人核心蛋白聚糖真核表达载体的构建及其体内外抗瘤效应研究
王桂琴, 兰 晶, 于培霞, 邓志华 (山西医科大学微生物学免疫学教研室, 太原 0000;军事医学科学院微生物学流行病学研究所;山西大医院检验科;山西医科大学第二临床医学院消化科)
Decorin belongs to an expanding gene family of the secreted,small leucine-rich proteoglycans(SLRPs),which is composed of a leucine-rich core protein and a single glycosaminoglycan(GAG)chain.Decorin gene is located on human chromosome 12q21-q22 whose molecule weight is 95-130 kD with 40 kD of the core protein.Many studies indicated that DCN inhibited fibrosis and scar-formation by neutr alized TGF-β and interfered the binding of TGF-β with its receptor,which induced ectopic deposition of extracellular matrix[1].Increasing evidences showed that decorin can exert significant growth inhibitory effect on various types of tumor cells and is considered as a promising candidate for tumor gene therapy[2-9].The purpose of this study was to construct a eukaryotic expression vector of decorin and investigate its antitumor effect against sarcoma cells in vitro and in vivo,and then provide evidence for further studies on the tumor-suppressing activity and clinical application of hDCN in various tumors.
1 Materials and methods
1.1 Design of primers
According to the cDNA sequence of human decorin reported by GeneBank,two specific primers were designed with the recognition sequence of EcoRⅠand NotⅠas being added to the sense and antisense primers,respectively,which were synthesized by the Shanghai Bio-Technology Co,Ltd.The sense primer was 5'-GGC GAA ATG AAG GGC ACT ATG CTC - 3'.The antisense primer was 5'-GTT GCG GCT TAC TAA TAG CCG AGT TG -3'.
1.2 Bacteria and reagents
E.coli strain DH-5α,template pPIC9K-DCN and eukaryotic expression vector pcDNA3.1(+)were conserved in our laboratory.S180 cells were given as a present by professor Liang-Qi Zhao in the Institution of Life and Science in Shanxi University.LipofectamineTM2000 and G418 were purchased from Invitrogen Corporation(Invitrogen,CA).
1.3 Construction and identification of the recombinant vector pcDNA3.1(+)-DCN
The decorin was amplified by PCR with the primers described above and pPIC9K-DCN as the template.The PCR product and the target vector pcDNA3.1(+)were digested with double enzyme EcoRⅠand NotⅠfollowed by connection with the T4DNA ligase.The positive recombinants were screened in Luria-Bertain(LB)supplemented with ampicillin after transformed into host of competent E.coli DH5α cells.The sequence of the recombinant plasmid pcDNA3.1(+)-DCN extracted from the DH-5α was identified by double enzyme digestion with EcoRⅠand NotⅠ,PCR and sequencing analysis.The recombinant plasmid pcDNA3.1(+)-DCN and the empty plasmid were extracted and stored at-20℃ for later use.
1.4 Liposome transfection
The abdominal dropsy(containing tumor cells S180)from a Kunming mouse was diluted ten times with the RPMI1640 medium.After centrifuged,dropped away the supernatant,washed several times in phosphatebuffered saline(PBS)and filtered by the cell sieve with 200 wells,the primary cells S180 were cultured in RPMI1640 medium supplemented with 10%inactivated fetal bovine serum(FBS)in the condition of 37℃,5% CO2.Recombinant expression vector containing DCN and the empty vector pcDNA3.1(+)were transfected into S180 cells with LipofectamineTM2000 reagent according to manufacturer’s instruction.Cells then were screened with 900 μg/ml G418 which was found to be the best screening concentration to generate decorin-expressing sublines and vector control sublines.After screening for two weeks,the positive clones were harvested successfully.
1.5 Verification of the positive cell clones
The reverse transcription-polymerase chain reaction(RT-PCR)was used to verify the positive cell clones after isolating the total RNA from 5×106S180 cells transfected with pcDNA3.1(+)-DCN under the same conditions as before.The product was observed in the 1.0%agarose gel electrophoresis.
1.6 Cell cycle and apoptosis analysis
Transfected recombinant plasmid,transfected empty plasmid and untransfected S180 cells were harvested,washed in PBS and fixed over 2 h at 4℃in 70%ethanol.After centrifuged and dropped away the ethanol,the cells were treated with propidium iodide(PI)and water bathed at 37 ℃ for 15 min.Cell cycle and apoptosis indices were analyzed using a flow cytometer,and the data were analyzed with CellQuest and Modfit software.
1.7 Tumor growth of mice
Eighteen female Kunming mice(5 - 7-week old,weighing 25 g),were randomized into three groups to be inoculated subcutaneously on the right flank with 5×106pcDNA3.1(+)-DCN/S180,pcDNA3.1(+)/S180 and S180 cells,respectively.One week later,the mice of each group were monitored to observe the growth status daily,the growth rate of tumor and the tumor volumes,which were measured in two dimensions using a vernier caliper and calculated using the formula:(length×width2)×0.5.
1.8 Pathological evaluation of tumor tissues section
At 21 d after inoculation,all mice were sacrificed by cervical dislocation under anesthesia,and then were stripped of their tumor tissues to perform pathological section stained with hematoxylin and eosin for morphologic examination.
1.9 Statistical analysis
2 Results
2.1 Results of PCR using the pPIC9K-DCN as template
The 1.0%agarose gel electrophoresis analysis of the PCR products with the pPIC9K-DCN as template showed a specific band of 1 000 bp which was consistent with the molecular mass of DCN(Fig 1).The result of sequencing analysis was also consistent with what reported in the GeneBank.2.2 Identification of recombinant plasmid with restriction enzyme

1.DCN;M.DNA marker DL2000Fig 1 The gene fragment of human DCN amplified by PCR
The 1.0%agarose gel electrophoresis analysis of the recombinant pcDNA 3.1(+)-DCN digested with restriction enzymes EcoRⅠand NotⅠshowed two specific bands of 54 kb and 1 000 bp which were consistent with the molecular mass of vector pcDNA3.1(+)and DCN,respectively(Fig 2).

1.pcDNA3.1(+)-DCN;2.pcDNA3.1(+);M.DNA marker DL2 000Fig 2 Identification of the recombinant plasmid with restriction enzyme digestion
2.3 Identification of recombinant plasmid with PCR The 1.0%agarose gel electrophoresis analysis of the PCR products of the recombinant plasmid pcDNA3.1(+)-DCN as template showed a specific band of 1 000 bp which was consistent with the molecular mass of DCN,but there was no target band found in the empty plasmid control(Fig 3).

1,2.pcDNA3.1(+)-DCN;3,4.pcDNA3.1(+);M.DNA marker DL2000Fig 3 Identification of recombinant plasmid pcDNA3.1(+)-DCN with PC
2.4 Identification of positive clone
The 1.0%agarose gel electrophoresis analysis of the RT-PCR products of the RNA isolated from the transfected positive cells using as template showed a specific band of 1 000 bp which was consistent with the molecular mass of DCN(Fig 4).

M.DNA marker DL2000;1.DCN;2.β-actinFig 4 Identification of positive clones with RT-PCR
2.5 Results of flow cytometer
2.5.1 Analysis of apoptotic indices
The apoptotic index in pcDNA3.1(+)-DCN/S180 group was significantly higher than those in other control groups,and those in both pcDNA3.1(+)/S180 and lipsome/S180 groups were higher than that in S180 group(Fig 5 and Tab 1).

Fig 5 Cell apoptotic index measured by FCM
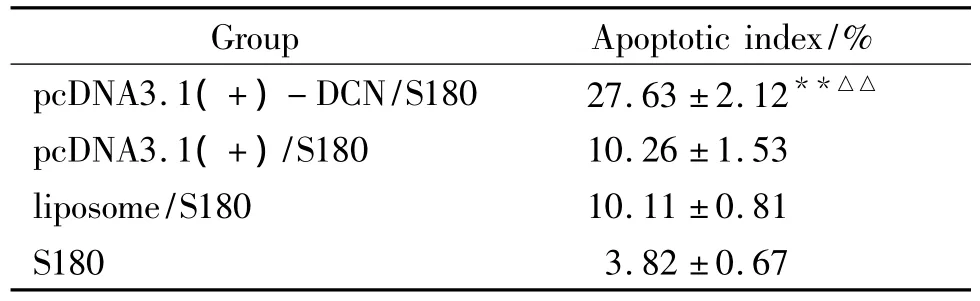
Tab 1 Apoptotic index in each group
2.5.2 Cell cycle analysis
Representative cell-cycle profiles of transfected recombinant,empty vector or liposome cells and untransfected S180 cells are shown in Fig 6 and Tab 2.The group of pcDNA3.1(+)-DCN/S180 cells had a significantly higher proportion of cells in the G0/G1phase and a lower proportion of cells in the G2/M and S phase compared to controls(Fig 6 and Tab 2).
2.6 Result of animal experiments
2.6.1 Result of tumor growth of mice
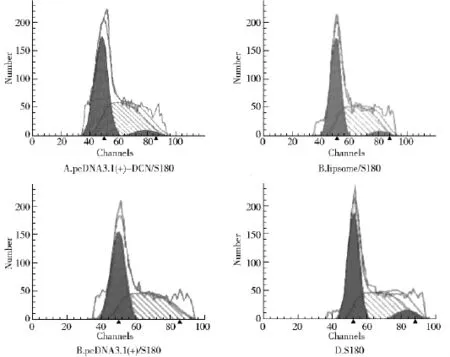
Fig 6 Cell cycles measured by FCM
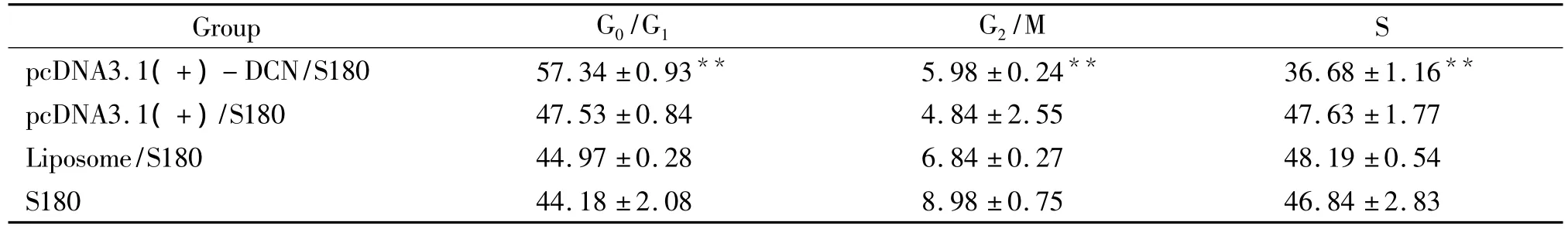
Tab 2 Cell cycle distribution of cells in each group (%)
The analysis at day 7 after the different treatment revealed an apparent difference among the groups in incidence and size of tumors.The tumors in different diameter ranging from rice grains to soybean were found in four cases in S180 group,three cases in pcDNA3.1(+)/S180 group and no case in pcDNA3.1(+)- DCN/S180 group.At day 10,tumors were seen in all six cases in S180 and pcDNA3.1(+)/S180 groups but no more tumors were found in the other cases in pcDNA3.1(+)-DCN/S180 group,and the size of tumors in pcDNA3.1(+)- DCN/S180 group was significantly smaller than that in the other control groups.Some symptoms in different degree were observed in the mice after inoculation,such as piloerection,less activity and bad response and so on,which were more remarkable in S180 and pcDNA3.1(+)/S180 groups than that in pcDNA3.1(+)-DCN/S180 group(Fig 7 and Tab 3).

Fig 7 Tumor growth of the mice injected with equal cells of pcDNA3.1(+)- DCN/S180,pcDNA3.1(+)/S180 and S180
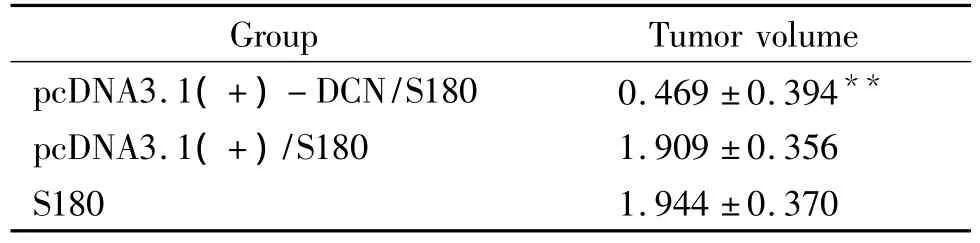
Tab 3 Tumor volume of mice in each group (cm3)
2.6.2 Pathological evaluation of tumor tissue
The pathological examination of the tumor tissues from the three groups revealed typical tumor cells proliferation.Round or oval,big hyperchromatic nuclei,a decrease in caryoplasmic ratio,karyokinesis and invasive growing cells with the normal tissues being destroyed were seen in all the visual fields under light microscope.Especially,the proliferation of tumor cells in pcDNA3.1(+)- DCN/S180 group was reduced and there was more inflammatory cells infiltrated in tumor tissues than those in other control groups.
3 Discussion
Decorin(DCN)is a member of the small leucine-rich proteoglycan gene family,also a multifunctional molecule of the extracellular matrix.The proteoglycan decorin putatively inhibits cell proliferation,adhesion and migration on various extracellular matrix substrates through affecting the biology of various integrins,such as beta-1 and alpha-2[10].Importantly,decorin has been consistently shown to exert growth inhibitory effect on various tumor types,and thus considered to be a strong candidate for tumor gene therapies.Some results from a research demonstrated that decorin play a novel role in the reduction or prevention of tumor metastases in breast cancer model and could eventually improve the therapies for metastatic breast cancer[5].
In this study,after the eukaryotic expression vector carrying DCN gene was constructed successfully,the recombinant plasmid pcDNA3.1(+)-DCN was transfected into tumor cells S180 with transfected empty plasmid and untransfected cells as control groups to observe its antitumor activity in vitro and in vivo.The 5 ×106cells/ml of each groups was harvested to analyze the apoptotic indices and alteration of cell cycles with flow cytometer.The results showed the group of pcDNA3.1(+)-DCN/S180 cells had a significantly higher apoptotic index,a higher proportion of cells in the G0/G1phase and a lower proportion of cells in the G2/M and S phase than those in the other control groups.These results suggested that transfected decorin can induce cell apoptosis and cell cycle arrest in the G0/G1phase.In vivo study,one week later after inoculated mice with pcDNA3.1(+)-DCN/S180,pcDNA3.1(+)/S180 and S180 cells,respectively,the lower growth rate of tumor in pcDNA3.1(+)-DCN/S180 group was observed with tumor volumes being smaller than those in two other groups.The routine pathological section of tumor tissues stained with hematoxylin and eosin also showed that the proliferation of tumor cells in pcDNA3.1(+)-DCN/S180 group was less active but inflammatory cell infiltration in tumor tissues was more active than those in the other groups.The results of in vivo studies showed a good correlation with those observed in proliferation inhibition of tumor cell in vitro.All these results consistently indicated that transfected DCN can effectively induce tumor cells apoptosis in vitro and inhibit tumor growth in vivo.These findings will lay a good foundation for further studying the antitumor activity of DCN and provide a strong support for gene therapy and the clinical application of DCN in various tumors.
[1] Ma W,Tan Y,Cai S,et al.The action of decorin in anti-fibrosis and anti-cancer[J].J Biomed Eng,2007,24(1):222 -225.
[2] Hu Y,Sun H,Owens RT,et al.Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways[J].Neoplasia,2009,11(10):1042 - 1053.
[3] Seidlera DG,Goldonia S,Agnewa C,et al.Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation[J].J Biol Chem,2006,281(36):26408 -26418.
[4] ZHAO Su-lian,WANG Gui-qin,TIAN Shu-hua.Effect of decorin on hepatoma cell line[J].China J Cancer Prev Treat,2002,9(3):235-238.
[5] Araki K,Wakabayashi H,Shintani K,et al.Decorin suppresses bone metastasis in a breast cancer cell line[J].Oncology,2009,77(2):92-99.
[6] Mlakar V,Berginc G,Volavsek M,et al.Presence of activating KRAS mutations correlates significantly with expression of tumor suppressor genes DCN and TPM1 in colorectal cancer[J].BMC Cancer,2009,9:282.
[7] Goldoni S,Seidler DG,Heath J,et al.An antimetastatic role for decorin in breast cancer[J].Am J Pathol,2008,173(3):844 -855.
[8] Augoff K,Grabowski K,Rabczynski J,et al.Expression of decorin in esophageal cancer in relation to the expression of three isoforms of transforming growth factor-beta(TGF-beta1,-beta2,and -beta3)and matrix metalloproteinase-2 activity[J].Cancer Invest,2009,27(4):443 -452.
[9] Bi X,Tong C,Dockendorff A,et al.Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation[J].Carcinogenesis,2008,29(7):1435 -1440.
[10] Ferdous Z,Peterson SB,Tseng H,et al.A role for decorin in controlling proliferation,adhesion,and migration of murine embryonic fibroblasts[J].J Biomed Mater Res A,2010,93(2):419 -428.

