一种可作为FCC基质的新型改性镁铝尖晶石材料
许孝玲 李春义 山红红
(中国石油大学(华东)化学化工学院,重质油国家重点实验室,山东青岛266555)
一种可作为FCC基质的新型改性镁铝尖晶石材料
许孝玲 李春义 山红红
(中国石油大学(华东)化学化工学院,重质油国家重点实验室,山东青岛266555)
镁铝尖晶石作为硫转移剂被广泛应用于流体催化裂化(FCC)过程中,可降低再生烟气中的SO2排放.通过将镁铝尖晶石和Y分子筛纳米簇溶液混合在酸性条件下再次晶化的方法改性镁铝尖晶石,改性后尖晶石的酸性明显提高.改性尖晶石材料的酸性和碱性可通过改变体系的pH值得以调节.实验表明,此改性方法可以将分子筛的结构单元引入到尖晶石中,从而使尖晶石的微孔比表面、酸性和水热稳定性明显提高.与原尖晶石相比,改性尖晶石裂化减压瓦斯油(VGO)的活性和产物选择性均得到改善,同时还保留了部分碱性活性位,仍具有一定的脱硫活性.改性尖晶石裂化VGO的反应性能优于高岭土,同时以改性尖晶石作为部分基质的FCC催化剂的反应性能也优于以高岭土为主要基质的FCC催化剂.
镁铝尖晶石;Y分子筛纳米簇;硫转移;催化裂化;水热稳定性
1 Introduction
Magnesium-aluminate spinel(MgAl2O4)is a composite oxide with high melting point(2135°C)and other attractive properties.1-4It has been extensively applied as a sulfur-transfer agent in FCC units for reduction of SOxemission since 1980s.5-9The sulfur-transfer agent first captures SOxas an un-stable sulfate under oxidation atmosphere of the regenerator; then it moves to the reactor and the formed sulfate is reduced to H2S which can be further treated in a modified Claus process,producing elemental sulfur.10-15Usually some rare earth metal oxides and/or transition metal ions are introduced into the structures of MgAl2O4to improve the desulfurization (De-SOx)activity and the reducibility of the sulfate formed by the adsorbed SO2.5,7,8,11,13-16
However,few previous papers have addressed on the performance of magnesium-aluminate spinel in catalytic cracking of hydrocarbons.Similar to other metal oxides,both Lewis acidic sites(unsaturated coordinated metal cations)and Lewis basic sites(oxygen anions)exist on the surface of magnesium-aluminate spinel.17The Mg-Al-O mixed oxides have been widely studied as base catalysts for double-bond isomerization,polymerization of propylene oxide and propiolactone,aldol condensation,and dehydrogenation of 2-propanol.18It has been reported that reactions on metal oxides usually involve the hydrogen abstraction by basic oxygen sites and the formation of anionic species.19Some researchers suggested that the basic sites of Mg-Al-O may catalyze cyclization and aromatization reactions in the case of n-hexane dehydrogenation over the Pt/Mg-Al-O catalysts.18Hence it should be expected that the stronger basic property for the spinel makes it less active for cracking hydrocarbons even compared with conventional inert matrices of FCC catalysts such as clays.It was found that the activity of the FCC catalyst was obviously reduced and the selectivity deteriorated when only 5%(w)of sulfur transfer agent was added to an equilibrium catalyst.20Due to the higher sulfur content in crude oil and the more stringent environmental regulations,the amount of sulfur-transfer agents required for effective SOxemission reduction was increased and their dilution effect on FCC catalysts would be stronger.
Here an alternative method was proposed.If the acidity of spinel was improved,then its dilution effect would be weakened and it could be used as an FCC matrix component,resulting in a sufficient amount of spinel present in FCC units for effective SOxremoval.As the spinel is applied in FCC units,hydrothermal stability is another important factor that should be considered.The conventional methods such as doping of divalent and trivalent transition metal ions into the spinel could induce a certain degree of acidity.21,22But the dehydrogenation route could be enhanced by the inclusion of reducible cations,22which would favor the formation of H2and coke in the catalytic cracking of hydrocarbons.Besides,whether the acidity can resist the severe hydrothermal treatment is another problem. Fortunately,an effective approach which involves incorporating the mesoporous materials with primary or secondary zeolitic units has been developed to improve the acidity and hydrothermal stability of mesoporous materials.Do et al.23-25have successfully produced zeolite-coated mesoporous SBA-15,siliceous mesostructured cellular foams(MCFs),and an amorphous aluminosilicate.Liu et al.26have described the synthesis and properties of a typical hexagonal aluminosilicate mesostructure derived from seeds that normally nucleate the crystallization of faujasitic zeolite type Y.
Bao et al.27,28have synthesized bimodal micro-mesoporous aluminosilicates(B-MASs)by assembling preformed zeolite Y nanoclusters using a triblock polymer as template and hierarchical macro-meso-micro-porous composite materials via in situ assembly of preformed zeolite Y nanoclusters on substrates(Kaolin)using a cationic surfactant as template.The resulting micro-mesoporous materials all showed much higher acidity and enhanced hydrothermal stability.Here a similar method was used to modify the magnesium-aluminate spinel for increasing its acidity.For preliminary research,only MgAl2O4was adopted as a simple object of study despite its relatively low De-SOxactivity and low sulfate reduction capacity.
It is expected that its cracking ability of heavy oil would be improved and the remaining basic sites would still be active for SO2uptake.The improved activity for cracking of heavy oil would make the modified spinel potential matrix of FCC catalysts.Moreover,as matrix of FCC catalysts,the presence of much larger amount of spinels than the conventional case would offer higher contact odds for SO2in the FCC regenerator.
In this paper,a series of modified spinels were prepared by recrystallizing the mixture of MgAl2O4with zeolite Y naonoclusters in acidic medium.The effects of the pH values of the synthesis system on the properties of the resulting materials and their catalytic performance of VGO cracking and SO2uptake were investigated.Also,the modified spinel as a possible substitute of conventional matrix of a FCC catalyst,i.e.,Kaolin clay,was tentatively explored.
2 Experimental
2.1 Preparation of catalysts
The synthesis of modified spinels comprised of three parts. The first step was the preparation of the magnesium-aluminate spinel.Secondly zeolite Y nanoclusters were synthesized.Finally the zeolite nanocrystals were incorporated into the spinel surface by using the prepared zeolite nanoclusters.
First,the preparation of MgAl2O4was as follows:pseudo-boehmite powder containing 68%(w)Al2O3(Shandong Aluminum Co.,Ltd.)was suspended in distilled water in a vessel, and then a suitable amount of HCl solution was added dropwise under vigorous stirring at 80°C until the pH value fell into the range of 3-4.The magnesium nitrate solution was prepared by dissolving the desired amount of magnesium nitrate(≥99.0%,Sinopharm Chemical Reagent Co.,Ltd.)in deionized water.Then the nitrate solution was added slowly into the pseudo-boehmite gel under vigorous stirring for a further 2 h.The gel obtained was then dried at 120°C overnight.Finally they were calcined at 800°C for 4 h.The resulting solids were crushed and sieved to powders of 160-200 mesh.
Then the four solutions of zeolite Y nanoclusters with the same composition(n(Si)/n(Al)=7.4)were obtained as follows:272.7 g aluminum sulfate(≥99.0%,Sinopharm Chemical Reagent Co.,Ltd.)and 3.75 g sodium hydroxide(≥96.0%,Sinopharm Chemical Reagent Co.,Ltd.)were first dissolved in 14.4 mL deionized water.Then a sodium silicate solution of 13.04 g containing 27.5%(w)of SiO2and 6.5%(w)of Na2O (Zibo Yifeng Water Glass Ltd.)was added dropwise into the above solution under vigorous stirring.The obtained four solutions were transferred into four autoclaves and then aged at 75°C for 24 h.

Table 1 Properties of Daqing vacuum gas oil(VGO)
Finally,12 g MgAl2O4powder was added to the resulting four zeolite Y nanoclusters solutions under stirring,and the pH values of the mixtures were adjusted to 7.0,5.0,3.0,2.0,respectively,by dropwise adding HNO3solutions of 2.5 mol·L−1. These slurries were further agitated for 0.5 h and then transferred into the autoclaves to heat at 100°C for 24 h.The resulting solid product was filtered,washed with distilled water and dried at 120°C.The obtained modified spinels were denoted as MgAl2O4/Y-x,in which‘x’represented the pH value of the synthesis system.
All of the H-form samples were obtained by a threefold ion exchange in ammonium nitrate solutions of 1 mol·L-1and subsequent calcination at 550°C.The H-form samples were hydrothermally treated at 800°C under 100%steam flow for 4 h to obtain the corresponding aged samples for catalytic tests.
2.2 Characterization
X-ray diffraction(XRD)was carried out on the XʹPert PRO MPD(DANalytical Co.)diffractometer with Cu-Kαradiation (40 kV,40 mA).BET surface area,pore size,and pore volume were measured by nitrogen adsorption using the ASAP 2020 V3.02 H porosimeter made by Micromeritics Co.The micropore data were obtained by the t-plot method.The composition of the samples was characterized by X-ray fluorescence(XRF).
The Fourier transform infrared(FTIR)spectra of the samples were obtained on a Nicolet Nexus-IR spectrometer.The acidity was measured by pyridine adsorption.First the samples were evacuated in situ in an IR cell at 300°C for 4 h.After the temperature decreased to room temperature,the IR spectra were then recorded and used as the background.Next pyridine was introduced into the cell and allowed to saturate the sample surface for 1 h.Finally,the FTIR spectra were recorded at 200 and 350°C subtracting the background to discover the relative amount of acid sites with different strengths.The basicity was measured by a similar procedure by adsorption of hexafluoro-2-propanol except that the FTIR spectra were only recorded at 200°C.
Transmission electron microscopy(TEM)measurements were carried out on a JEM-2100 microscope(JEOL)with an acceleration voltage of 200 kV.
2.3 Catalytic evaluation of catalysts
Vacuum gasoil catalytic cracking tests were carried out in a fixed micro-reactor unit based on American Society for Testing and Materials(ASTM)D3907-80.29The feedstock was preheated before injecting into the stainless steel reactor.After injecting the feedstock,the catalyst bed was purged with a stream of 30 mL·min-1N2for 600 s to a total gas volume that was determined by the amount of displaced water.The products were quenched using ice bath and separated into liquid and gas products.The mass of feedstock was 1 g and the oil was injected for 75 s.The catalyst to oil mass ratio was 5 and reaction temperature was 540°C.The properties of Daqing vacuum gas oil (VGO)feedstock are shown in Table 1.
As a reference,the Kaolin clay(Suzhou,China)steamed at the same condition as that of the other prepared samples was also evaluated.
Varian 3800 graphite electrode(GC)was used to examine the composition of the cracking gas.The liquids obtained in the experiments were examined using a simulated distillations gas chromatogram with the ASTM D2887 standard to obtain the percentage weight of gasoline,diesel oil(LCO),and heavy oil(HCO).The amount of coke deposited on the catalysts after cracking was determined by analyzing the amount of CO2obtained from the burning of coke in situ using the chromatogram.
The SO2uptake tests were conducted in a fixed bed reactor using 0.6 g of catalyst.Astream of 235 mL·min-1with 800 μL· L-1of SO2,20%of O2,and N2balance was passed over the catalyst at 700°C.
The SO2uptake capacity of the catalyst was obtained by measuring the SO2concentration of the gas mixture at the exit of the reactor under different reaction times with a flue gas analyzer Testo350EPA.
3 Results and discussion
3.1 Characterization of the catalysts
The XRD patterns of MgAl2O4and MgAl2O4/Y-x samples before and after hydrothermal treatment were shown in Fig.1.All of the samples presented well-resolved diffraction peaks characteristic of MgAl2O4.Also,minor peaks assigned to the free MgO were observed for the parent MgAl2O4samples(fresh and aged ones).However,the absence of peaks ascribed to MgO for MgAl2O4/Y-x samples was most likely to be related with the acidic medium which may dissolve the residual MgO during the synthesis procedure.As for MgAl2O4/Y-x samples, there were no other new peaks present,suggesting that no bulky zeolite crystals were formed during the synthesis.Besides,the broad“bulges”in the 2θ region of 20°-30°for MgAl2O4/Y-x samples indicated that some amorphous species arising from the zeolite Y nanoclusters existed in those samples.

Fig.1 XRD patterns of the fresh and aged parent MgAl2O4and modified MgAl2O4

Fig.2 FTIR spectra of the fresh parent MgAl2O4and modified MgAl2O4
With respect to the parent MgAl2O4,the peaks of the aged sample were apparently higher and narrower than those of the fresh one.This would be attributed to the agglomeration and growth of the spinel crystals during the severe hydrothermal treatment.Whereas in the case of MgAl2O4/Y-x samples,much less distinct differences were detected when comparing the XRD spectra of fresh samples with those of aged ones.This was indicative of the improvement in hydrothermal stability for the spinel phase in the modified samples.

Fig.3 FTIR spectra of the parent Y nanocluster(a), Y nanocluster at pH 7.0(b)and pH 5.0(c)
The FTIR spectra of the parent MgAl2O4and modified MgAl2O4samples(Fig.2)revealed that they all had two broad bands at 510 and 700 cm-1,which were both ascribed to the AlO6groups.30However,no additional band at 570-580 cm-1typically ascribed to the six-member rings of Y type zeolite27,28for the modified MgAl2O4samples was observed.Instead,another band at around 1030 cm-1appeared in the spectra of MgAl2O4/Y-x samples,which corresponded to the asymmetric stretching vibrations of Si―O―Al in the zeolite Y framework31or amorphous aluminosilicate.This gave a hint of two possibilities:one was that the structural building units of zeolite Y may have been incorporated into the mesoporous structure of MgAl2O4;the other was that the zeolite Y nanoclusters were transformed into amorphous aluminosilicate during the synthesis and then inserted into the mesoporous spinels.In the case of the former assumption,the absence of the band at 570-580 cm-1may probably be rationalized by the assumption that this band was somewhat overlapped with the broad band ofAlO6groups at 510 cm-1.
For further clarification,the solid products obtained by filtrating the parent nanocluster solution and nanocluster solutions at acidic conditions were also characterized by FTIR.The parent nanocluster showed all bands typical of faujasite crystals.The spectra of nanocluster solutions treated under acidic conditions(Fig.3)also revealed some representative bands of zeolite Y,including the band at 575 cm-1.Thus it can be concluded that the structural building units of zeolite Y were introduced into the spinel under acidic conditions.

Fig.4 TEM images of the calcined MgAl2O4(a,c)and MgAl2O4/Y-5(b,d)
The TEM images of the parent MgAl2O4and modified sample MgAl2O4/Y-5 were shown in Fig.4.The micrograph of MgAl2O4showed the presence of crystals mostly well below the size of 20 nm that were agglomerated together.With regard to the modified sample,some spots having an interplanar spac-ing of about 0.7 nm validated the existence of microporosity. Since the mesoporous structure of the spinel is greatly different from other typical ordered mesoporous materials such as MCM-41,and the spinel particles are aggregates of nanocrystallites,the microporosity is difficult to be clearly recognized.
The parent MgAl2O4and modified MgAl2O4samples either for fresh or aged ones exhibited type IV adsorption-desorption isotherms typical of mesoporous materials with H2hysteresis loops(Fig.5),probably attributed to an ink-bottle-like pore structure.In contrast with the parent MgAl2O4,steep increases at low relative pressure p/p0for the modified samples suggested the presence of microporous structure.This could also be affirmed by the markedly larger micropore surface areas and micropore volumes of the modified samples than those of the parent MgAl2O4(Table 2).
For the fresh samples,with decreasing the pH value of the synthesis system from 7 to 5,the micropore surface area increased from 60.4 to 130.5 m2·g-1,while the micropore volume increased from 0.025 to 0.076 cm3·g-1.When the pH value was further lowered,the loss of microporosity was observed instead.This may be interpreted by the more primary or secondary building units derived from zeolite Y nanoclusters in more acidic medium,which resulted in the incorporation of more zeolite structural units into the mesoporous spinel and endowed the corresponding MgAl2O4/Y-x samples with increased microporosity.But when the synthesis medium was too acidic, the primary or secondary building units of zeolite may also be further dissolved,giving rise to less effective incorporation and slightly lower microporosity.The change in surface areas of the aged samples with the pH value followed a similar way to the fresh samples.Furthermore,the mean pore diameter was reduced with the incorporation of zeolite Y nanoclusters,reflecting the blockage of the mesopores.
Table 3 presented the chemical composition of the modified spinels.As observed in XRD analysis of the modified samples, the residual free MgO was dissolved in the acidic medium during the synthesis procedure.Thus the mass of spinel present in the modified samples was calculated according to the stoichiometric formula.It showed that the amount of inserted Si species varied with the pH value similarly to the microporosity. But the spinel content continuously decreased with decreasing the pH value.Furthermore,it should be noted that the spinel content in the modified samples was lower than that in the starting composition.This may indicate that some species in the parent spinel(such as free MgO,amorphous Mg and Al species which were not completely transformed into the spinel phase)were dissolved in strongly acidic environments and the amount of dissolved species increased with lowering the pH value.

Fig.5 N2adsorption-desorption isotherms of the fresh(a)and aged(b)parent MgAl2O4and modified MgAl2O4samples

Table 2 Surface areas and pore properties of the fresh and aged parent MgAl2O4and modified MgAl2O4samples
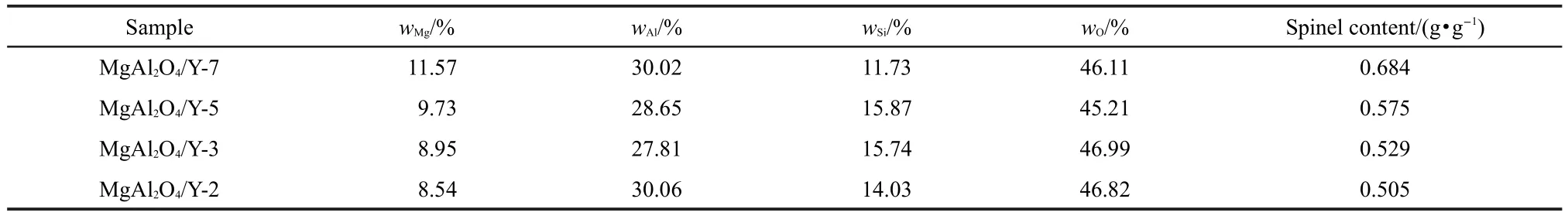
Table 3 Chemical compositions of the modified spinels
The FTIR spectra of pyridine adsorbed on the H-form samples after evacuation at different temperatures were employed to elucidate the change in the acidity of the modified samples. The two bands at about 1540 and 1450 cm-1correlate with pyridinium ions(pyridine chemisorbed on Brönsted acid sites)and coordinatively bound pyridine(pyridine interacting with Lewis acid sites),respectively.32-34The quantitative information ofBrönsted(B)and Lewis(L)acid sites calculated from pyridine adsorption at 200 and 350°C was listed in Table 4.

Table 4 Amount of B and Lacid sites determined by FTIR of pyridine adsorption for the aged samples at different desorption temperatures

Fig.6 FTIR spectra of pyridine adsorbed on different aged samples after desorption at 200°C

Fig.7 FTIR spectra of pyridine adsorbed on different aged samples after desorption at 350°C
Apparently,for the parent MgAl2O4,only a minor amount of weak Lewis acid sites was present(Fig.6 and Fig.7).In comparison with the acidity of MgAl2O4,both Brönsted and Lewis acid sites of the modified samples were remarkably increased. The total amount of Brönsted and Lewis acid sites first increased with decreasing the pH value of the synthesis system and reached a maximum for the sample MgAl2O4/Y-5,but then decreased as the pH value was further reduced.This tendency was consistent with the order of the microporosity obtained from the N2adsorption results.Moreover,the results indicated that the introduction of zeolite nanoclusters contributed more Lewis acid sites than Brönsted acid sites.
On the other hand,it was clear that the increased Brönsted acidity was mostly strong and the weak Lewis acidity was in major proportion of the total Lewis acidity for MgAl2O4/Y-x samples.As is known,the strong Lewis acidity predominates in the total Lewis acidity for the amorphous aluminosilicate species which have both Brönsted and Lewis acid sites.35So the fact that the increased Lewis acidity was primarily weak suggested that the incorporation of zeolitic structural units rather than amorphous aluminosilicate species would be responsible for the increased acidity of the MgAl2O4/Y-x samples.And the amorphous species that existed in the modified samples were mostly amorphous almina or silica that had little acidity.
Concerning the Kaolin clay,the Brönsted acidity was higher than that of MgAl2O4,but the acidity was far lower than those of MgAl2O4/Y-x samples.
It has been reported that adsorption of the probe molecule (hexafluoro-2-propanol,hereinafter HFIP)occurs dissociatively selectively on basic sites for a number of oxides,such as TiO2,MgAl2O4,and ZnAl2O4.36-38The sites responsible for the dissociation of HFIP may be identified as exposed oxide ions.
After the adsorption of HFIP on the parent MgAl2O4,a new sharp band at 1376 cm-1due to the CH bending vibration was observed(Fig.8).Simultaneously,the stretching vibration bands of CF3were detected at 1290,1230,1193,and 1095 cm-1.As for all the modified samples,only two distinct bands at 1376 and 1290 cm-1were observed,implying much weaker basicity than the parent MgAl2O4.In addition,different from MgAl2O4/Y-7 and MgAl2O4/Y-5,the two samples MgAl2O4/Y-3 and MgAl2O4/Y-2 showed two weak bands at around 1230 and 1193 cm-1,demonstrating slightly more basic sites for the latter two samples than those for the former.Concerning the Kaolin clay,no pronounced peaks were detected,giving proof of negligible basic sites.
3.2 VGO catalytic cracking performance
The results of VGO cracking over MgAl2O4and MgAl2O4/ Y-x samples were listed in Table 5,in which the data of the Kaolin clay were also included as a reference.Evidently,MgAl2O4was provided with much lower conversion than that of Kaolin clay.But the overall conversions of the MgAl2O4/Y-x samples were enhanced considerably compared to the parent MgAl2O4. And the sequence of the conversions ranked almostly the same as the order of the acidity.Moreover,it was noteworthy that the two samples MgAl2O4/Y-7 and MgAl2O4/Y-2 had conversions close to that of Kaolin clay in spite of the much higher acidity for the former samples.
Regarding the selective hydrogen transfer coefficient HTCa clearly larger value was observed for MgAl2O4than that for Kaolin clay.But the HTC values of the modified spinels were significantly decreased relative to that of MgAl2O4.Combined the lower HTC values of the MgAl2O4/Y-x samples than Kaolin clay with the much higher hydrogen transfer activity of the amorphous aluminosilicate species with Brönsted acidity than Kaolin clay,35the possibility of the incorporation of the amorphous aluminosilicate species into the mesoporous spinels was further excluded.However,in this case the phenomenon that the higher acidity resulted in lower HTC value seemed discrepant with the general knowledge that the acid density was proportional to the hydrogen transfer activity.An assumption was that the basic sites in MgAl2O4would favor hydrogen transfer reactions.A similar result was reported that MgAl2O4was effective in reducing olefins in gasoline by promoting hydrogen transfer reactions.41As the acidity increased in the case of MgAl2O4/Y-x samples,the basicity was weakened,leading to the reduction in HTC values.
On the other hand,the non-selective hydrogen transfer activity can be represented by the selectivity of coke and the sum of H2and CH4.The much higher values for the parent MgAl2O4than those of Kaolin clay may also be related to the basicity of MgAl2O4in consideration of its weak acidity.This may be related to the cyclization and aromatization reactions which proceed via dehydrogenation route catalyzed by the basic sites of Mg―Al―O.18Likewise,the non-selective hydrogen transfer reactions were drastically suppressed by introduction of zeolite nanoclusters.
To sum up,the incorporation of zeolitic microporosity into the mesopores of the spinel not only increased the acidity and hydrothermal stability of the modified samples but also enhanced the cracking ability of the heavy feedstock.What’s more,the non-selective hydrogen transfer reactions were suppressed and the product selectivity was improved.As the nature of basic sites in heterogeneous catalysis is still poorly understood in typical base catalyzed reactions,it would be even harder to illustrate the role of basic sites in catalytic cracking.
For checking the potential use of the modified spinels as part of an FCC catalyst formulation,a formulation was simulated with the modified spinel,MgAl2O4/Y-3(M),as partial matrix,and compared with a catalyst containing Kaolin clay as reference(K).The catalyst K was comprised of 30%(w)of USY zeolite,20%(w)of alumina,and 50%(w)of Kaolin clay. While for the catalyst M,30%(w)of Kaolin clay was substituted by MgAl2O4/Y-3 and the other components remained unchanged.Both FCC catalysts were steamed at the same condition for those spinels before microactivity(MA)test.The catalytic results were given in Table 6,and the catalyst M showed apparently higher conversion,LPG,and gasoline yields than the reference catalyst K,reaffirming the higher cracking activi-ty of the modified spinel.As known from our previous work,29the presence of spinel in the catalyst formulation was detrimental to the zeolite.However,the result here indicated that the higher cracking ability of the modified sample than Kaolin clay outbalanced the negative effect of Mg on the zeolite.

Table 5 VGO cracking over different aged samplesa

Table 6 Catalytic behavior of MgAl2O4/Y-3(M)as matrix in simulated FCC catalyst formulation compared to that of a reference Kaolin clay(K)as matrix for VGO cracking

Table 7 The amount of SO2uptake over different aged samples
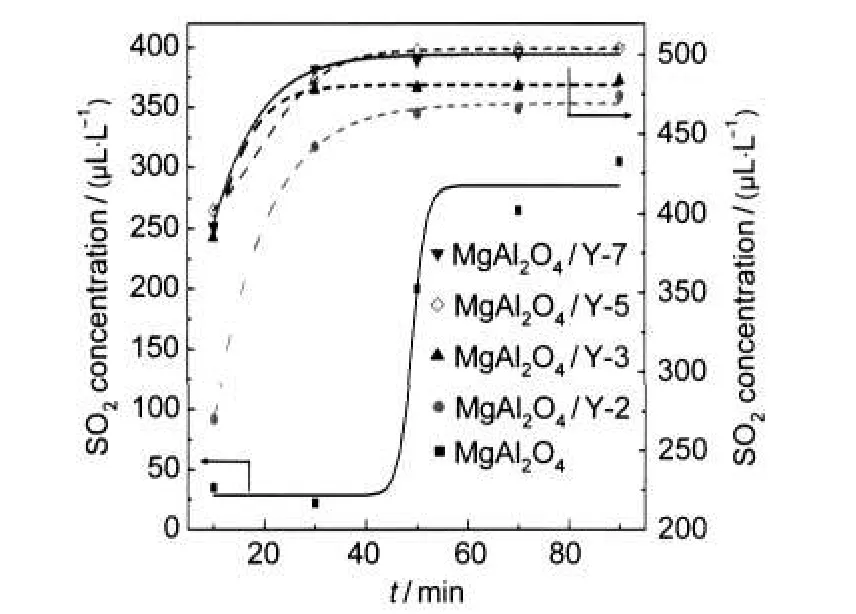
Fig.9 De-SOxactivities of different aged samplesreaction temperature:700°C;catalyst mass:0.6 g;flow rate:235 mL·min-1; SO2concentration:800 μL·L-1
3.3 SO2uptake capacity
The performance of the studied catalysts in the De-SOxprocess was compared in Fig.9.The amount of SO2uptake per unit mass of the composite was calculated by integration method and listed in Table 7.It can be observed that the modified samples presented lower SO2pick-up capacity than the parent MgAl2O4,which was in agreement with the weaker basicity of the modified samples.And the amounts of SO2removed for samples MgAl2O4/Y-3 and MgAl2O4/Y-2 were larger than the other modified samples.
However,the amount of SO2uptake per unit mass of spinel present in the composite better reflected the real capacity of SO2uptake.The results suggested that the incorporation of zeolitic component decreased the capacity of SO2uptake for the spinel part but the extent of the decrease was lower as the synthetic condition was more acidic.The decrease in the De-SOxcapacity for the spinel part may be ascribed to the surface basic sites of the spinel phase deactivated by the inserted species. While the larger recoverable SO2uptake capacity for the spinel part in more acidic environments may imply that the linkage between the spinel and inserted species in the modified samples was looser in more acidic conditions.
4 Conclusions
Compared with Kaolin clay,the typical inert matrix of an FCC catalyst,the general basic material MgAl2O4applied as a sulfur transfer agent exhibited considerably lower cracking ability and higher selective or non-selective hydrogen transfer activity.The higher HTC value,larger coke and hydrogen selectivities for MgAl2O4in VGO cracking tests were probably conduced by promoting hydrogen transfer reactions and dehydrogenation reactions catalyzed by basic sites rather than the usual bimolecular hydrogen transfer reactions associated with acid sites.In light of this situation,it is necessary to enhance the hydrothermally stable acidity and improve the cracking activity for MgAl2O4.The improved activity for cracking of heavy oil would make the modified spinel to be potential matrix of FCC catalysts.
The results indicated that processing the spinels with clear zeolite Y gel solutions was an effective way to improve the cracking performance by increasing both Lewis and Brönsted acid sities.On the other hand,the basic sites and SO2uptake capacity of the spinel were obviously reduced by modification. Still,some basic sites were remained in the modified spinels. However,it should be noted that,in practice,some rare earth metal oxides and/or transition metal ions are introduced as promoters of MgAl2O4considering its relatively low De-SOxactivity.The doping of promoters to the modified spinels would make the De-SOxactivity more acceptable.This will be further studied in the future.
Moreover,the improved cracking ability of heavy oil made the modified spinels interesting FCC matrices superior to the Kaolin clay.Also,the much larger amount of the modified spinels as FCC matrices compared to the conventional sulfurtransfer agent could compensate the decrease in the SO2pickup capacity.Consequently,the modified MgAl2O4samples with both basic and acid sites will be potential FCC matrix components attached with the function of SOxremoval.
(1) Păcurariu,C.;Lazău,I.;Ecsedi,Z.;Lazău,R.;Barvinschi,P.; Mărginean,G.J.Eur.Ceram.Soc.2007,27,707.
(2) Prabhakaran,K.;Patil,D.S.;Dayal,R.;Gokhale,N.M.; Sharma,S.C.Mater.Res.Bull.2009,44,613.
(3)Guo,J.;Lou,H.;Zhao,H.;Wang,X.;Zheng,X.Mater.Lett. 2004,58,1920.
(4) Iqbal,M.J.;Farooq,S.Mater.Sci.Eng.B 2007,136,140.
(5) Bhattacharyya,A.A.;Woltermann,G.M.;Yoo,J.S.;Karch,J. A.;Cormier,W.E.Ind.Eng.Chem.Res.1988,27,1356.
(6) Yoo,J.S.;Bhattacharyya,A.A.;Radlowski,C.A.Ind.Eng. Chem.Res.1991,30,1444.
(7)Yoo,J.S.;Bhattacharyya,A.A.;Radlowski,C.A.Ind.Eng. Chem.Res.1992,31,1252.
(8) Wang,J.;Li,C.Mater.Lett.1997,32,223.
(9) Wang,J.A.;Chen,L.F.;Limas-Ballesteros,R.;Montoya,A.; Dominguez,J.M.J.Mol.Catal.A-Chem.2003,194,181.
(10) Wang,J.;Li,C.Appl.Surf.Sci.2000,161,406.
(11) Wang,J.;Chen,L.;Li,C.J.Mol.Catal.A-Chem.1999,139, 315.
(12) Corma,A.;Palomares,A.E.;Rey,F.Appl.Catal.B 1994,4,29.
(13) Corma,A.;Palomares,A.E.;Rey,F.;Márques,F.J.Catal. 1997,170,140.
(14) Cantú,M.;López-Salinas,E.;Valente,J.S.;Montiel,R. Environ.Sci.Technol.2005,39,9715.
(15) Palomares,A.E.;Lópes-Nieto,J.M.;Lázaro,F.J.;Lópes,A.; Corma,A.Appl.Catal.B 1999,20,257.
(16) Pereira,H.B.;Polato,C.M.S.;Monteiro,J.L.F.;Henriques, C.A.Catal.Today 2010,149,309.
(17) Busca,G.Catal.Today 1998,41,191.
(18) Shen,J.;Kobe,J.M.;Chen,Y.;Dumesic,J.A.Langmuir 1994, 10,3902.
(19) Iglesla,E.;Barton,D.G.;Biscardi,J.A.;Gines,M.J.L.;Soled, S.L.Catal.Today 1997,38,339.
(20) Wang,L.;Guo,H.;Qi,W.;Su,S.;Deng,X.;Liu,J.;Liu,S. Sulfur TransferAdditive for Catalytic Cracking of Hydrocarbons and a Catalytic Cracking Process of Hydrocarbons Using the Same.US Patent 20030121824A1, 2003-01-21.
(21) de Morais,B.A.H.;Ramos,F.S.O.;Pinheiro,T.B.;Lima,C. L.;de Sousa,F.F.;Barros,E.B.D.;Filho,J.M.;de Oliveira,A. S.;de Souza,J.R.;Valentini,A.;Oliveira,A.C.Appl.Catal.AGen.2010,382,148.
(22) Valente,J.S.;Hernandez-Cortez,J.;Cantu,M.S.;Ferrat,G.; López-Salinas,E.Catal.Today 2010,150,340.
(23) Do,T.O.;Nossov,A.;Springuel-Huet,M.A.;Schneider,C.; Bretherton,J.L.;Fyfe,C.A.;Kaliaguine,S.J.Am.Chem.Soc. 2004,126,14324.
(24) Do,T.O.;Kaliaguine,S.J.Am.Chem.Soc.2003,125,618.
(25) Do,T.O.;Kaliaguine,S.Angew.Chem.2002,114,1078.
(26) Liu,Y.;Zhang,W.Z.;Pinnavaia,T.J.J.Am.Chem.Soc.2000, 122,8791.
(27) Tan,Q.;Liu,H.;Song,T.;Shi,G.;Shen,B.;Bao,X.AIChE J. 2008,54,1850.
(28) Tan,Q.;Bao,X.;Song,T.;Fan,Y.;Shi,G.;Shen,B.;Liu,C.; Gao,X.J.Catal.2007,251,69.
(29) Xu,X.;Ran,X.;Cui,Q.;Li,C.;Shan,H.Energy Fuels 2010, 24,3754.
(30) Olhero,S.M.;Ganesh,I.;Torres,P.M.C.;Ferreira.J.M.F. Langmuir 2008,24,9525.
(31) Ju,Y.N.;Shen,Z.H.;Zhao,J.;Zhao,J.Q.;Wang,X.L.Acta Phys.-Chim.Sin.2006,22,28.[鞠雅娜,沈志虹,赵 佳,赵俊桥,王秀林.物理化学学报,2006,22,28.]
(32) Tonetto,G.M.;Ferreira,M.L.;Atias,J.A.;de Lasa,H.I. AIChE J.2006,52,754.
(33) Jiang,G.;Zhang,L.;Zhao,Z.;Zhou,X.;Duan,A.;Xu,C.;Gao, J.Appl.Catal.A-Gen.2008,340,176.
(34) Lercher,J.A.;Jndling,C.G.;Eder-Mirth,G.Catal.Today 1996, 27,353.
(35) Song,H.T.;Jiang,W.B.;Da,Z.J.Acta Petrolei Sin.(Pet. Process.Sect.)2003,19,14.[宋海涛,蒋文斌,达志坚.石油学报(石油加工),2003,19,14.]
(36) Rossi,P.F.;Busca,G.;Lorenzelli,V.;Saur,O.;Lavalley,J.C. Langmuir 1987,3,52.
(37) Rossi,P.F.;Busca,G.;Lorenzelli,V.;Waqif,M.;Saw,O.; Lavalley,J.C.Langmuir 1991,7,2677.
(38) Lavalley,J.C.Catal.Today 1996,27,377.
(39) Wallenstein,D.;Harding,R.H.Appl.Catal.A-Gen.2001,214, 11.
(40) Wang,G.;Xu,C.;Gao,J.Fuel Process.Technol.2008,89,864.
(41) Ren,Y.J.;Shi,L.China Inorg.Chem.Ind.2008,40,17. [任彦瑾,施 力.无机盐工业,2008,40,17.]
March 30,2011;Revised:May 24,2011;Published on Web:June 28,2011.
Novel Modified Magnesium-Aluminate Spinels as Potential FCC Matrix Components
XU Xiao-Ling LI Chun-Yi*SHAN Hong-Hong
(State Key Laboratory of Heavy Oil Processing,College of Chemistry and Chemical Engineering,China University of Petroleum(East China),Qingdao 266555,Shandong Province,P.R.China)
Modified magnesium-aluminate spinels(MgAl2O4)were prepared by recrystallizing a mixture of MgAl2O4and zeolite Y nanoclusters in acidic medium to improve the acidity of MgAl2O4,which was commonly used as a sulfur transfer agent in fluid catalytic cracking(FCC)units.The acidity and basicity of these samples can be tuned by varying the pH value of the synthesis system.From the characterization and catalytic cracking tests the introduction of zeolitic building units into the spinels contributed to the increased microporosity,acidity,and hydrothermal stability.The catalytic results indicate that the activities and the product selectivities of the modified spinels for vacuum gas oil(VGO)cracking improved remarkably compared to the parent spinel.These samples exhibited even better performance than Kaolin clay for VGO cracking while retaining a part of the basic sites for oxidative SO2uptake.Moreover,the FCC catalyst prepared using the modified spinel as a partial matrix,after equilibration,also gave superior catalytic behavior compared to a reference FCC catalyst with Kaolin clay as the main matrix.
Magnesium-aluminate spinel;Zeolite Y nanocluster;Sulfur transfer; Catalytic cracking;Hydrothermal stability
∗Corresponding author.Email:chyli@upc.edu.cn;Tel:+86-532-86981862.
The project was supported by the Creation Group Foundation of the Ministry of Education of China.
国家教育部创新基金资助项目
O643
- 物理化学学报的其它文章
- Micellization Behavior of an Amphiphilic Drug Promethazine Hydrochloride-Surfactant System in an Aqueous Medium
- Synthesis of a Novel Thiadiazine Derivative and Electrochemical Properties for Pb2+Transfer across Water/1,2-Dichloroethane Interface
- YLuAG:Ce粉体的发光及闪烁特征:制备方法及缺陷效应
- 纳米碳纤维载铂作为质子交换膜燃料电池阳极催化剂
- 乙烯基噻吩共轭螺噁嗪化合物的密度泛函理论研究
- 用于单分子动力学实验的微流控混合器

