The remedial effect of soluble interleukin-1 receptor type II on endometriosis in the nude mouse model☆
Liying Go, Ling Sun, Yugui Cui, Zhen Hou, Li Go, Jing Zhou,Yundong Mo, Suping Hn, Jiyin Liu*
aCenter of Clinical Reproductive Medicine, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029
bSuzhou Municipal Hospital & Suzhou Medical Center for Maternal and Child Health, Suzhou 215002, China.
INTRODUCTION
Endometriosis is a common gynecological disorder that affects 10%-15% of women of reproductive age and up to 50% of infertile patients. It is characterized by pelvic pain, dyspareunia and infertility as a result of the dystopic location of endometrial cells in tissues other than the uterine cavity[1]. The origin of endometriotic cells remains elusive and the most widely accepted theory on the aetiology of endometriosis is retrograde menstruation[2]. However,this phenomenon has also been described in healthy women and can be viewed as a physiological process[3,4]. Thus factors other than retrograde menstruation are necessary for the development of endometriosis. Several lines of evidence indicate that the cytokines in the peritoneal fluid (PF)of women with endometriosis establishes a microenvironment for the development of the disease and undergoes a number of pathological changes, including inflammatory processes with locally and systemically altered functions of the immune system[5-7]. Increased levels of Interleukin (IL)-1ß mRNA expression have been identified in the peritoneal macrophages of patients with mild endometriosis[8]. IL-1 is a major proinflammatory cytokine which has been isolated from the PF of patients with endometriosis. It is mainly produced by monocytes and macrophages, and is believed to have an important role in endometriosis pathophysiology.
The IL-1 family consists of two distinct molecular forms, IL-1α and IL-1ß, which are encoded by different genes, but have comparable biological activities[9]. Cell activations in response to IL-1 are mediated via the IL-1 receptor type Ⅰ (IL-1 RⅠ),the functional signaling receptor[10]. However, IL-1 receptor type Ⅱ (IL-1 RⅡ)is not a signaling molecule and is reported to be a decoy target of IL-1. Moreover,IL-1 RⅡ could be shed from the cell surface as a soluble molecule (sIL-1 RⅡ)that captures IL-1 and inhibits IL-1 from binding to IL-1 RⅠ, thereby neutralize the bioactivity of IL-1[11,12,13]. Recent studies have indicated that local expression of sIL-1 RⅡin endometrial tissue decreased in women suffering from endometriosis, and defective IL-1 RⅡ was more significant in infertile women than in fertile women with endometriosis, which suggests its involvement in endometriosis associated infertility[14,15].
The purpose of this study was to evaluate the therapeutic potential of sIL-1 RⅡ in counteracting the effect of IL-1 using an in vivo nude mouse model of experimentally-induced endometriosis.
MATERIALS AND METHODS
Acquisition of human tissues
Eutopic endometrial tissues at the proliferative phase of the menstrual cycle were obtained from 5 women (aged from 23 to 35 years)undergoing laparoscopy for endometriosis. Endometriosis in these 5 women was staged as Ⅱ or Ⅲ during the laparoscopy, according to the revised American Fertility Society classification system[20]. All participants had regular menstrual cycles, had not received hormonal treatment, and had not been pregnant, breast-feeding, or using an intrauterine device for the past 3 months. Endometrial biopsies were placed immediately into a culture medium and transferred to the laboratory. Written informed consent was obtained from each patient using consent forms and protocols approved by the Ethics Committee of the First Affiliated Hospital of the Nanjing Medical University.
Transplantation of the endometrial fragments into nude mice
Nineteen 6- 8-week-old female nude mice purchased from the Shanghai Laboratory Animal Corporation were maintained in a barrier unit in a well-controlled pathogen-free environment with regulated cycles of light/dark (12 h/12 h).All equipment and food entering the barrier was autoclaved. Mice had free access to food and water.Approval from the Animal Ethics Committee of the Nanjing Medical University was obtained before the study.
Mice were weighed, and anesthetized with intraperitoneal injections of pentobarbital. The endometrial tissue fragments were washed three times with PBS and cut into 2-3 mm2sections that were implanted with a suture of polypropylene 3/0, in the parietal peritoneum in two sections on each side of the midline incision with a separation of 0.5-1.0 cm.The abdominal wall was then closed with Prolene 3-0 non-absorbable polypropylene sutures (Holycon,Nantong, Jiangsu, China). Each mouse received an intramuscular injection of benzestrofol (2 mg/Kg)every 4 days from the day of tissue transplantation for 2 weeks. All animal studies were approved and conducted in accordance with Institutional Animal Care Committee guidelines.
Drug administration
EDA(Electronic Design Automation)即电子设计自动化,EDA软件可以大大降低科研人员在原理图的绘制,性能计算,产品组装,设备调试等环节中的工作强度。并极大地提高数据计算,检测数据的可靠性。
After transplantation with endometrial tissue,nineteen nude mice were randomly divided into three groups: group A (n = 7)was treated with only sIL-1 RⅡ for two weeks, group B(n = 6)was treated with only IL-1 for the same time period and group C(n = 6)was treated with saline as control. The optimal dosage was unknown due to the lack of previous studies on sIL-1 RⅡ for experimental endometriosis. Therefore,we based the dosage on sIL-1 RⅡ treatment in the experimental orthodontic tooth movement in rats,namely 2 μg/215 g[21]. Each group of mice was treated with an intraperitoneal injection of 0.2 ml of their respective treatments every other day for 2 weeks.Treatment with human recombinant IL-1 (5 ng/kg)(R&D System, USA)and human recombinant sIL-1 RⅡ (25 μg/kg)(R&D System)was started at day 1 after tissue transplantation. Mice in the control group received the same volume of saline.
Evaluation of ectopic uterine tissue
Two weeks after the beginning of treatment,animals were sacrificed and the lesions were collected to determine the growth of each implant based on the area of lesions. The growth of lesions per mouse was measured using the average size of the four implants, which were calculated by measuring two perpendicular diameters (a×b, length and width)with a caliper and the area of each lesion was calculated by the formula of an ellipse (a×b×∏/4)[22]. The person performing the laparotomy and the evaluation of the endometriotic implants had no prior knowledge of the animal group allocation.
After measuring, the lesions were immediately fixed in 4% paraformaldehyde and then paraffin embedded. The presence of viable endometiosis was confirmed by conventional hematoxylin and eosin staining in one 5 μm thickness section per implant.The histologic diagnosis of endometriosis was based on the morphologic identification of endometrial glandular tissue and stroma. An individual other than the authors evaluated three sections from each implant in a blinded fashion.
Finally, the expression of Bcl-2 and VEGF were detected in serial sections by immunohistochemistry according to the laboratory protocol. Sections were deparaffinized in xylene and rehydrated through graded alcohols to distilled water. In sections used to detect VEGF this was followed by microwaving in 0.01 mol/L sodium citrate buffer for antigen retrieval. Incubation with 0.01% trypsin for 30 min at 37°C was used to detect Bcl-2, and steam-heat incubation with EDTA for 30 min at 97°C pH 8 was used for VEGF. Endogenous peroxidase was removed by treatment with 0.3% hydrogen peroxide for 30 min at 37°C, after which nonspecific binding was blocked by incubation with normal goat serum.Tissue sections were incubated with anti-human Bcl-2 rabbit polyclonal antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA)or VEGF anti-human rabbit monoclonal antibody (1:200,Zeta, Sierra Madre, CA, USA)overnight at 4°C,then incubated with anti-rabbit-peroxidase conjugate(Zhongshan Ltd., Beijing, China)for 60min at 37°C.Binding was visualized by incubating sections with 3, 3'-Diaminobenzidine (DAB). After rinsing, slides were counterstained with hematoxylin, dehydrated through a graded ethanol series to xylene, and then coverslips put in place. Negative control specimens were processed by omitting the primary antibody. The intensity of Bcl-2 and VEGF staining was assessed in a blind fashion at 200 magnifications by microscopy(Leica, Germany). Images were captured and the gray value was measured using Leica Qwin, standard V2.8 software. A quantitative assessment method was used to compare the gray value of 3 randomly selected fields of each tissue specimen.
Measure of IL-8 and VEGF
At the end of the experimental period, animals were sacrificed, the blood and PF were collected, and PF and serum levels of VEGF and IL-8 were measured by standard cytokine-specific enzyme-linked immunosorbent assay (ELISA)using commercial kits (Adlitteram Diagnostic Laboratories, USA). All measurements were performed in duplicate, according to the manufacturer’s instructions. The sensitivity of the kits was approximately 1 pg/ml for both VEGF and IL-8.
Statistical analysis
All data are expressed as mean ±SD. Differences among groups were analyzed by one-way analysis of variance (ANOVA), and the post hoc Student-Newman-Keuls (SNK)method was used for multiple comparison. The P-value reported was two-sided and value of P < 0.05 was considered statistically significant. All analyses were performed using the SPSS software (Version 11.0, SPSS Inc., USA).
RESULTS
The behavior of the mice during the experiment was normal. No evidence of toxicity was noted in both the sIL-1 RⅡ group and IL-1 group. The body weights of three groups (20.1±1.9 g of sIL-1 RⅡgroup, 19.7±2.4 g of IL-1 group, and 20.3±2.2 g of control)were similar (P > 0.05).
Of a total of 19 mice which received endometrial fragments, all mice developed 4 endometriotic lesions in the peritoneum at 2 weeks (Fig. 1 A).Macroscopically, the deposits appeared as reddish cystic areas and were found to be well vascularized and attached to the abdominal wall peritoneum (Fig.1 B). Histological sections stained with hematoxylin and eosin detected the lesions taken from all groups at day 14 after transplantation revealed typical endometriotic lesions; the morphology of endometriotic lesions in all treatment groups was diverse, with typical endometrium glands and stroma as well as tubal metaplasia with large cysts and flattened epithelium. The observed morphology of the lesions was similar to that seen in human endometriosis (Fig. 1 C and 1D).

Fig.1 The lesions on day 14 after transplantation (A), The lesion viewed by stereo microscope, 12×(B). The morphology of the lesions at day 14 after transplantation, 200×(C)and 400×(D).
Effects of sIL1-RⅡon the growth of endometriotic implants
After two weeks of treatment, the average area of ectopic endometrial lesions in the sIL-1 RⅡ treated nude mice decreased compared with the other two groups, but there was no significant difference among the three groups (Fig. 2).

Fig.2 The average area of lesions per mouse in each of the three groups (sIL-1R Ⅱ treated group, IL-1 treated group and control group treated with saline)at day 14 after transplantation (P = 0.645).
Treatment of sIL1-RⅡinhibits the expression of VEGF and Bcl-2 in endometriotic lesions
The immunohistochemical staining of the slides were reviewed on three separate occasions by different combinations of investigators. The Bcl-2 and VEGF were mainly located in the plasma membrane and membranes of intracellular organelles. Expression of Bcl-2 was present in both epithelial and stromal cells of the endometrium (Fig. 3), while the expression of VEGF was mostly limited to the epithelium (Fig.4). The negative control sections showed an absence of specific staining. Fig. 5 showed the gray value of the lesions' glandular epithelium staining for each of the three groups. The expression of protein was the inverse of the gray value, so the expression of VEGF and Bcl-2 were highest in the IL-1 group, but lowest in the sIL-1 RⅡ group and both proteins in each group were significantly different (P < 0.05)from the control group values.
The IL-8 and VEGF levels in the peritoneal fluid and serum
In the PF and serum, the concentrations of IL-8 and VEGF were lowest in the sIL1-RⅡgroup and highest in the IL-1 group. The differences in IL-8 concentration between any two groups reached statistical significance (P < 0.05), but the VEGF did not (P > 0.05)(Fig. 6).
DISCUSSION
It has been demonstrated that the expression of IL-1ß by peritoneal macrophages was elevated during the initial stages of endometriosis but the IL-1ra was usually elevated during the late stages of the disease[15]. An up-regulation of IL-1 expression was recently observed in the eutopic endometrial tissue of endometriosis patients[23]. It has also been found that sIL-1 RⅡ binds and blocks processing of the IL-1ß precursor, inhibits its maturation, loses affinity for the IL-1ra and does not therefore interfere with IL-1ra mediated inhibition of IL-1 effects[24]. Finally, sIL-1 RⅡ appears to act by sequestering IL-1ß within the extracellular compartment, thus restricting its availability and interaction with IL-1 RI.
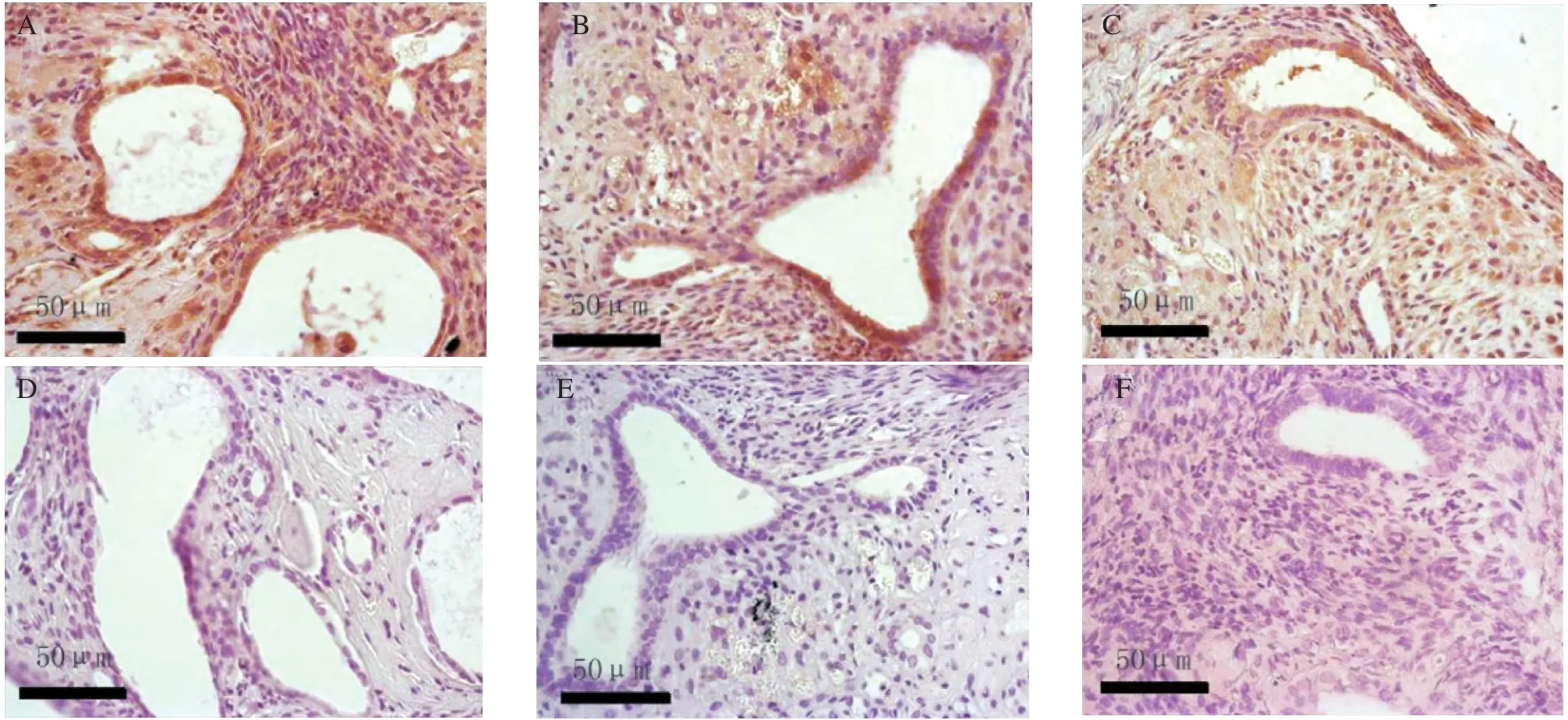
Fig.3 The expression of Bcl-2 on day 14 after transplantation in the 3 groups: the IL-1 treated group (A)and the negative control (D),the saline treated control group (B)and the negative control (E), and the sIL-1 RⅡ treated group (C)and the negative control (F)

Fig.4 The expression of VEGF on day 14 after transplantation in the 3 groups: the IL-1 treated group (A)and the negative control (D),the saline treated control group (B)and the negative control (E), and the sIL-1 RⅡ treated group (C)and the negative control (F).
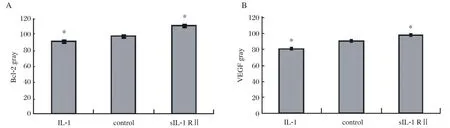
Fig.5 The gray values (inversely proportional to protein expression)of Bcl-2 (A)and VEGF (B)of the immunohistochemical slides of the 3 groups. Significantly different from the control (*P < 0.05).

Fig.6 The IL-8 and VEGF concentrations in the PF and serum for each group of animals. Significantly different from control(*P < 0.05). A: The IL-8 concentration in PF; B: The IL-8 concentration in serum; C:The VEGF concentration in PF; D: The VEGF concentration in serum.
IL-1 induces an angiogenic phenotype in endometriotic cells and stimulates the secretion of VEGF. Recently, several studies have proposed that angiogenesis represents an important step during the process of endometriosis, because similar to tumour metastases, endometriotic implants require neovascularization to establish, proliferate and invade[25-27]. The endometrium has angiogenic potential[28], and endometriotic lesions are larger in areas with a rich blood supply[29]. In a recent study about the growth of human menstrual endometrium in nude mice, a high VEGF score was observed in stromal cells as early as 5 days after transplantation,suggesting that an active vascular network is a necessary condition for the survival of the graft[30]. Our results suggest that sIL-1 RⅡ treatment throughout the development of endometriosis decreases the expression of VEGF protein, which then affects the angiogenesis of the lesions. Although, the change of VEGF in PF and serum showed no significant difference (P > 0.05)between the three groups after two weeks of treatment of nude mice, this may be caused by the short treatment time.
In endometriosis, abnormal adhesion of endometrial fragments to ectopic sites could be due to an increase in the adhesive and proliferative properties of the menstrual endometrium, a decrease in apoptosis, and anomalities in the target tissue microenvironment.Previous studies demonstrated that endometrial cells(ECs)from women with endometriosis exhibited low apoptosis, when compared to ECs from women without endometriosis[31]. Dmowski and colleagues[32], using a cell death detection ELISA assay, demonstrated that endometrial apoptosis in the eutopic endometrium from women with endometriosis was lower than controls, and that endometrial apoptosis in the ectopic endometrium further decreased when compared to the eutopic endometrium. The evidence suggested that the normal balance between proliferative and apoptotic cyclicity of the endometrium was disturbed in women with endometriosis[33], with a reduced apoptosis which was observed primarily in the early proliferative and late secretory phases of the menstrual cycle[34].The disturbed expression of IL-1 RⅡ on the eutopic endometrium resulted in disturbances in normal macrophage trafficking into the eutopic endometrium in women with endometriosis. There may also be a positive correlation between apoptosis and macrophage numbers in the eutopic endometrium[35].These results provided further evidence for an intrinsic abnormality in apoptotic sensitivity of the endometrium of women with endometriosis. The Bcl-2 is defined as a new class of proto-oncogenes that block cell death without promoting cell proliferation[36]. Under normal conditions, epithelial cells require interactions with the extracellular matrix to survive and grow. Apoptosis is thought to prevent the dissemination and attachment of differentiated epithelial cells at inappropriate locations when they lose contact with the matrix. It is likely that in healthy women, ECs and tissue fragments expelled during menses do not survive in ectopic locations because of programmed cell death. Disruption of cell matrix interactions induces cell death through a process called anoikis[37]. This might prevent detached normal cells from implanting in a new and inappropriate location. Endometriotic lesions show an increase in Bcl-2 expression compare with the endometrium and such an elevated Bcl-2 expression could promote the survival of endometriotic tissue at ectopic sites[38]. Our results demonstrate that in the formed lesions, sIL-1 RⅡ decreased the expression of Bcl-2 and VEGF compared with both control and IL-1 groups, and may contribute to decreasing the size of already formed lesions in the endometriosis nude mouse model.
IL-8 is elevated in the PF of women with endometriosis[39-41], and the IL-8 level is also correlated with the severity of the disease[40]. It has recently been shown that IL-8 stimulates the adhesion of endometrial cells to fibronectin[42]. Thus, IL-8 may be relevant for stimulating the attachment of endometrial implants in the pathogenesis of endometriosis, and the adherence of ECs induces further IL-8 expression by an integrin-dependent mechanism[43].
Our previous results demonstrated that IL-8 secretion induced by IL-1ß was significantly decreased after infection with recombinant adenovirus expressing IL-1RⅡ[18]. Now, we have found that in an endometriosis nude mice model, the IL-8 concentration in the peritoneal fluid and serum of the sIL-1 RⅡ treated group is significantly lower than the other two groups, and is in fact increased in the IL-1 treated group. Although the cytokine network in the endometrium is complex, our results provide further insights into the mechanism of immunologic modulation of sIL-1 RⅡ during the process of endometriosis.
Taken together, these findings further support the contention that the pathogenesis of endometriosis is multifactorial and involves many mediators.Presently, endometriosis is mainly treated by estrogen antagonist, which only suppresses symptoms, but will not eradicate the ectopic implants. Moreover, there are significant side effects. Alternatively, endometriosis can be treated surgically, but symptoms recur in time in a majority of women. An effective therapeutic agent for endometriosis would be a compound that not only prevents the development of endometriotic lesions but is also effective against the growth of established lesions. Our results suggest that sIL-1 RⅡmay provide a new clinical strategy for the treatment of endometriosis.
[1]Goldman MB, Cramer DW. The epidemiology of endometriosis. Prog Clin Biol Res 1990;323:15-31.
[2]Brosens IA, Brosens JJ. Endometriosis. Eur J Obstet Gynecol Reprod Biol 2000;90:159-64.
[3]Halme J, Hammond MG, Hulka JF, Raj SG, Talbert IM.. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984;64:151-4.
[4]Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, et al. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril 2002;78:787-95.
[5]Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril 2001;75:1-10.
[6]Seli E, Arici A. Endometriosis: interaction of immune and endocrine system. Semin Reprod Med 2003;21:135-44.
[7]Wu MY, Ho HN. The role of cytokines in endometriosis.Am J Reprod Immunol 2003;49:285-96.
[8]Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K,Tamaya Tl. Expression of interleukin-1 (IL-1)beta messenger ribonucleic acid (mRNA)and IL-1 receptor antagonist mRNA in peritoneal macrophages from patients with endometriosis. Fertil Steril 1992;57:535-42.
[9]Dinarello CA. Biologic basis for interleukin-1 in disease.Blood 1996; 87: 2095-147.
[10]Sims JE, Gayle MA, Slack JL, Alderson MR, Bird TA,Giri JG, et al. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA 1993;90:6155-9.
[11]Colotta F, Dower SK, Sims JE, Mantovani A. The type II ‘decoy’ receptor: a novel regulatory pathway for interleukin 1. Immunol Today 1994;15:562-6.
[12]Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1)receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proc Natl Acad Sci USA 1995;92:1714-18.
[13]Dinarello CA. Interleukin-1, interleukin-1 receptor’s and interleukin-1 receptor antagonist. Int Rev Immunol 1998;16:457-99.
[14]Kharfi A, Boucher A, Akoum A. Abnormal interleukin-1 receptor type II gene expression in the endometrium of women with endometriosis. Biol Reprod 2002;66:401-6.
[15]Akoum A, Jolicoeur C, Kharfi A, Aube M. Decreased expression of the decoy interleukin-1 receptor type Ⅱ in human endometriosis. Am J Pathol 2001;158:481-9.
[16]Simon C, Frances A, Piquette G. The immune mediator interleukin-1 receptor antagonist (IL-1ra)prevents embryo implantation. Endocrinology 1994;134:521-8.
[17]Akoum A, Lawson C, McColl S, Villeneuve Ml. Ectopic endometrial cells express high concentrations of interleukin (IL)-8 in vivo regardless of the menstrual cycle phase and respond to oestradiol by up-regulating IL-1-induced IL-8 expression in vitro. Mol Hum Reprod 2001; 7: 859-66.
[18]Hou Z, Zhou J, Ma X, Fan L, Liao L, Liu JY. Role of Interleukin-1 receptor type II in the pathogenesis of endometriosis. Fertil Steril 2008;89:42-51.
[19]Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O,Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril 2000;74:760-6.
[20]American Fertility Society. Revised American Fertility Society classification of endometriosis. Fertil Steril 1985;43:351-2.
[21]Jager A, Zhang D, Kawarizadeh A, Tolba R, Braumann B,Lossodrfer S, et al. Soluble cytokine receptor treatment in experimental orthodontic tooth movement in the rat.Eur J Orthod 2005;27:1-11.
[22]Becker CM, Sampson DA, Short SM, Javaherian K,Folkman J, et al. Short synthetic endostatin peptides inhibit endothelial migration in vitro and endometriosis in a mouse model. Fertil Steril 2006;85:71-7.
[23]Bergqvist A, Bruse C, Carlberg M, Carlstrom K..Interleukin 1 beta, interleukin-6, and tumor necrosis factor-alpha in endometriotic tissue and in endometrium.Fertil Steril 2001;75:489-95.
[24]Dunne A, O’Neill LA. The interleukin-1 receptor/Tolllike receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003;171:3-17.
[25]Fujishita A, Hasuo A, Khan KN, Masuzaki H, Nakashima H, Ishimaru T. Immunohistochemical study of angiogenic factors in endometrium and endometriosis.Gynecol Obstet Invest 1999;48:36-44.
[26]Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci 2002;955:89-100.
[27]Gescher DM, Haensel A, Meyhofer-Malik A, Malik E.The importance of angiogenesis for the pathogenesis of endometriosis. Zentralbl Gynakol 2003;125:243-6.
[28]Maas JW, Groothuis PG, Dunselman GA, de Goeij AF, Struyker-Boudier HA, Evers JL. Endometrial angiogenesis throughout the human menstrual cycle.Hum Reprod 2001;16:1557-61.
[29]Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J. Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril 1993;59:681-4 .
[30]Nisolle M, Casanas-Roux F, Donnez J. Early-stage endometriosis:adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril 2000;74:306-12.
[31]Gebel HM, Braun DP, Tambur A, Frame D, Rana N,Dmowski WP.. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril 1998;69:1042-7.
[32]Dmowski WP, Gebel H, Braun DP. Decreased apoptosis and sensitivity to macrophage mediated cytolysis of endometrial cells in endometriosis. Hum Reprod Update 1998;4:696-701.
[33]Braun DP, Ding J, Shaheen F, Willey JC, Rana N,Dmowski WP. Quantitative expression of apoptosisregulating genes in endometrium from women with and without endometriosis. Fertil Steril 2007;87:263-8.
[34]Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB,Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis.Hum Reprod 2001;16:1802-8.
[35]Braun DP, Ding J, Shen J, Rana N, Fernandez BB,Dmowski WP. Relationship between apoptosis and the number of macrophages in eutopic endometrium from women with and without endometriosis. Fertil Steril 2002;78:830-5.
[36]Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood 1992;80:879-86.
[37]Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994;124:619-26.
[38]Jones R, Searle R, Bulmer J. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod 1998;13:3496-3502.
[39]Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL.Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukine-8 expression in human mesothelial cells. Mol Hum Reprod 1996;2:40-5.
[40]Gazvani MR, Christmas S, Quenby S, Kirwan J, Johnson PM, Kingsland CR. Peritoneal fluid concentrations of interleukine-8 in women with endometriosis: relationship to stage of disease. Hum Reprod 1998;13:1957-61.
[41]Iwabe T, Harada T, Tsudo T, Tanikawa M, Onohara Y,Terakawa N. Pathogenic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril 1998;69:924-30.
[42]Garcia-Velasco JA, Arici A. Interleukin-8 stimulates the adhesion of endometrial stromal cells to fibronectin.Fertil Steril 1999;72:336-40.
[43]Garcia-Velasco JA, Arici A. Interleukin-8 expression in endometrial stromal cells is regulated by integrindependent cell adhesion. Mol Hum Reprod 1999;5:1135-40.
 THE JOURNAL OF BIOMEDICAL RESEARCH2010年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2010年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Reduction of G0 phase cells of colon cancer caco-2 cells may enhance 5-fluorouracil efficacy☆
- The potential of carcinoembryonic antigen, p53, Ki-67 and glutathion Stransferase-π as clinico-histopathological markers for colorectal cancer☆
- Left ventricular hypertrophy amplifies the QT, and Tp-e intervals and the Tp-e/ QT ratio of left chest ECG
- The preparation and characterization of folate-conjugated human serum albumin magnetic cisplatin nanoparticles ☆
- Tinea incognito due to microsporum gypseum
