Reduction of G0 phase cells of colon cancer caco-2 cells may enhance 5-fluorouracil efficacy☆
Lin Ye, Kaixiong Tao, Yang Yu, Guobin Wang
Department of Laproscope Surgery, Union Hospital affiliated to HuaZhong University of Science and Technology,WuHan 430022, China
INTRODUCTION
Colon cancer is still a leading cause of cancerrelated mortality in the world. Although its incidence and mortality have decreased[1]due to remarkable improvements in surgical technique, radiation therapy,and systemic therapies[2], chemoresistance is still a hurdle in the treatment of colon cancer. The lack of responsiveness to chemotherapy[3]is one of the most important problems that needs to be resolved.
The chemotherapy unresponsiveness of colon cancer cells is most likely due to a multiplicity of causes[4]. One plausible reason is the large number of cells in the G0phase, cells that are less vulnerable to cell cycle dependent chemotherapy[5]. Furthermore,these cells may be activated anytime, anywhere by growth factors[6]or cytokines[7,8], and they may be a time bomb for tumor recurrence. This poses a hurdle in the goal of modern cancer treatment where the goal is the prolongation of disease free survival. The aim of this study is to reduce the number of cells in the G0phase and thus enhance the chemotherapy efficacy in a colon cancer model.
Epidermal growth factor receptor (EGFR)was reported to be overexpressed in colon cancer[9]. The activation of this receptor plays an important role in the regulation of tumor growth[10]. In this study, we use epidermal growth factor (EGF), a key trigger in activating the EGFR signaling pathway[11-13], to stimulate caco-2 colon carcinoma cells which were tested to overexpress EGFR. We found that the percentage of tumor cells in G0phase was reduced and more tumor cells were synchronized in the S phase and G2/M phase. Meanwhile, in the presence of EGF, the chemosensitivity was enhanced nearly threefold. This result may provide a novel strategy for future therapy.
MATERIALS AND METHODS
Cell culture
Cells from the caco-2 human colon cancer cell line were purchased from the China Center for Type Culture Collection, and were grown in High Glucose DMEM medium (Hyclone, USA)containing 10%fetal bovine serum (Gibco, USA)with penicillin and streptomycin antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, respectively)at 37°C in a humidified incubator with 5% CO2in air.
Cell cycle analysis
Tumor cells (2×105)were seeded in 6-well plates.After overnight incubation, the medium was changed with serum-free medium containing various amounts of EGF (PeproTech, USA)and incubated for 48h.Cells were then harvested and stained with propidium iodide for DNA cell cycle analysis using standard FACS techniques and FACSort (BD, USA)The results were analyzed and expressed as percentages of total gated cells using the Modfit LTTMSoftware (BD,USA).
Western blot analysis
Briefly[14], whole cell extracts (50 μg/lane)were electrophoresed through 10% sodium dodecyl sulfate(SDS)-polyacrylamide gel and were transferred onto a Hybond-C-super nitrocellulose membrane (Dako,Denmark). Prestained molecular weight markers(Dako)were included. Membranes were blocked for 1h in Tris-buffered saline (TBS, pH 7.5)with 0.5%Tween-20 (TBST)and 5% nonfat dry milk. After blocking, membranes were incubated for 2 h with proliferating cell muclear antigen (PCNA)(1:100 dilution, Wuhan Boster, China). After incubation with horseradish peroxidase conjugated secondary antibody(1:5000 dilution, Boster), the membranes were scored by the enhanced chemiluminescence (ECL)detection system (Amersham, USA).
MTT assay
Cells in 100 μl of culture medium per well were seeded into 96-well plates (Dakewe, Shenzhen,China)and cultured at 37°C for 24 h. Then the culture medium was replaced with serum-free medium and 10 μl of medium containing various amounts of 5-fluorouracil (5-FU)(HaoranBio, China)and EGF (PeproTech, USA)prepared using serum free medium, was added to each well. After additional incubation at 37°C for 48 h, 10 μl of MTT (Boster)dissolved in PBS at a concentration of 5 mg/ml was added to each well, and the plates were incubated at 37°C for 4 h. The medium was removed, 150 μl of dimethylsulfoxide was added to each well, and the plates were agitated for 5 min. The absorbance was then read at 570 nm in a scanning spectrophotometer.
Statistical analysis
All data are expressed as mean ±SD. Differences among groups were analyzed by one-way analysis of variance (ANOVA), and Fisher’s Least Significant Difference (LSD)method was used for multiple comparison. The P-value reported was two-sided and a value of P < 0.05 was considered statistically significant. All analyses were performed using the SPSS software (Version 11.0, SPSS Inc., USA).
Results
Cell cycle transition by stimulation with EGF
We stimulated tumor cells with EGF of different concentrations. As shown in Fig. 1A-1F, the cell cycle transitions were EGF concentration dependent at concentrations at or below 100 ng/ml. At the concentration of 100 ng/ml, the percentage of cells in the G0/G1phase was reduced by approximately 20% compared to the control group (P < 0.05), and the percentage of cells in the S and G2/M phases increased. The percentage of cells transitioning out of the G0/G1phase did not significantly change further when the concentration of EGF was above 100 ng/ml. Then we stimulated tumor cells with an EGF co ncentration of 100 ng/ml for different time periods(Fig. 1G), and found that the number of G0/G1phase cells was reduced significantly at 48 h by 10%compared to 24 h(P < 0.05).
Expression of PCNA following stimulation with EGF
PCNA correlates with the proliferation of cells in many human tumors, including colon cancer. Levels increase in late G1phase and peak in the S phase of the cell cycle, and the antigen is not detectable in quiescent cells. In our experiment, the expression of PCNA increased with increasing concentrations of EGF and the maximum increase was > 2 fold (Fig.2)when the EGF concentration was≥100 ng/ml.This demonstrated the dormant cells (G0phase)were reduced and were recruited into an activated phase.


Fig. 2 Effect of EGF on the expression of PCNA. The expression of PNCA was EGF concentration dependent (0~1 000 ng/ml), reaching a maximum level of > 2 times that of the control group (P < 0.05). F = 83.733,P < 0.001,within subject effects P1-2 = 0.692,P1-3 < 0.001,P1-4< 0.001,P1-5 < 0.001,P4-5 = 0.134, and 1 to 5 represent the EGF concentrations of 0 to 1 000 ng/ml respectively.
Chemosensitivity enhanced by stimulation with EGF
We evaluated the synergistic effect of EGF and 5-FU using an MTT assay. The relative sensitivity was judged by the 50% inhibiting concentration (IC50). The growth of caco-2 cells was inhibited in a concentrationdependent manner by 5-FU over the concentration range 1.25 to 1 250 μg/ml (Fig. 3). The sensitivity of caco-2 cells to 5-FU was significantly enhanced by a combination with EGF, and this sensitivity effect was increased nearly threefold compared to the group that was treated with 5-FU alone (Table 1).

Fig. 3 Effect of 5-FU on growth of caco-2 cells. Cells were seeded in 96-well microplates and incubated in DMEM medium over night. Then various concentrations of 5-FU (1.25~1250 μg/ml)were added. After further incubation for different time periods (24~96h), cell viability was evaluated by MTT assay.Means±SD of triplicate experiments. When incubated for more than 72 h, a 5-FU concentration of less than 12.5 μg/ml did not inhibit the tumor cell growth, and at the incubation of 24 h, the concentrations of 5-FU higher than 125 μg/ml did not achieve the maximal effect. (Effect of time F = 40.127, P < 0.001; effect of 5-FU levels: F = 3767.636, P < 0.001).

Table 1 Combined effect of 5-FU and EGF on growth of caco-2 cells.
Effect of 5-FU and the synergistic use of EGF on cell cycle transition
As shown in Fig. 4, although the cells in the G0/G1phase increased nearly 5% when treated with 5-FU (1 250 μg/ml)plus EGF (100 ng/ml)compared to when treated with the 5-FU alone,there was no significant difference on cell cycle transition (P > 0.05).
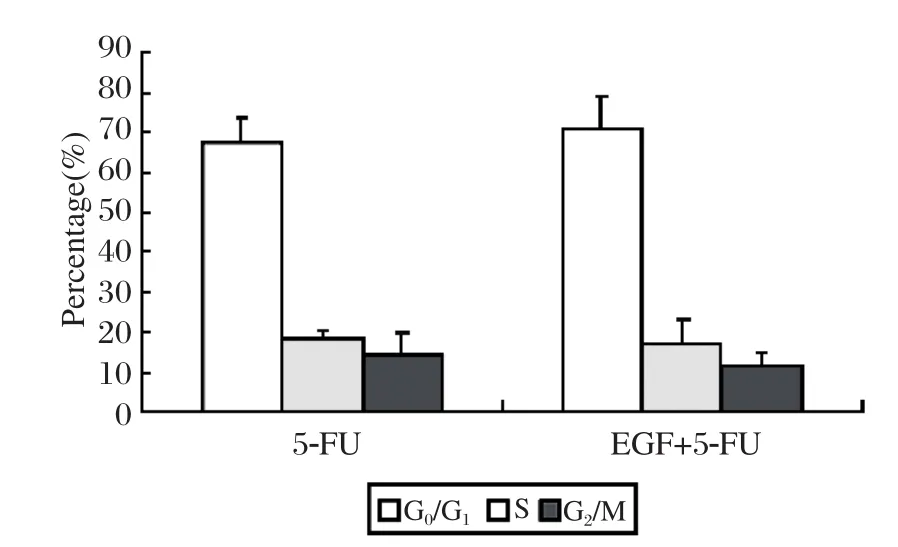
Fig. 4 Effect of 5-FU (1 250 ug/ml)on cell cycle transition.Although the cells in G0/G1 phase increased nearly 5% when treated with 5-FU (1 250 μg/ml)plus EGF (100 ng/ml)compared to when treated with 5-FU alone, there was no significant difference in cell cycle distribution (P > 0.05).
DISCUSSION
Chemo-resistance and recurrence become the major problems in the treatment of colon cancer.Conventional therapies which target actively dividing cells may substantially reduce tumor bulk, but often times do not prevent tumor regrowth, presumably because conventional therapy does not destroy the G0cells which are in a dormant state[15]. Thus the longterm effect of chemotherapy may be poor as any activation of G0phase cells may result in recurrence.
There are few studies dealing with the G0phase cells. Some investigators reported that the unresponsiveness of leukemic cells to chemotherapy could be due to their residence in the resting G0phase of the cell cycle[3,5], and recruitment of leukemic cells from the dormant phase into an activated phase of the cycle by activation or induction of proliferation restored their sensitivity. Hambek and coworkers[6]found that the toxicity of docetaxel in head and neck cancer treatment could be enhanced by stimulation of G0cells which were resistant to chemotherapy.
In our study, we found that the number of G0phase cells was reduced and more tumor cells were recruited into an activated phase by EGF, while the toxicity of 5-FU was enhanced nearly threefold. These results support our contention that caco-2 cells become more vulnerable to chemotherapy when there is a reduction of dormant cells by stimulation of EGF. The same result was found in another colon cancer cell line(sw480). With this cell line the 5-FU chemosensitivity was nearly doubled by the synergistic use of 5-FU with EGF compared to the use of 5-FU alone (data not shown).
To date, many studies on the treatment of colon cancer mainly target signaling molecules which manipulate the key signaling pathways regulating tumor growth[16-19]. Although clinically meaningful antitumor effects were observed in patients with advanced or metastatic colon cancer in some clinical trials[20,21], the dormant cells (G0phase)were still ignored. As a result, the risk of recurrence remains high. However, the results of our study may resolve this problem by demonstrating that we can make tumor cells become vulnerable to chemotherapy by causing a reduction of dormant cells by stimulation with a growth factor. This may provide a novel therapeutic protocol in the treatment of colon cancer,while decreasing the potential risk for recurrence.
Reference
[1]Weitz J, Koch M, Debus J. Colorectal cancer. Lancet 2005;445:106-10.
[2]Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005;352:476-87.
[3]Jedema I, Barge RM, Frankel AE. Acute myeloid leukemia cells in G0phase of the cell cycle that are unresponsive to conventional chemotherapy are sensitive to treatment with granulocyte-macrophage colonystimulating factor/diphtheria toxin fusion proteins. Exp Hematol 2004;32:188-94.
[4]Houghton J, Morozov A, Smirnova I. Stem cells and cancer. Semin Cancer Biol 2007;17:191-203.
[5]Jedema I, Barge RM, Nijmeijer BA. Recruitment of leukemic cells from G0 phase of the cell cycle by interferons results in conversion of resistance to daunorubicin. Leukemia 2003;17:2049-51.
[6]Hambek M, Werner C, Baghi M. Enhancement of docetaxel efficacy in head and neck cancer treatment by G0cell stimulation. Eur J Cancer 2007;43:1502-7.
[7]Banes AK, Shaw SM, Tawfik A. Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin.Am J Physiol Cell Physiol 2005;288:805-12.
[8]Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signaling by interleukin-6 in prostate carcinoma cells.Nature 1998;393:83-5.
[9]Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol 2002;18:1-13.
[10]Spano JP, Lagorce C, Atlan D. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 2005;16:102-8.
[11]Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell 2008;13:7-9.
[12]Dassonville O, Bozec A, Fischel JL. EGFR targeting therapies: monoclonal antibodies versus tyrosine kinase inhibitors. Similarities and differences. Crit Rev Oncol Hematol. 2007;62:53-61.
[13]Aaronson DS, Muller M, Neves SR. An androgen-IL-6-Stat3 autocrine loop re-routes EGF signal in prostate cancer cells. Mol Cell Endocrinol 2007;270:50-6.
[14]Lin Q, Lai R, Chirieac LR. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines:inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol 2005;167:969-80.
[15]Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol 2004;51:1-28.
[16]Mocellin S, Lise M, Nitti D. Targeted therapy for colorectal cancer. Trends Mol Med. 2005;11:327-35.
[17]Xiong H, Zhang ZG, Tian XQ. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest,and reduces tumor cell invasion in colorectal cancer cells. Neoplasia 2008;10:287-97.
[18]Li L, Shaw PE. Autocrine-mediated activation of STAT3 correlates with cell proliferation in breast carcinoma lines. J Biol Chem 2002;277:17397-405.
[19]Ni Z, Lou W, Leman ES. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res 2000;60:1225-8.
[20]Cerea G, Ricotta R, Schiavetto I. Cetuximab for treatment of metastatic colorectal cancer. Ann Onco. 2006;17:66-7
[21]Schrag D. The price tag on progress: chemotherapy for colorectal cancer. N Engl J Med 2004; 351:317-9.
 THE JOURNAL OF BIOMEDICAL RESEARCH2010年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2010年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The remedial effect of soluble interleukin-1 receptor type II on endometriosis in the nude mouse model☆
- The potential of carcinoembryonic antigen, p53, Ki-67 and glutathion Stransferase-π as clinico-histopathological markers for colorectal cancer☆
- Left ventricular hypertrophy amplifies the QT, and Tp-e intervals and the Tp-e/ QT ratio of left chest ECG
- The preparation and characterization of folate-conjugated human serum albumin magnetic cisplatin nanoparticles ☆
- Tinea incognito due to microsporum gypseum
