吉西他滨对人胰腺癌SW1990和BxPC3细胞株Notch信号通路的诱导作用
程宪永 熊光苏 李祥苏 吴叔明
·论著·
吉西他滨对人胰腺癌SW1990和BxPC3细胞株Notch信号通路的诱导作用
程宪永 熊光苏 李祥苏 吴叔明
目的观察吉西他滨对人胰腺癌细胞(SW1990和BxPC3)Notch信号通路活性的影响,探讨其与胰腺癌对吉西他滨化疗耐药的关系。方法不同浓度吉西他滨处理人胰腺癌SW1990和BxPC3细胞株48 h,实时定量PCR检测Notch信号通路受体Notch1、2、3、4,配体Jagged1、2和下游靶基因Hes1 mRNA的表达,Western blotting测定细胞Hes1蛋白表达。结果2 μmol/L吉西他滨作用胰腺癌细胞株48 h,SW1990细胞的Notch1、2、3、Jagged1、2和Hes1 mRNA表达量分别为8.26±0.48、39.12±4.87、0.84±0.06、105.8±17.92、6.59±0.32和17.30±2.96,均较未处理细胞的1.02±0.15、15.25±1.28、0.12±0.02、32.66±1.98、1.88±0.29和5.02±0.64明显升高(P<0.05或P<0.01);BxPC3细胞上述各项表达量分别为7.87±0.59、109.4±10.98、0.74±0.19、62.73±13.50、2.09±0.16和15.38±1.06,也均较未处理细胞的1.14±0.43、58.96±2.63、0.10±0.02、16.95±3.79、0.98±0.02和2.04±0.16,明显升高(P<0.05或P<0.01)。1、2 μmol/L吉西他滨作用胰腺癌细胞株48 h,SW1990细胞Hes1蛋白表达量分别为0.30±0.03、0.42±0.03;BxPC3细胞分别为0.33±0.02、0.45±0.03,均较未处理细胞显著增高(0.13±0.01、F=33.71;0.09±0.02、F=38.54,P值均<0.01)。结论吉西他滨可明显激活SW1990和BxPC3细胞的Notch信号通路,这可能是胰腺癌细胞获得化疗耐受性的机制之一。
胰腺肿瘤; 吉西他滨; Notch受体; 信号转导
吉西他滨单药化疗是晚期胰腺癌标准的一线治疗,但疗效较差,耐药是化疗失败的主要原因之一[1]。研究发现,Notch信号的异常与多种肿瘤的发生有关,其中包括胰腺癌[2]。并且,Notch的激活与多种肿瘤耐药性的产生有关,阻断Notch信号可以明显增强化疗药物的敏感性[3-4]。Hes1是Notch信号通路特异的下游靶基因,可以作为Notch活性的标志[5]。最近文献报道,吉西他滨抵抗胰腺癌细胞株较敏感株Notch信号分子Notch2和Jagged1明显上调[6],提示Notch的激活可能与吉西他滨的耐药有关。本研究应用吉西他滨处理胰腺癌细胞株,观察Notch信号通路各分子表达水平的变化,探讨Notch的活性与化疗耐药的关系。
材料和方法
一、细胞培养
胰腺癌细胞株SW1990和BxPC3由本实验室保存,常规培养传代。
二、Notch信号通路各分子mRNA表达的检测
采用实时定量PCR方法。应用0、2μmol/L吉西他滨(江苏豪森药业公司)处理SW1990和BxPC3细胞48 h后收集细胞,采用Trizol法抽提总RNA。引物序列见表1,由上海生工生物工程技术服务公司合成。先逆转录为cDNA, PCR反应:95℃ 30s,95℃ 5 s、60℃ 31 s,40个循环,95℃ 15 s, 60℃ 60,95℃ 15 s,60℃ 15 s;以SDS2.3软件分析Ct值。根据2-△△Ct法计算mRNA表达量,△△Ct=(Ct目的基因-Ct内参基因)实验组-(Ct目的基因-Ct内参基因)对照组。
三、Hes1蛋白表达检测
采用Western blotting方法检测。应用0、1、2 μmol/L吉西他滨处理SW1990和BxPC3细胞48 h后采用细胞裂解液冰浴裂解收集细胞,BCA法测蛋白浓度。鼠抗人Hes1单克隆抗体购自Abnova公司;鼠抗α-tublin抗体和辣根过氧化物酶标记羊抗鼠二抗购自美国Sigma公司。最后DAB显色,扫描仪扫描,以目的条带与内参条带灰度值比表示目的蛋白相对表达量,实验重复3次。
四、统计学分析
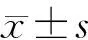
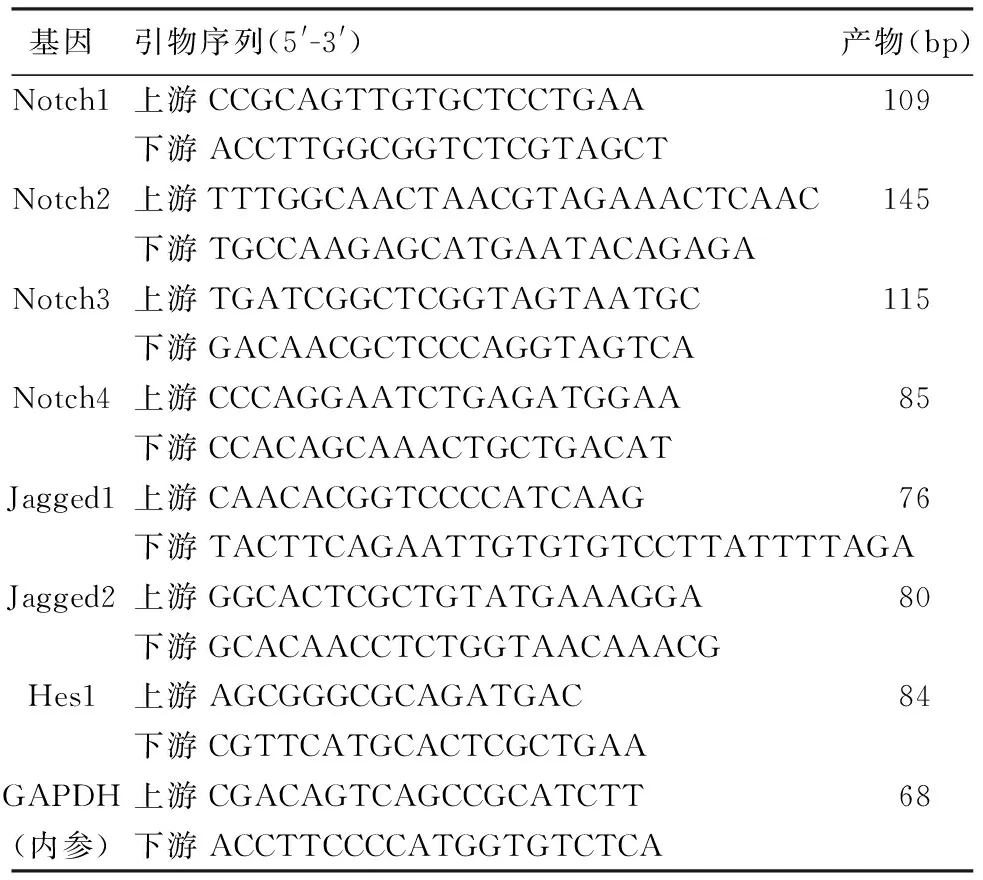
表1 Q-PCR所用引物序列
结 果
一、Notch信号通路mRNA表达的变化
SW1990和BxPC3细胞都不同程度表达Notch信号通路受体(Notch1、2、3)、配体(Jagged1、2)和下游靶基因Hes1。其中Notch2及Jagged1表达水平最高;Notch3表达很微弱,Notch4未见表达。吉西他滨处理细胞48 h,各分子mRNA表达水平均较未处理细胞明显升高(P<0.05或P<0.01,表2)。
二、Hes1蛋白表达的变化
应用1、2 μmol/L吉西他滨处理细胞48 h后,SW1990的Hes1蛋白表达量分别为0.30±0.03、0.42±0.03;BxPC3分别为0.33±0.02、0.45±0.03,均较未处理细胞的0.13±0.01和0.09±0.02显著增高(F=33.71,F=38.54,P值均<0.01;图1),并均与吉西他滨浓度呈正相关(P<0.01)。
讨 论
Notch信号通路在胰腺发育和成熟胰腺组织稳态中发挥重要作用[7]。人胰腺癌和动物模型的癌及癌前病变组织均有Notch受体、配体以及下游靶基因的中到高度表达[8-9]。用γ分泌酶抑制剂阻断Notch信号通路,可以明显抑制小鼠移植瘤的生成,并可阻断癌前病变向胰腺癌的进展[5]。
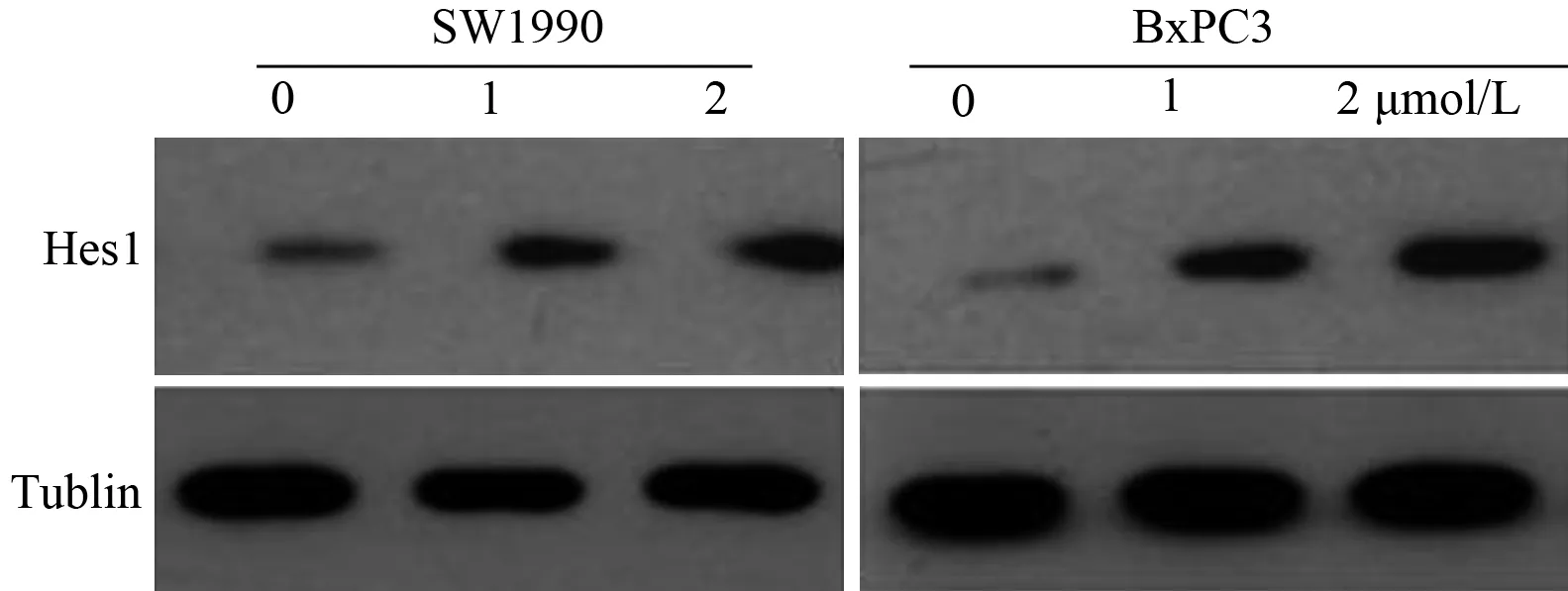
图1SW1990和BxPC3细胞经吉西他滨处理前后Hes1蛋白表达的变化
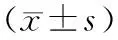
表2 吉西他滨处理前后SW1990和BxPC3细胞Notch信号通路各基因mRNA表达的变化
注:与处理前比较,aP<0.05,bP<0.01
Mungamuri等[10]首先发现Notch激活可引发下游促生存信号PI3K/Akt、Bcl-Xl和Survivin等的表达,使肿瘤细胞免于化疗药物诱导的凋亡。阻断Notch信号通路可以增加结肠癌、T淋巴细胞白血病、黑色素瘤细胞对化疗药物的敏感性[3-4]。而一些化疗药如奥沙利铂、5-FU、SN-38都可以激活结肠癌细胞Notch信号通路[3]。
最近有人发现,吉西他滨抵抗胰腺癌细胞株较敏感株Notch信号分子Notch2和Jagged1明显上调[6],本研究结果显示,吉西他滨干预胰腺癌细胞株48 h, Notch信号通路受体、配体和靶基因Hes1的mRNAs表达水平均明显上调,Hes1蛋白的表达也明显上升,且与吉西他滨浓度呈正相关。因此,我们认为Notch的激活可能在胰腺癌对化疗耐药产生中起着重要作用,提示针对Notch信号通路的靶向干预治疗可能有助于提高胰腺癌的化疗敏感性。
[1] Fryer RA,Galustian C,Dalgelish AG. Recent advances and developments in treatment strategies against pancreatic cancer.Curr Clin Pharmacol,2009,4:102-112.
[2] Miyamoto Y,Maitra A,Ghosh B,et al.Notch mediates TGF alpha induced changes in epithelial differentiation during pancreatic tumorigenesis.Cancer Cell,2003,3:565-576.
[3] Meng RD,Shelton CC,Li YM,et al.gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity.Cancer Res,2009,69:573-582.
[4] Real PJ,Tosello V,Palomero T,et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia.Nat Med,2009,15:50-58.
[5] Plentz R,Park JS,Rhim AD,et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma.Gastroenterology,2009,136:1741-1749.
[6] Wang Z,Li Y,Kong D,et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway.Cancer Res,2009,69:2400-2407.
[7] Nakhai H,Siveke JT,Klein B,et al.Conditional ablation of Notch signaling in pancreatic development.Development,2008,135:2757-2765.
[8] Habbe N,Shi G,Meguid RA,et al.Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice.Proc Natl Acad Sci USA,2008,105:18913-18918.
[9] De La O JP,Emerson LL,Goodman JL,et al.Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia.Proc Natl Acad Sci USA,2008,105:18907-18912.
[10] Mungamuri SK,Yang X,Thor AD,et al.Survival signaling by Notch1:mammalian target of rapamycin(mTOR)-dependent inhibition of p53.Cancer Res,2006,66:4715-4724.
2009-11-09)
(本文编辑:吕芳萍)
GemcitabineinducesNotchsignalingpathwayactivationinpancreaticcancercelllinesSW1990andBxPC3
CHENGXian-yong,XIONGGuang-su,LIXiang-su,WUShu-ming.
DepartmentofGastroenterology,ShanghaiInstituteofDigestiveDisease,RenjiHospital,MedicalSchool,ShanghaiJiaotongUniversity,Shanghai200001,China
WUShu-ming,Email:wushuming@vip.sina.com
ObjectiveTo investigate the changes of Notch signaling pathway activity in human pancreatic cancer cell lines(SW1990,BxPC3)after gemcitabine induction, and to study its relationship with pancreatic cancer resistant to gemcitabine chemotherapy.MethodsThe pancreatic cancer cell lines SW1990 and BxPC3 were cultured with different concentrations of gemcitabine for 48 hours. The Notch signaling pathway receptors (Notch1, Notch2, Notch3, Notch4), ligands (Jagged1, Jagged2) and downstream target Hes1 mRNAs expression were detected by quantitative real-time PCR (Q-PCR). Protein levels of Hes1 were determined by Western blotting.ResultsAfter treatment with 2 μmol/L gemcitabine for 48 hours, the expression of Notch1, Notch2, Notch3, Jagged1, Jagged2 and Hes1 mRNAs in SW1990 cells were 8.26±0.48, 39.12±4.87, 0.84±0.06, 105.8±17.92, 6.59±0.32 and 17.30±2.96, which were significantly elevated when compared with those without gemcitabine treatment (1.02±0.15, 15.25±1.28, 0.12±0.02, 32.66±1.98, 1.88±0.29 and 5.02±0.64,P<0.05 orP<0.01); the expression in BxPC3 cells was 7.87±0.59, 109.4±10.98, 0.74±0.19, 62.73±13.50, 2.09±0.16 and 15.38±1.06, which were significantly elevated when compared with those without gemcitabine treatment (1.14±0.43, 58.96±2.63,0.10±0.02, 16.95±3.79, 0.98±0.02 and 2.04±0.16,P<0.05 orP<0.01). The expressions of Hes1 protein in SW1990 cells after 1, 2 μmol/L gemcitabine treatment for 48 h were 0.30±0.03, 0.42±0.03; and the expressions in BxPC3 cells were 0.33±0.02, 0.45±0.03, which were significantly increased when compared with those without gemcitabine treatment (0.13±0.01,F=33.71,0.09±0.02,F=38.54,P<0.01).ConclusionsThe Notch signaling pathway is significantly activated in pancreatic cancer cells SW1990 and BxPC3 by gemcitabine, which may be one of the mechanisms of chemoresistance.
Pancreatic neoplasms; Gemcitabine; Notch receptors; Signal transducing
10.3760/cma.j.issn.1674-1935.2010.05.011
200001 上海,上海交通大学医学院附属仁济医院,上海市消化疾病研究所
吴叔明,Email:wushuming@vip.sina.com

