大黄附子汤对急性坏死性胰腺炎大鼠小肠黏膜屏障功能的保护作用
路小光 战丽彬 康新 刘国辉 董云 范治伟 白黎智 刘莉 纪春阳 王小周
·论著·
大黄附子汤对急性坏死性胰腺炎大鼠小肠黏膜屏障功能的保护作用
路小光 战丽彬 康新 刘国辉 董云 范治伟 白黎智 刘莉 纪春阳 王小周
目的探讨大黄附子汤对急性坏死性胰腺炎(ANP)大鼠小肠黏膜屏障功能的影响及意义。方法将60只SD大鼠按数字表法随机分为假手术组(19只)、ANP组(21只)和大黄附子汤治疗组(20只)。经胰胆管逆行注入4%牛磺胆酸钠1 ml/kg体重建立ANP模型,同时行空肠造瘘。治疗组于制模后0.5 h经空肠造瘘管注入大黄附子汤2 ml,隔4、8 h再注入2 ml;其他两组注入等容积生理盐水。术后24 h经腹主动脉取血,测定血淀粉酶、内毒素、D-乳酸含量及二胺氧化酶(DAO)活性。取胰腺、小肠组织行病理学检查,测定肠上皮损伤指数,观察小肠黏膜超微结构改变。结果假手术组大鼠血淀粉酶、内毒素、D-乳酸含量及DAO活性分别为(152±32)U/L、(6.95±2.10)pg/L、(3.96±1.08)μg/ml和(14.26±2.67)μg/ml,ANP组分别为(1549±93)U/L、(40.48±3.41)pg/L、(12.34±1.23) μg/ml和(80.28±3.54)μg/ml,治疗组分别为(655±49)U/L、(19.55±2.50)pg/L、(6.75±1.36)μg/ml和(20.69±7.53)μg/ml,ANP组较假手术组明显升高,而治疗组较ANP组显著降低,但仍高于假手术组(P<0.05或<0.01)。ANP组小肠黏膜厚度、绒毛高度分别为(389.44±29.87)μm、(16.52±3.73)μm,显著低于治疗组的(501.95±45.38)μm、(27.82±5.17)μm,更显著低于假手术组的(658.72±57.49)μm和(35.49±6.43)μm;而肠上皮损伤指数为3.72±0.65,显著高于治疗组的2.12±0.37和假手术组的0.85±0.24。同时,大黄附子汤治疗后小肠黏膜组织学和超微结构改变亦均较ANP组明显减轻。结论大黄附子汤可明显减轻ANP大鼠小肠黏膜屏障功能损害的程度。
胰腺炎,急性坏死性; 大黄附子汤; 内毒素类; 二胺氧化酶; 肠道屏障功能障碍
重症急性胰腺炎(SAP)早期易并发肠屏障功能障碍(intestine barrier functional disturbance,IBFD),造成肠源性细菌和内毒素易位引起继发感染,导致全身炎症反应综合征(SIRS)和多器官功能障碍综合征(MODS) 等严重并发症的发生[1-5]。因此,如何保护肠黏膜屏障功能的完整性,减轻和防止发生肠源性感染,成为控制SAP发展、减少并发症的关键。本实验应用大黄附子汤干预急性坏死性胰腺炎(ANP)大鼠,观察其对小肠屏障功能的保护作用,为大黄附子汤临床应用提供理论依据。
材料和方法
一、实验动物及分组
清洁级健康雄性SD大鼠60只购自大连医科大学实验动物中心,体重(280±20)g。用抽签法将动物随机分为假手术组(19只)、ANP组(21只)和大黄附子汤治疗组(治疗组,20只)。采用逆行胰胆管缓慢注入4%牛磺胆酸钠1 ml/kg体重的方法制备ANP模型,假手术组大鼠仅触摸胰腺数次。另选取空肠起始部以下约2 cm处置入造瘘管,经由皮下引出体外,妥善固定。
二、药物制备与给药方法
将附子9 g(先煎)、大黄9 g(后下)、细辛3 g常规水煎制成悬浊液,置100℃水浴消毒80 min,冷却后置4℃冰箱。治疗组大鼠于制模后0.5 h开始,每隔4 h经瘘管注入大黄附子汤2 ml,共3次;假手术组和ANP组大鼠于同时间点注入等容积生理盐水。
三、检测指标及方法
1.血淀粉酶、内毒素、D-乳酸含量及二胺氧化酶(DAO)活性测定:术后24 h经腹主动脉取血,分离血清或血浆。全自动生化仪器测定大鼠血清淀粉酶含量,分光光度法测定血清D-乳酸含量(试剂盒购自Sigma公司),比浊法测定血浆内毒素含量(试剂盒购自天津一瑞生物工程有限公司),活性比色法测定血浆DAO活性(试剂盒购自上海杰美基因医药有限公司)。
2.胰腺、小肠黏膜病理组织学检查及肠上皮损伤指数测定:取胰腺及距幽门10 cm处约2 cm长小肠,常规固定病理检查,测20个小肠绒毛的绒毛高度及小肠黏膜厚度。参照文献[6]将小肠黏膜损伤分为6级:0级,正常黏膜绒毛结构;Ⅰ级,肠黏膜绒毛顶端上皮下间隙增宽;Ⅱ级,绒毛上皮下间隙进一步扩大,绒毛顶端上皮抬高与固有膜剥离;Ⅲ级,绒毛两边上皮成块脱落;Ⅳ级,绒毛上皮完全脱落,仅存固有膜结构;V级,黏膜固有膜崩解,出现出血和溃疡。
3.小肠黏膜超微结构观察:取约2 cm长小肠,置2.5%戊二醛溶液中固定,经乙醇脱水,环氧树脂包埋,美兰染色后光学显微镜下定位, 醋酸铀、柠檬酸铅染色,透射电镜(JEM-200EX,JEOL公司)下观察并摄片。
四、统计学处理
结 果
一、血清淀粉酶、D-乳酸、内毒素含量及DAO活性的变化
ANP组大鼠血淀粉酶、D-乳酸、内毒素含量及DAO活性均明显高于假手术组(P<0.01)。治疗组大鼠血淀粉酶、D-乳酸、内毒素含量及DAO活性较ANP组明显降低(P<0.01或<0.05),但仍显著高于假手术组(P<0.05,表1)。

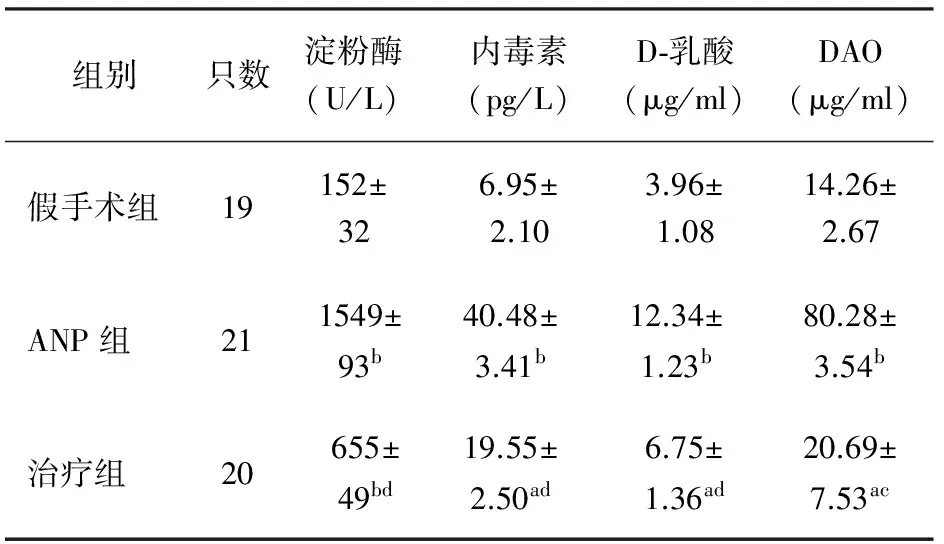
组别只数淀粉酶(U/L)内毒素(pg/L)D⁃乳酸(μg/ml)DAO(μg/ml)假手术组19152±326.95±2.103.96±1.0814.26±2.67ANP组211549±93b40.48±3.41b12.34±1.23b80.28±3.54b治疗组20655±49bd19.55±2.50ad6.75±1.36ad20.69±7.53ac
注:与假手术组比较,aP<0.05,bP<0.01;与ANP组比较,cP<0.05,dP<0.01
二、胰腺和小肠病理组织学变化
假手术组胰腺及小肠黏膜组织均无明显变化。ANP组胰腺组织不同程度出血、坏死、炎性细胞浸润和胰周组织脂肪坏死;小肠黏膜上皮糜烂,大片坏死、脱落及缺损,大量炎细胞浸润,绒毛样结构减少。治疗组胰腺水肿、出血、坏死,组织间隙略增宽,少量炎性细胞浸润;小肠黏膜轻度水肿,片状坏死,绒毛部分脱落及倒伏(图1)。
三、小肠黏膜厚度、绒毛高度及肠上皮损伤指数变化
ANP组大鼠小肠黏膜厚度和绒毛高度均较假手术组明显降低(P<0.01),小肠上皮损伤指数较假手术组明显增加(P<0.01);治疗组小肠黏膜厚度和绒毛高度较ANP组显著增加(P<0.05或<0.01),而小肠上皮损伤指数较ANP组明显降低(P<0.01),但与假手术组仍差异显著(P<0.05或<0.01,表2)。
四、小肠黏膜超微结构改变
假手术组大鼠小肠黏膜上皮细胞表面微绒毛排列整齐,柱状上皮细胞结构完整,细胞质内细胞器丰富,结构未见异常。ANP组大鼠小肠黏膜上皮微绒毛稀疏、部分缺如,上皮细胞萎缩,细胞核浓缩,染色质边聚,线粒体高度肿胀,嵴缺失,内质网肿胀。治疗组大鼠小肠黏膜上皮微绒毛排列不规则,上皮细胞线粒体、内质网轻度肿胀(图2)。

图1假手术组(a)、ANP组(b)和治疗组(c)胰腺(左列,HE ×400)和小肠黏膜(右列,HE ×100)病理变化
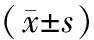
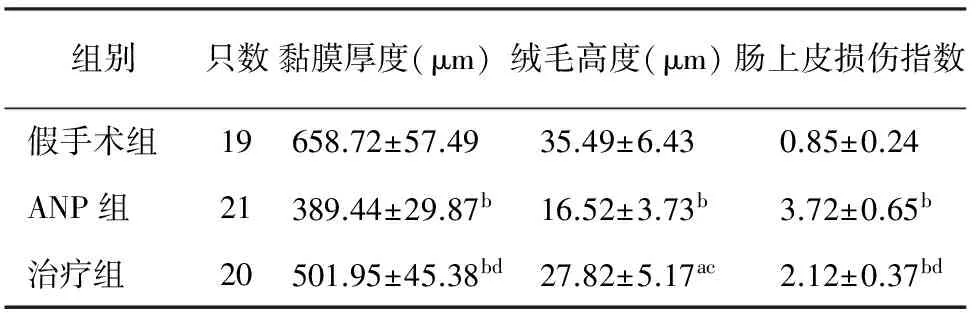
组别只数黏膜厚度(μm)绒毛高度(μm)肠上皮损伤指数假手术组19658.72±57.4935.49±6.430.85±0.24ANP组21389.44±29.87b16.52±3.73b3.72±0.65b治疗组20501.95±45.38bd27.82±5.17ac2.12±0.37bd
注:与假手术组比较,aP<0.05,bP<0.01;与ANP组比较,cP<0.05,dP<0.01

图2假手术组(a)、ANP组(b)和治疗组(c)小肠黏膜超微结构改变(×5000)
讨 论
SAP在中医学中属于“胃脘痛”、“脾心痛”等范畴。因肝胆疏泄、胆汁郁滞不畅,致脾胃运化失常,痰湿内生,阳气被遏。日久,湿从热化,湿热壅结,以致寒热错杂,痰淤凝滞,腑气不通,不通则痛而发本病。大黄附子汤源自汉·张仲景《金匮要略》,由大黄、附子、细辛组方,有效成分包括大黄素、乌头碱以及细辛醚等。大黄性苦寒,味重浊,直降下行,走而不守。《本经》中记载大黄可荡涤肠胃,推陈出新,通利水谷。附子、细辛性温热,散寒止痛,温运脾胃。三者合用,寒热并投,刚柔并用,可避免大黄苦寒凝滞之弊,同时增加疏通之力。本方具有辛开苦降,清热解毒,化淤散结之功,使脏腑通畅,气血调和,邪去正安,危病得愈。我们曾报道[7],大黄附子汤可抑制ANP大鼠血清内毒素、TNF-α、IL-1β释放,上调抗炎因子IL-4水平,从而减轻ANP损伤的程度。本实验结果显示,大黄附子汤治疗后大鼠小肠黏膜的厚度、绒毛高度及肠上皮损伤指数均较ANP组得到明显改善,说明大黄附子汤对ANP大鼠小肠黏膜具有良好的保护作用。
DAO是存在于肠黏膜上层绒毛细胞中具有高度活性的细胞内酶,以空、回肠活性最高。肠黏膜细胞受损、坏死后该酶释放入血,导致血浆DAO活性增高。D-乳酸是肠道细菌酵解的固有代谢产物,正常情况下很少被吸收。当肠黏膜通透性增加时,肠道内D-乳酸进入血流,而哺乳动物无将其分解的酶系统,因此血浆D-乳酸含量升高[8]。内毒素是肠道内革兰阴性菌裂解产物,由于健全的肠道屏障作用,正常肠道内游离内毒素处于高水平[9]。因此,血DAO活性、D-乳酸和内毒素含量均是肠黏膜上皮细胞损伤及肠道屏障破坏的指标。本实验结果显示,大黄附子汤治疗后大鼠血浆DAO活性、D-乳酸和内毒素含量均较ANP组明显降低,说明大黄附子汤可明显减轻小肠黏膜上皮细胞的损伤,保护肠道屏障功能。
[1] 李能平,杨欣,顾永峰,等.急性胰周积液和胰腺坏死对急性胰腺炎预后的影响.中华胰腺病杂志,2009,9:79-81.
[2] Pearce CB,Zinkevich V,Beech I,et al.Using the polymerase chain reaction coupled with denaturing gradient gel electrophoresis to investigate the association between bacterial translocation and systemic inflammatory response syndrome in predicted acute severe pancreatitis.World J Gastroenterol,2005,11:7142-7147.
[3] 苗彬,崔乃强,赵二鹏,等.重症急性胰腺炎复发相关因素分析.中华胰腺病杂志,2009,9:150-152.
[4] Mofidi R,Patil PV,Suttie SA,et al.Risk assessment in acute pancreatitis.Br J Surg,2009,96:137-150.
[5] Vege SS,Gardner TB,Chari ST,et al.Low mortality and high morbidity in severe acute pancreatitis without organ failure:a case for revising the Atlanta classification to include "moderately severe acute pancreatitis".Am J Gastroenterol,2009,104:710-715.
[6] Nieuwenhuijzen GA,Haskel Y,Lu Q, et al.Macrophage elimination increases bacterial translocation and gut-origin septicemia but attenuates symptoms and mortality rate in a model of systemic inflammation.Ann Surg,1993,218:791-799.
[7] 路小光,战丽彬,刘伟光,等.大黄附子汤对重症急性胰腺炎肺损伤大鼠肺组织Toll样受体4/核转录因子-κB的影响.中国中西医结合急救杂志,2009,16:14-16.
[8] Yuan ZQ,Peng YZ,Li XL,et al.Induction of heat shock protein 70 by sodium arsenite attenuates burn-induced intestinal injury in severe burned rats.Burns,2008,34:247-253.
[9] Wang YL,Zheng YJ,Zhang ZP,et al.Effects of gut barrier dysfunction and NF-kappaB activation on aggravating mechanism of severe acute pancreatitis.J Dig Dis,2009,10:30-40.
2010-04-16)
(本文编辑:吕芳萍)
2011年第1期 曲衍清、梁锐、王博、王立明、高振明“胰腺神经内分泌癌一例”一文的通信作者为:高振明,Email:gaozhenmingdl@163.com。特此补充说明。
ProtectiveeffectsofDahuangfuzidecoctionontheintestinebarrierfunctionalofacutenecrotizingpancreatitisinrats
LUXiao-guang,ZHANLi-bin,KANGXin,LIUGuo-hui,DONGYun,FANZhi-wei,BAILi-zhi,LIULi,JIChun-yang,WANGXiao-zhou.
DepartmentofEmergency,ZhongshanHospital,DalianUniversity,Dalian116001,China
KANGXin,Email:kangxinhao1@yahoo.com.cn
ObjectiveTo observe the effects of Dahuangfuzi decoction on the intestine barrier functional of acute necrotizing pancreatitis in rats.MethodsThe 60 rats were randomly divided into sham operation group (n=19), ANP group (n=21), and Dahuangfuzi treatment group (n=20). The rats of ANP group were induced by injecting 1ml/kg of 4% sodium taurocholate into the pancreatiobiliary duct, and jejunal fistula was esablished. The rats of treatment group
Dahuangfuzi decoction (2 ml, repeated at 4 and 8 h) through jejunum distal stoma tube 0.5 h after ANP induction. The other 2 groups received same amount of normal saline. Blood sample was collected through abdominal aorta, 24 h after ANP induction, and the serum amylase, endotoxin, D-lactate, plasma diamine oxidase (DAO) were detected. Pancreas, small intestine tissue was harvested for pathologic examination, index of intestinal epithelial damage was measured and ultrastructural changes in small intestinal mucosa was observed.ResultsThe expression of serum amylase, endotoxin, D-lactate, DAO in sham operation group was (152±32)U/L, (6.95±2.10)pg/L, (3.96±1.08)μg/ml and (14.26±2.67)μg/ml, while the corresponding values were (1549±93)U/L, (40.48±3.41)pg/L,(12.34±1.23) μg/ml and (80.28±3.54)μg/ml in ANP group, and they were (655±49)U/L, (19.55±2.50)pg/L,(6.75±1.36)μg/ml and (20.69±7.53)μg/ml in treatment group. The values in ANP group were significantly higher than those in sham operation group. The values in treatment group were significantly lower than those in ANP group, but significantly higher than those in sham operation group (P<0.05 orP<0.01). The thickness and height of intestinal mucosa in ANP group were (389.44±29.87)μm and (16.52±3.73) μm, which were significantly lower than those in treatment group [(501.95±45.38)μm, (27.82±5.17)]μm, and in sham operation group [(658.72±57.49)μm, (35.49±6.43)μm,Index of intestional epitholial donage in ANP group was 3.72±0.65 which is significently higher than those in theatment (2.12±0.37) and in sham operation group (0.85±0.24). The intestinal mucosa histological and ultrastructural changes in Dahuangfuzi treatment group were better than those in ANP group.ConclusionsDahuangfuzi decoction can significantly decrease the damage of intestine barrier function in ANP rats.
Pancreatitis, acute necrotizing; Dahuangfuzi decoction; Endotoxins; Diamine oxidase; Intestine barrier functional disturbance
10.3760/cma.j.issn.1674-1935.2011.01.012
国家自然科学基金项目(30672767、30971626);辽宁省科技厅(2007225005)
116001 大连,大连大学附属中山医院急诊科(路小光、康新、董云、范治伟、白黎智、刘莉、纪春阳、王小周);大连医科大学附属二院中医科(战丽彬);吉林大学附属第一医院急诊科 (刘国辉)
共同第一作者:战丽彬
康新,Email:kangxinhao1@yahoo.com.cn

