吡格列酮对急性坏死性胰腺炎大鼠胰腺PPARγ mRNA表达的影响
徐萍 李清华 王静 徐凯 姜景平 陈令全
·论著·
吡格列酮对急性坏死性胰腺炎大鼠胰腺PPARγ mRNA表达的影响
徐萍 李清华 王静 徐凯 姜景平 陈令全
目的观察过氧化物酶增殖物激活受体γ (peroxisome proliferator-activated receptors,PPARγ)激动剂吡格列酮对急性坏死性胰腺炎(ANP)大鼠PPARγ mRNA表达的影响。方法54只SD大鼠按完全随机法分成假手术组、ANP组、吡格列酮组。采用牛磺胆酸钠逆行胰胆管内注射建立ANP模型,于造模后3、6、12 h检测血清淀粉酶含量,观察胰腺病理学改变,并采用RT-PCR法检测胰腺组织PPARγ mRNA的表达。结果ANP组6 h点的血清淀粉酶及胰腺组织病理学评分分别为(7171±1636)U/L和13.00±2.36,均较假手术组的(523±166)U/L和1.67±2.34显著增高(P<0.01),而PPARγ mRNA在两组中表达均较弱,无显著差异(0.18±0.05对0.22±0.03,P>0.05);吡格列酮组6 h的血清淀粉酶水平、胰腺病理学评分分别为(4504±1901)U/L和9.00±0.89,均较ANP组明显下降(P<0.05),PPARγ mRNA表达较ANP组增强(0.56±0.05对0.18±0.05,P<0.05)。结论吡格列酮对ANP大鼠的保护作用可能与上调PPARγ基因的表达密切相关。
胰腺炎,急性坏死性; 过氧化物酶体激活物受体; 吡格列酮
过氧化物酶增殖物激活受体γ (peroxisome proliferator-activated receptors,PPARγ)是一类由配体激活的核转录因子,主要参与细胞的增殖、分化以及糖脂代谢的平衡。近年来研究发现,PPARγ激动剂能够明显抑制炎症反应,然而其对重症急性胰腺炎(severe acute pancreatitis,SAP)的作用机制仍不十分清楚。因此,本文观察PPARγ激动剂吡格列酮对急性坏死性胰腺炎(ANP)大鼠PPARγ mRNA表达的影响,探讨其抗炎机制。
材料与方法
一、实验动物及分组
健康雄性SD大鼠54只,清洁级,鼠龄2~2.5个月,体重160~200 g,由南昌大学医学院动科部提供。按完全随机法分为假手术组、ANP组和吡格列酮组,各18只。以胰胆管逆行注射5%牛磺胆酸钠溶液1 ml/kg体重(Sigma公司)制备ANP模型;吡格列酮组在制模前2 h腹腔注射吡格列酮50 mg/kg体重(成都宇洋高科技发展公司);假手术组开腹后以钝器轻划胰腺3次。术后3、6、12 h腹主动脉取血,分离取血清;留取胰腺组织,部分于10%中性甲醛固定,部分置液氮罐保存。
二、指标的测定
1.血清淀粉酶:采用全自动生化分析仪检测。
2.胰腺组织病理学检查:胰腺固定标本采用常规病理学检查,并按照Schmidt等[1]标准评分。
3.胰腺组织PPARγmRNA表达检测:采用RT-PCR法。应用Trizol(Promega公司)抽提总RNA。PPARγ(642 bp)上游引物5′-TGACCACTCCCATTCCTTTG-3′,下游引物5′-TTTCCTGTCAAGATCGCCCT-3′;β-actin (228 bp)上游引物5′-AGCCATGTACGTAGCCATCC-3′,下游引物5′-CTCTCAGCTGTGGTGGTGAA-3′。引物由上海杰瑞基因技术公司合成。PCR条件:95℃ 5 min,95℃ 1 min、53℃(PPARγ)或55℃(β-actin)1 min、72℃ 1 min,29个循环,72℃ 5 min。产物经电泳分离,图像分析软件扫描,以PPARγ/β-actin比值表示mRNA 相对表达量。
三、统计学处理
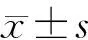
结 果
一、血清淀粉酶的变化
ANP组血清淀粉酶活性于术后3 h急剧上升,6 h达峰值,显著高于假手术组(P<0.01);吡格列酮组血清淀粉酶含量较ANP组同时点均降低,其中6、12 h降低显著(P值均<0.05,表1)。
二、胰腺组织病理学改变
假手术组大鼠胰腺组织结构清晰,腺泡小叶完整,偶见间质区轻度水肿(图1a);ANP组胰腺水肿,部分腺泡呈孤岛状,伴大片腺泡细胞坏死,胰腺实质及间质内见大量红细胞、炎症细胞浸润(图1b),病理评分较假手术组明显升高(P<0.01);吡格列酮组镜下表现较ANP组减轻(图1c),6、12 h的病理评分较ANP组明显降低(P<0.05,表1)。
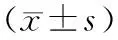
表1 各组血清淀粉酶及胰腺病理评分的变化
注:与假手术组比较,aP<0.01;与ANP组比较,bP<0.05
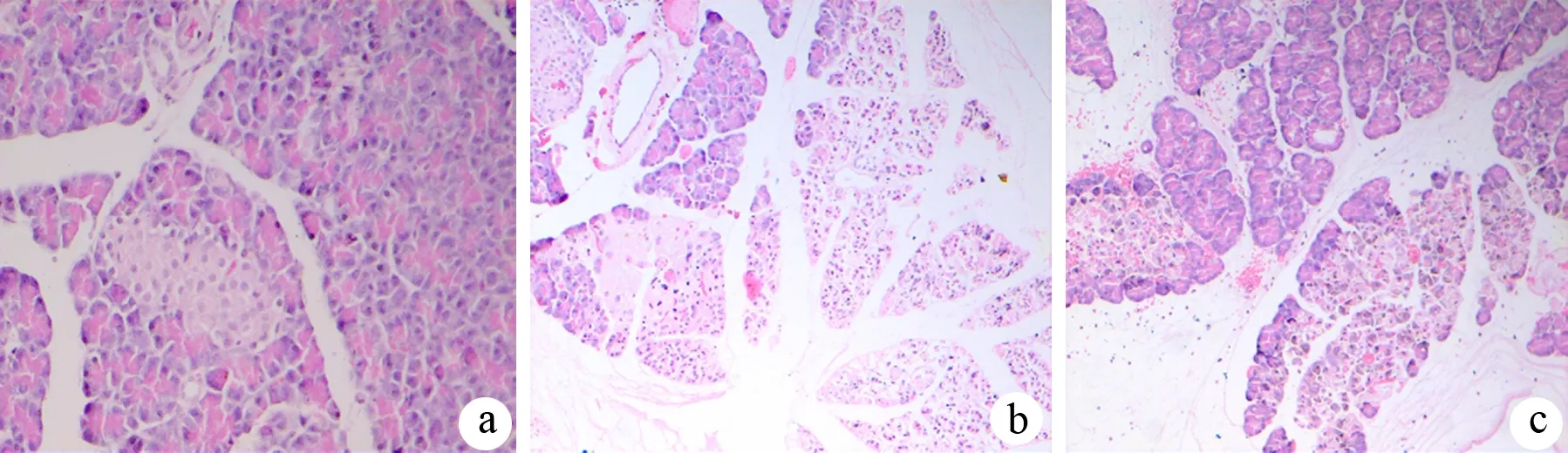
图1假手术组(a)、ANP组(b)和吡格列酮组(c)12 h的胰腺病理改变(HE ×200)
三、胰腺组织PPARγ mRNA表达的变化
假手术组和ANP组PPARγ mRNA表达均较弱,两组无明显差异(P>0.05);吡格列酮组PPARγ mRNA表达较假手术组及ANP组均明显增强(P值均<0.05,表2、图2)。

1:假手术组;2:ANP组;3~5:吡格列酮组(3、6、12 h组)

组别只数术后3h6h12h假手术组60.23±0.040.22±0.030.20±0.04ANP组60.21±0.030.18±0.050.15±0.03吡格列酮组60.40±0.03ab0.56±0.05ab0.65±0.04ab
注:与假手术组比较,aP<0.05;与ANP组比较,bP<0.05
讨 论
PPAR有三种表型:PPARα、PPARβ、PPARγ,其中PPAR-γ的研究最为深入。PPARγ激动剂可抑制NF-κB、AP-1等信号的活化,降低促炎因子、黏附分子表达以及环氧合酶、一氧化氮合酶、基质金属蛋白酶-9的活性,负性调节炎症反应[2-3]。目前认为,PPARγ激动剂可能通过PPARγ依赖和非依赖性途径,从不同水平调控细胞内多条涉及炎症反应的信号通路,实现抗炎作用。PPARγ依赖性机制可能包括:(1)直接与NF-κBp65/p50结合形成复合物,降低NF-κB的DNA结合活性,抑制下游靶基因的表达;(2)竞争性结合NF-κB、AP-1的辅助活化因子,阻止这些转录因子的激活;(3)抑制IκB激酶,阻止IκB的降解;(4)降低JNK活性,减少AP-1的活化[4-5]。PPARγ非依赖性途径目前不太清楚。
近年来,PPARγ激动剂已被广泛应用于急性胰腺炎(AP)的实验研究,它对AP的保护作用得到不断地验证,有望成为一种治疗AP的崭新手段。Cuzzocrea等[6]采用罗格列酮干预雨蛙素诱导的AP小鼠,其胰腺组织中性粒细胞的浸润、ICAM-1和硝基酪氨酸的表达均较AP组显著下降。Hashimoto等[7]报道,PPARγ天然激动剂-15d-PGJ2可明显减轻AP大鼠的胰腺组织损伤,减少IL-6的释放及COX-2的表达,并抑制IκB蛋白的降解。Konturek等[8]研究显示,吡格列酮对AP的抗炎作用呈剂量依赖性,并可促进热休克蛋白-70的表达。我们前期实验结果亦证实,吡格列酮能有效减轻ANP大鼠胰腺组织病理损伤,降低胰腺NF-κBp65/p50和ICAM-1蛋白的表达[9]。
然而,PPARγ激动剂在AP中的作用机制至今尚未完全阐明。有研究者发现,AP可导致胰腺组织中PPARγ蛋白表达下降,而15d-PGJ2能促进PPARγ蛋白表达,认为PPARγ激动剂表现出的抗炎特性有赖于PPARγ的参与[10]。有研究显示,炎症因子TNF-α、IL-1β可抑制PPARγ mRNA表达,而应用PPARγ激动剂则可使PPARγ 基因的转录恢复到正常水平,从而发挥调控炎症反应的生物学效应[11]。本实验结果显示,大鼠ANP胰腺组织中PPARγ mRNA的表达略下降,应用吡格列酮干预后则可明显增强PPARγ mRNA的表达,同时血清淀粉酶含量与胰腺组织学评分显著降低。因此,我们推测吡格列酮可能是通过上调PPARγ基因表达而发挥对胰腺炎的保护性作用。
[1] Schmidt J,Rattner DW,Lewandrowski K,et al.A better model of acute pancreatitis for evaluating therapy.Ann Surg,1992,215:44-56.
[2] Abdelrahman M,Sivarajah A,Thiemermann C.Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury,inflammation and shock.Cardiovasc Res,2005,65:772-781.
[3] Imamoto E,Yoshida N,Uchiyama K,et al.Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells.Biofactors,2004,20:37-47.
[4] Jennewein C,Kuhn AM,Schmidt MV,et al.Sumoylation of peroxisome proliferator-activated receptor gamma by apoptotic cells prevents lipopolysaccharide-induced NCoR removal from kappaB binding sites mediating transrepression of proinflammatory cytokines.J Immunol,2008,181:5646-5652.
[5] Hamblin M,Chang L,Fan Y,et al.PPARs and the cardiovascular system.Antioxid Redox Signal,2009,11:1415-1452.
[6] Cuzzocrea S,Pisano B,Dugo L,et al.Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma,reduces acute inflammation.Eur J Pharmacol,2004,483:79-93.
[7] Hashimoto K,Ethridge RT,Saito H,et al.The PPAR-gamma ligand,15d-PGJ2,attenuates the severity of cerulein-induced acute pancreatitis.Pancreas,2003,27:58-66.
[8] Konturek PC,Dembinski A,Warzecha Z,et al.Pioglitazone,a specific ligand of peroxisome proliferator-activated receptor-gamma,protects pancreas against acute cerulein-induced pancreatitis.World J Gastroenterol,2005,11:6322-6329.
[9] Xu P,Zhou XJ,Chen LQ,et al.Pioglitazone attenuates the severity of sodium taurocholate-induced severe acute pancreatitis.World J Gastroenterol,2007,13:1983-1988.
[10] Rollins MD,Sudarshan S,Firpo MA,et al.Anti-inflammatory effects of PPAR-gamma agonists directly correlate with PPAR-gamma expression during acute pancreatitis.J Gastrointest Surg,2006,10:1120-1130.
[11] Rollins MD,Sudarshan S,Firpo MA,et al.Anti-inflammatory effects of PPAR-gamma agonists directly correlate with PPAR-gamma expression during acute pancreatitis.J Gastrointest Surg,2006,10:1120-1130.
2010-02-08)
(本文编辑:屠振兴)
EffectofpioglitazoneonPPARγmRNAexpressioninratswithacutenecrotizingpancreatitis
XUPing,LIQing-hua,WANGJing,XUKai,JIANGJing-ping,CHENLing-quan.
DepartmentofGastroenterology,SongjiangHospital,MedicalShchool,ShanghaiJiaotongUniversity,Shanghai201600,China
XUPing,Email:yfyxp@yahoo.com.cn
ObjectiveTo observe the effect of pioglitazone on peroxisome proliferators activated receptors (PPARγ) mRNA expression in rats with acute necrotizing pancreatitis (ANP).Methods54 rats were randomly divided into sham group, ANP group, and pioglitazone group. ANP was induced by the retrograde injection of sodium taurocholate into pancreatic duct. The levels of serum amylase at 3 h, 6 h, and 12 h after ANP induction were determined and pancreatic pathological scores were measured, and the expression of pancreatic PPARγ mRNA was determined by RT-PCR.ResultsThe levels of serum amylase and pancreatic pathological scores at 6 h in ANP group were (7171±1636)U/L and 13.00±2.36; which were significantly higher than those in sham group [(523±166)U/L and 1.67±234,P<0.01]. While the expressions of PPARγ mRNA were weakly expressed in ANP gyoup and sharm group(0.18±0.05vs0.22±0.03,P>0.05). The levels of serum amylase and pancreatic pathological scores at 6 h in pioglitazone group were (4504±1901)U/L and 9.00±0.89, which were significantly lower than those in ANP group (P<0.05). While the expressions of PPARγ mRNA was higher than that in ANP group (0.56±0.05vs0.18±0.05,P<0.05).ConclusionsThe protective role of pioglitazone on ANP rats was closely correlated with up-regulation of PPARγ gene expression.
Pancreatitis, acute necrotizing; Peroxisome proliferator-activated receptors; Pioglitazone
10.3760/cma.j.issn.1674-1935.2010.05.016
201600 上海,上海交通大学附属上海市第一人民医院松江分院消化内科
徐萍,Email:yfyxp@yahoo.com.cn

