晚期糖基化终末产物受体在急性坏死性胰腺炎大鼠中的表达
苏晓菊 汪鹏 满晓华 蒋斐 杜奕奇 郑永志 金晶 龚燕芳 高军 湛先保 李兆申 邹多武
·论著·
晚期糖基化终末产物受体在急性坏死性胰腺炎大鼠中的表达
苏晓菊 汪鹏 满晓华 蒋斐 杜奕奇 郑永志 金晶 龚燕芳 高军 湛先保 李兆申 邹多武
目的观察急性坏死性胰腺炎(ANP)大鼠发病过程中胰腺组织及血清中晚期糖基化终末产物受体(the receptor for advanced glycation end products, RAGE)含量变化的规律。方法64只雄性SD大鼠按完全随机法分为对照组,ANP 6、18、24、36、48、72、96 h组,每组8只。采用腹腔注射20% L-精氨酸250 mg/100 g体重2次、间隔1 h方法制备ANP模型。对照组大鼠腹腔注射等容积生理盐水。胰腺组织行病理学检查并评分。检测腹水量、血清淀粉酶及RAGE浓度,实时PCR法检测胰腺组织RAGE mRNA表达。结果ANP组胰腺病理损伤随时间延长逐渐加重;腹水量从6 h的(1.98±0.64)ml增加到96 h的(8.69±0.62)ml;血清淀粉酶浓度从造模后6 h开始升高,18 h达(5069.88±603.25)U/L,36 h时恢复正常。对照组大鼠血清RAGE浓度及胰腺组织RAGE mRNA表达量分别为(18.33±2.99)ng/ml和0.41±0.13。ANP组6 h时两者开始升高,分别为(30.31±5.03)ng/ml和1.57±0.19,较对照组显著升高(P<0.05);24 h组达峰值,分别为(105.41±21.31)ng/ml和4.23±0.73,较ANP其他时间点显著升高(P值均<0.05);96 h下降至最低点,分别为(33.54±6.96)ng/ml和1.19±0.19,但仍高于对照组(P<0.05)。结论血清RAGE浓度及胰腺组织RAGE mRNA表达量在ANP发生36 h内逐渐增加,随后下降,但始终高于正常值。
胰腺炎,急性坏死性; 受体,晚期糖基化终产物; 胰腺组织; 炎症; 精氨酸; 大鼠
晚期糖基化终末产物受体(RAGE)属于细胞表面分子免疫球蛋白超家族多配体受体成员[1],广泛分布在单核-巨噬细胞、内皮细胞、平滑肌细胞、肾小球系膜细胞、肿瘤细胞、星形胶质细胞、T淋巴细胞等表面[2],可以与HMGB1、S100/钙结合蛋白,淀粉样肽等多种配体结合[3-4],激活多条细胞内信号转导途径,活化NF-κB[5],参与炎症反应过程。目前有研究提示,重症急性胰腺炎(SAP)合并器官衰竭时血清中RAGE水平显著升高。本实验观察急性坏死性胰腺炎(ANP)大鼠胰腺组织RAGE mRNA及血清RAGE浓度的动态变化,探讨RAGE的变化规律。
材料与方法
一、实验动物与分组
健康雄性SD大鼠,体重200~250 g,5~6周龄,清洁级,第二军医大学实验动物中心提供,动物合格证号:SCXK(沪)2007-0003。适应性喂养1周后按完全随机法分为对照组,ANP 6、18、24、36、 48、72、96 h组,每组8只。参照Tani等[6]方法,采用腹腔注射20% L-精氨酸(Sigma公司)250 mg/100 g体重2次、间隔1 h制备ANP模型。对照组大鼠腹腔注射等容积生理盐水。按各时间点处死大鼠,心脏取血,抽取腹水,留取胰腺组织。部分胰腺组织置液氮保存,部分置4%中性甲醛液固定。
二、观测指标及检测方法
1.血清淀粉酶、腹水量检测: 血清淀粉酶采用HITACHI-7150型自动生化分析仪测定,腹水量应用10 ml针筒抽吸测量。
2.胰腺组织病理学检查: 固定的胰腺组织常规石蜡包埋、切片、HE染色,采用盲法由病理科医师阅片,参照Rongione标准[7]进行评分。
3.血清RAGE浓度检测:采用RAGE 酶联免疫吸附法(ELISA)试剂盒 (上海威奥生物技术有限公司)检测,严格按照试剂盒说明书操作。
4.胰腺组织RAGE mRNA检测:采用实时PCR法。用Trizol试剂盒(上海晶美公司)提取胰腺组织总RNA。应用Primer5.0软件设计引物,RAGE引物:上游5′-AACACAGGAAGGACTGAAGC-3′,下游5′-AGACTCGGACTCGGTAGTTG-3′,片段长度190 bp;β-actin(内参)引物:上游5′-CCTGTACGCCAACAC-AGTGC-3′,下游5′-ATACTCCTGCTTGCTGATCC-3′,片段长度 251 bp。引物由上海英骏生物技术公司合成。逆转录cDNA后,使用 SYBR Premix Ex TagTM试剂盒,在ABI 7500PCR仪(Applied Biosystems公司)上采用两步法PCR扩增标准程序进行基因扩增。荧光定量PCR的结果以Ct值显示。以正常胰腺组织作为对照,△△Ct=△Ct样品-△Ct正常胰腺=(Ct样品-Ct内参)-(Ct正常胰腺-Ct内参),RQ=2-△△Ct。
三、统计学处理

结 果
一、血清淀粉酶、腹水量的变化
造模后6 h起,血清淀粉酶水平开始升高,18 h达峰值,以后下降,36 h恢复到正常值。腹水量随着时间推移逐渐增多,96 h达峰值(表1)。
二、大鼠胰腺组织病理改变
对照组胰腺未见明显损伤。ANP 6 h组胰腺组织出现间质水肿,有轻、中度炎细胞浸润;18 h组有大量炎细胞浸润,轻度实质坏死和出血;36 h组腺泡结构破坏较多,小叶排列紊乱,其间充斥大量炎性渗出;72 h组胰腺坏死加重,可见皂化斑;96 h组胰腺组织坏死最严重(图1)。ANP 6、18、24、36、48、72、96 h组胰腺组织病理分值分别为(3.38±1.30)、(6.88±0.64)、(9.50±1.77)、(10.25±1.04)、(12.00±1.31)、(12.38±1.06)、(13.50±0.93)分,均较对照组的(0.38±0.52)分显著升高(P值均<0.05)。
三、血清RAGE浓度的变化
ANP各组血清RAGE浓度均显著高于对照组(P值均<0.05),其中24 h组最高(表1)。
四、胰腺组织RAGE mRNA表达的变化
ANP各组胰腺组织RAGE mRNA表达量均显著高于对照组(P值均<0.05),其中24 h组的表达量最高(表1)。胰腺组织RAGE mRNA表达量与血清RAGE浓度变化的趋势完全一致(图2)。

表1 各组大鼠腹水量、血清淀粉酶及RAGE浓度、胰腺组织RAGE mRNA表达量的变化
注:与对照组比较,aP<0.05;与ANP 24 h组比较,bP<0.05
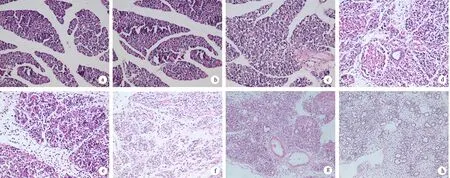
图1 对照组(a),ANP 6(b)、18(c)、24(d)、36(e)、48(f)、72(g)、 96 h(h)组胰腺组织病理改变(HE ×200)
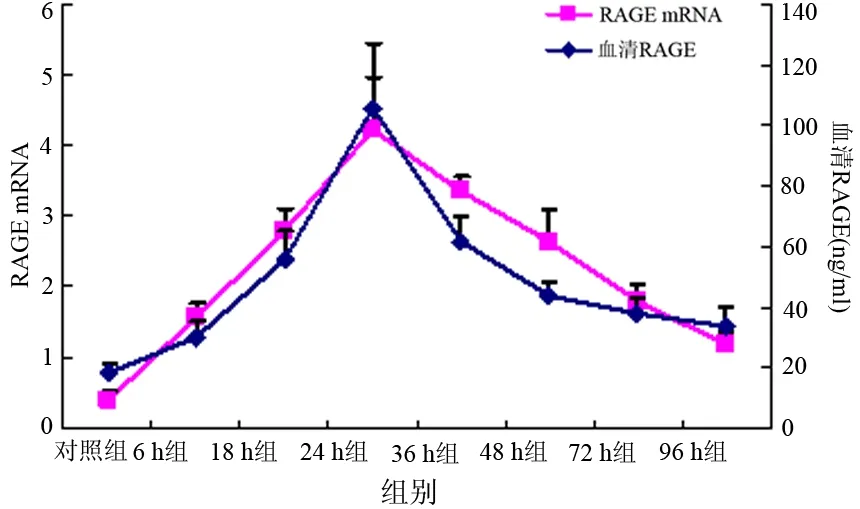
图2胰腺组织RAGE mRNA表达量与血清中RAGE浓度的变化趋势
讨 论
晚期糖基化终末产物受体(RAGE)最初在牛肺内皮细胞中发现,能结合多种配体[3-4]。它属于单跨膜片段受体,由400多个氨基酸组成,分为胞外段、跨膜段和胞内段,相对分子质量约25 000。其胞外段包含3个免疫球蛋白样结构(1个V区和2个C区),V区是RAGE胞外段与配体结合的主要位点,其后是疏水的跨膜段,最后是高度带电荷的胞内段[8]。在个体生长发育阶段,RAGE高水平表达于内皮细胞、单核巨噬细胞、外膜细胞、肾系膜细胞、平滑肌细胞的胞膜,尤其在心肌细胞、中枢神经系统表达丰富。在成熟的动物及人体中RAGE呈低水平表达。当细胞处于激活或应激状态时,细胞的RAGE表达急剧增加。
文献报道,吸烟相关性气道疾病、肉芽肿性炎症、间质性肺炎等几种持续性炎症性肺病患者的肺组织RAGE表达增强[9],晚期肾脏疾病和急性肺损伤患者血清RAGE水平升高[10-11]。Mullins等[12]报道,严重脓毒症或脓毒症休克患者入院时血清中RAGE浓度升高,但大多数患者在入院后96 h恢复至基线水平。
本结果显示,ANP组大鼠血清RAGE浓度及胰腺组织RAGE mRNA表达均较对照组明显增加,均在24 h时达峰值,此后逐渐下降,96 h时最低,但仍高于对照组,表明RAGE在ANP的病程中并不是线性增高的。但ANP大鼠的胰腺组织病理损伤随时间推移进行性加重,推测RAGE参与早期的炎症反应,还有其他炎症因子参与整个疾病过程。
[1] Wilson PG, Manji M, Neoptolemos JP. Acute pancreatitis as a model of sepsis. J Antimicrob Chemother, 1998, 41:51-63.
[2] Ulloa L, Tracey KJ. The cytokine profile: a code for sepsis.Trends Mol Med, 2005, 11:56-63.
[3] Goodwin GH, Shooter KV, Johns EW. Interaction of a non-histone chromatin protein (high-mobility group protein 2) with DNA. Eur J Biochem, 1975, 54:427-433.
[4] Melvin VS, Edwards DP. Coregulatory proteins in steroid hormone receptor action: the role of chromatin high mobility group proteins HMG-1 and -2. Steroids, 1999, 64:576-586.
[5] Bustin M. Regulation of DNA-dependent activities by functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol, 1999, 19:5237-5246.
[6] Tani S, Itoh H, Okabayashi Y, et al. New modeI of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci, 1990, 35:367-374.
[7] Rongione AJ, Kusske AM, Ashley SW, et al. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology, 1997, 112:960-967.
[8] Bicdlaus A, Hofmann MA, Ziegler R, et al. AGE and their interaction with AGE reception in vacular disease and diabetes mellitmi. Cardiovase Res, 1998, 37:586-600.
[9] Harashima A, Yamamoto Y, Cheng C, et al. Identification of mouse orthologue of endogenous secretory receptor for advanced glycation end-products: structure, function and expression. Biochem J, 2006, 396:109-115.
[10] Kalousova M, Jachymova M, Mestek O, et al. Receptor for advanced glycation end productsVsoluble form and gene polymorphisms in chronic haemodialysis patients. Nephrol Dial Transplant, 2007, 22:2020-2026.
[11] Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med, 2006, 173:1008-1015.
[12] Mullins G, Sunden-Cullberg J, Tokics L, et al. Increase of Soluble RAGE in Patients With Severe Sepsis/Septic Shock [PhD thesis]. Stockholm, Sweden: Karolinska University, Department of Medicine, 2005.
2011-05-25)
(本文编辑:吕芳萍)
Expressionofreceptorforadvancedglycationendproductsinratswithacutenecrotizingpancreatitis
SUXiao-ju,WANGPeng,MANXiao-hua,JIANGFei,DUYi-qi,ZHENGYong-zhi,JINJing,GONGYan-fang,GAOJun,ZHANXian-bao,LIZhao-shen,ZOUDuo-wu.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
ZOUDou-wu,Email:dwzou@smmu.edu.cn
ObjectivesTo explore the expression of the receptor for advanced glycation end products (RAGE) in pancreas and the serum concentration of RAGE in rats with acute necrotizing pancreatitis (ANP).MethodsSixty four male Spraque-Dawley rats were randomly divided into control group, ANP 6, 18, 24, 36, 48, 72, 96 h group with 8 rats in each group.The rat model of ANP was established by injecting 20% L-arginine intraperitoneally at the dose of 250mg/100g body weight for twice at the interval of 1 h. Rats in control group were injected intraperitoneally with the same amount of saline. The pancreas samples were histologically examined by light microscope and scored. The amount of ascites, levels of serum amylase and RAGE was determined; Real-time PCR was performed to detect the expression of RAGE mRNA in pancreatic tissue.ResultsPancreatic injuries aggravated with time. The amount of ascites increased from (1.98±0.64)ml at 6h to (8.69±0.62)ml at 96 h. Serum amylase level began to increase at 6h after L-arginine intraperitoneal injection and reached the peak value at 18 h [(5069.88±603.25)U/L], and returned to normal at 36 h. The serum concentration of RAGE and RAGE mRNA expression in pancreatic tissue were (18.33±2.99)ng/ml and 0.41±0.13 in the control group. The corresponding values increased at 6 h in ANP group, which were (30.31±5.03)ng/ml and 1.57±0.19, and they were significantly higher than those in the control group (P<0.05); they reached the peak value at 24 h [(105.41±21.31)ng/ml and 4.23±0.73], which were significantly higher than those in other ANP groups (P<0.05); at 96 h they decreased to the lowest point [(33.54±6.96)ng/ml and 1.19±0.19], which were still significantly higher than those in the control group (P<0.05).ConclusionsThe expression levels of RAGE in pancreatic tissues and serum level of RAGE increase within 36 h of ANP onset,then decrease gradually, but they are always higher than normal values.
Pancreatitis, acute necrotizing; Receptor, advanced glycation end products; Pancreatic tissue; Inflammation; Arginine; Rat
10.3760/cma.j.issn.1674-1935.2011.05.009
200433 上海,第二军医大学长海医院消化内科
邹多武,Email: dwzou@smmu.edu.cn

