.;Brevet,P.F.;Girault,H.H. J.Electroanal.Chem.2000,483,135.
(7) Kasumov,V.T.;Köksal,F.Spectrochim.Acta Part A 2005,61, 225.
(8) Hatay,I.;Su,B.;Li,F.;Partovi-Nia,R.;Vrubel,H.;Hu,X.; Ersoz,M.;Girault,H.H.Angew.Chem.Int.Edit.2009,48, 5139.
(9)Alemu,H.Pure Appl.Chem.2004,76,697.
(10) Herzog,G.;McMahon,B.;Lefoix,M.;Mullins,N.D.;Collins, C.J.;Moynihan,H.A.;Arrigan,D.W.M.J.Electroanal. Chem.2008,622,109.
(11) Gobry,V.;Ulmeanu,S.;Reymond,F.;Bouchard,G.;Carrupt,P. A.;Testa,B.;Girault,H.H.J.Am.Chem.Soc.1998,123,10684.
(12) Koryta,J.Electrochim.Acta 1979,24,293.
(13) Shao,Y.;Stewart,A.A.;Girault,H.H.J.Chem.Soc.Faraday Trans.1991,87,2593.
(14) Lagger,G.;Tomaszewski,L.;Osborne,M.D.;Seddon,B.J.; Girault,H.H.J.Electroanal.Chem.1998,451,29.
(15) Prasad,A.R.;Ramalingam,T.;Rao,A.B.;Diwan,P.V.;Sattur, P.B.Eur.J.Med.Chem.1989,24,199.
(16)Fotouhi,L.;Mosavi,M.;Heravia,M.M.;Nematollahi,D. Tetrahedron Lett.2006,47,8553.
(17) Rodrîguez-Fernândez,E.;Manzano,J.L.;Benito,J.J.; Hermosa,R.;Monte,E.;Criado,J.J.J.Inorg.Biochem.2005, 99,1558.
(18)El-Daway,M.A.;Omar,A.M.M.E.;Ismail,A.M.;Hazzaa,A. A.B.J.Pharm.Sci.1983,72,45.
(19) Witvrouw,M.;Arranz,M.E.;Pannecouque,C.;Declercq,R.; Jonckheere,H.;Schmit,J.C.;Vandamme,A.M.;Diaz,J.A.; Ingate,S.T.;Desmyter,J.;Esnouf,R.;Van Meervelt,L.;Vega, S.;Balzarini,J.;Clercq,D.Antimicrob.Agents Chemother. 1998,42,618.
(20) Mahajan,R.K.;Sood,P.;Mahajan,M.P.;Singh,P.Anal.Sci. 2004,20,1423.
(21)Adamo,M.A.;Adlington,R.M.;Baldwin J.E.;Day,A.L. Tetrahedron 2004,60,841.
(22)Akgemci,E.G.;Bingol,H.;Ersoz,M.;Stibor,I.Electroanalysis 2008,20,1354.
(23) Marecek,V.;Samec,Z.J.Electroanal.Chem.1985,185,263.
(24) Koryta,J.Electrochim.Acta 1984,29,445.
(25) Yuan,Y.;Gao,Z.;Guo,J.;Shao,Y.J.Electroanal.Chem.2002, 526,85.
(26) Katano,H.;Senda,M.Anal.Sci.1996,12,683.
(27) Tetko,V.;Gasteiger,J.;Todeschini,R.;Mauri,A.;Livingstone, D.;Ertl,P.;Palyulin,V.A.;Radchenko,E.V.;Zefirov,N.S.; Makarenko,A.S.;Tanchuk,V.Y.;Prokopenko,V.V.J.Comput. Aid.Mol.Des.2005,19,453.
(28) Steyaert,G.;Lisa,G.;Gaillard,P.;Boss,G.;Reymond,F.; Girault,H.H.;Carrupt,P.A.;Testa,B.J.Chem.Soc.Faraday Trans.1997,93,401.
(29) Shao,Y.;Osborne,M.D.;Girault,H.H.J.Electroanal.Chem. 1991,318,101.
(30) Cheng,Y.;Schiffrin,D.J.J.Electroanal.Chem.1997,429,37.

