纳米材料用于构建新型电化学生物传感器的研究进展
陈桂芳,梁志强,李根喜,
1.南京大学生物化学系,医药生物技术国家重点实验室,南京 210093;
2.上海大学生命科学学院,上海 200444
纳米材料用于构建新型电化学生物传感器的研究进展
陈桂芳1,梁志强2,李根喜1,2
1.南京大学生物化学系,医药生物技术国家重点实验室,南京 210093;
2.上海大学生命科学学院,上海 200444
生物传感器是目前生命科学及临床医学测试方法研究中最为活跃的领域之一,电化学生物传感器是其中一个重要分支,已广泛用于临床、工业、环境和农业分析等领域。进入21世纪,纳米科技的迅猛发展为新型生物传感器的研制提供了难得的机遇。本文简要介绍和总结近年来基于蛋白质和纳米材料发展新型电化学生物传感器的研究进展,着重探讨纳米材料在构建新型电化学生物传感器中的应用。
蛋白质;纳米材料;电化学生物传感器;酶传感器;免疫传感器
0 引 言
生物传感器一词已经被广泛用来描述一类用以监控生命体系或与之相关联的生物基元的器件。其完整的概念可以认为是由作为识别元件的固定化的生物敏感材料 (包括酶、抗体、微生物、细胞、组织、核酸等生物活性物质),以及作为物理换能器的适当的信号转换器 (如氧电极、光敏管、场效应管、压电晶体等)所组成的体系。当待测物质与分子识别元件发生特异性的结合后,所产生的复合物信号 (如光、热、电等)通过信号转换器转变成光、电等可输出信号,从而达到对待测物质进行分析检测的目的。从识别元件来看,蛋白质 (主要包括酶、抗体)相比其他材料而言,具有功能的多样性、对特定底物识别的专一性,以及分子本身的可操控性等不可比拟的优点,因此成为目前最为常用的识别元件。而从信号转换来看,电化学技术由于具备灵敏、快速、成本低廉、检测装置轻便、低能耗且易于微型化和集成化等优点,已成为生物传感器领域最为活跃的检测手段[1,2]。
进入21世纪,随着纳米技术的飞速发展,纳米材料在医学成像、疾病诊断、药物传输、基因治疗等多个领域显示了巨大的优势。对于生物传感领域而言,纳米材料在光学性能、电学性能、磁学性能、力学性能和化学活性等方面表现出的独特性能使其成为很好的换能器元件;另一方面,生物传感器中的分子运作本身就是基于纳米层次,纳米材料的参与可以将其优良的性能很好地整合到分子运作中,从而改进甚至革新分子运作的体系。鉴于以上特点,纳米材料在生物传感器中的应用引起了越来越多科研工作者的兴趣。本文从纳米材料的角度综述了近年来基于蛋白质与纳米材料发展新型电化学生物传感器方面的研究进展。
1 金属纳米材料
金属纳米材料良好的电子传递性能使其成为电化学生物传感器中最为常用的纳米材料之一,其中尤以纳米金的应用最为广泛。纳米金制备简单、性状稳定、生物相容性良好,而且易于进行表面化学修饰,因此,利用纳米金与生物分子进行组装并介导电子传递,是构建电化学生物传感器的良好方案。
Willner课题组[3]于2003年在Science上发表文章介绍了纳米金的经典应用。他们将葡萄糖氧化酶 (glucose oxidase,GOD)的辅基FAD修饰到纳米金表面,再通过双巯基化合物的连接将FAD修饰的纳米金固定到金电极上。当引入脱辅基的GOD后,由于FAD与酶的特异结合,完整的GOD可以最大限度地保持其原有结构和活性而组装到电极表面,从而催化葡萄糖的氧化。通过监测催化过程中FAD经纳米金介导传递到电极表面的电化学信号,可以对底物葡萄糖进行定量分析。此后他们又利用纳米金与聚阴离子组成的“微杆”介导得到了溶液中GOD的电化学响应,从而实现葡萄糖的高灵敏检测 (图1)[4]。结果显示,纳米金的使用一方面使得GOD与电极间的电子传递速率提高了25倍,另一方面,“微杆”结构所提供的巨大的比表面积使得即使在溶液状态下也能获得GOD的电化学信号 (图2)。近年来,基于纳米金与GOD所构建的各种葡萄糖生物传感器层出不穷,并发展出了一系列优秀的实验设计方案。比如,Sun等发展了GOD在纳米金表面的共价固定[5]以及基于层层组装技术 (layer-by-layer,LBL)的多层设计[6],Ju等[7]发展了掺入GOD和纳米金颗粒的碳糊电极,Chen等[8]发展了壳聚糖和纳米金的联合运用,还有许多实验方案不在此一一列举[9~16]。
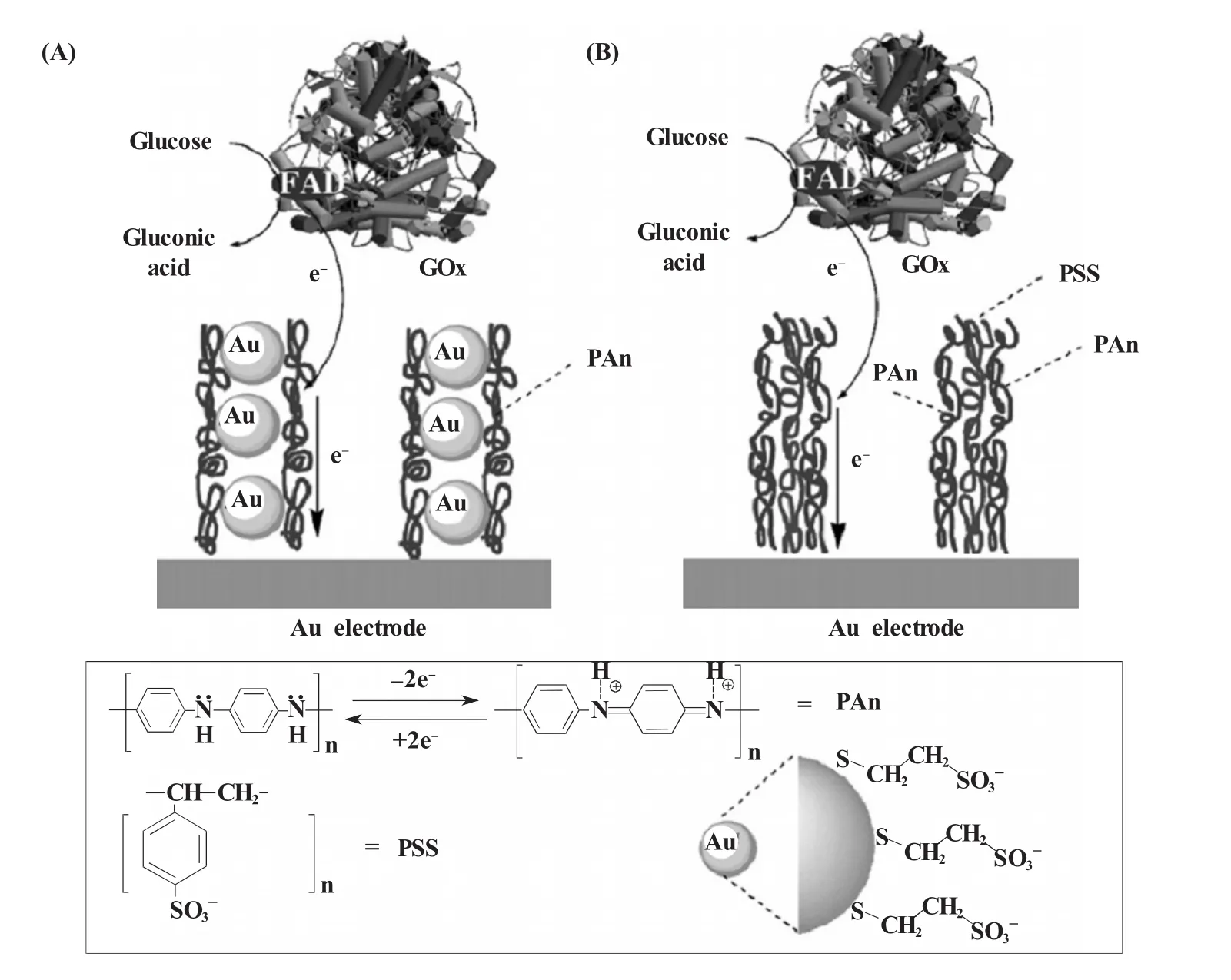
图1 葡萄糖氧化酶存在条件下,通过聚苯胺-纳米金微杆(A)和聚苯胺-聚苯乙烯磺酸盐微杆(B)介导的电化学催化氧化葡萄糖的示意图[4]Fig.1 Bioelectrocatalytic oxidation of glucose in the presence of GOx mediated by Polyaniline/Au-nanoparticles composite micro-rods(A)and polyaniline/polystyrene sulfonate composite micro-rods(B)[4]
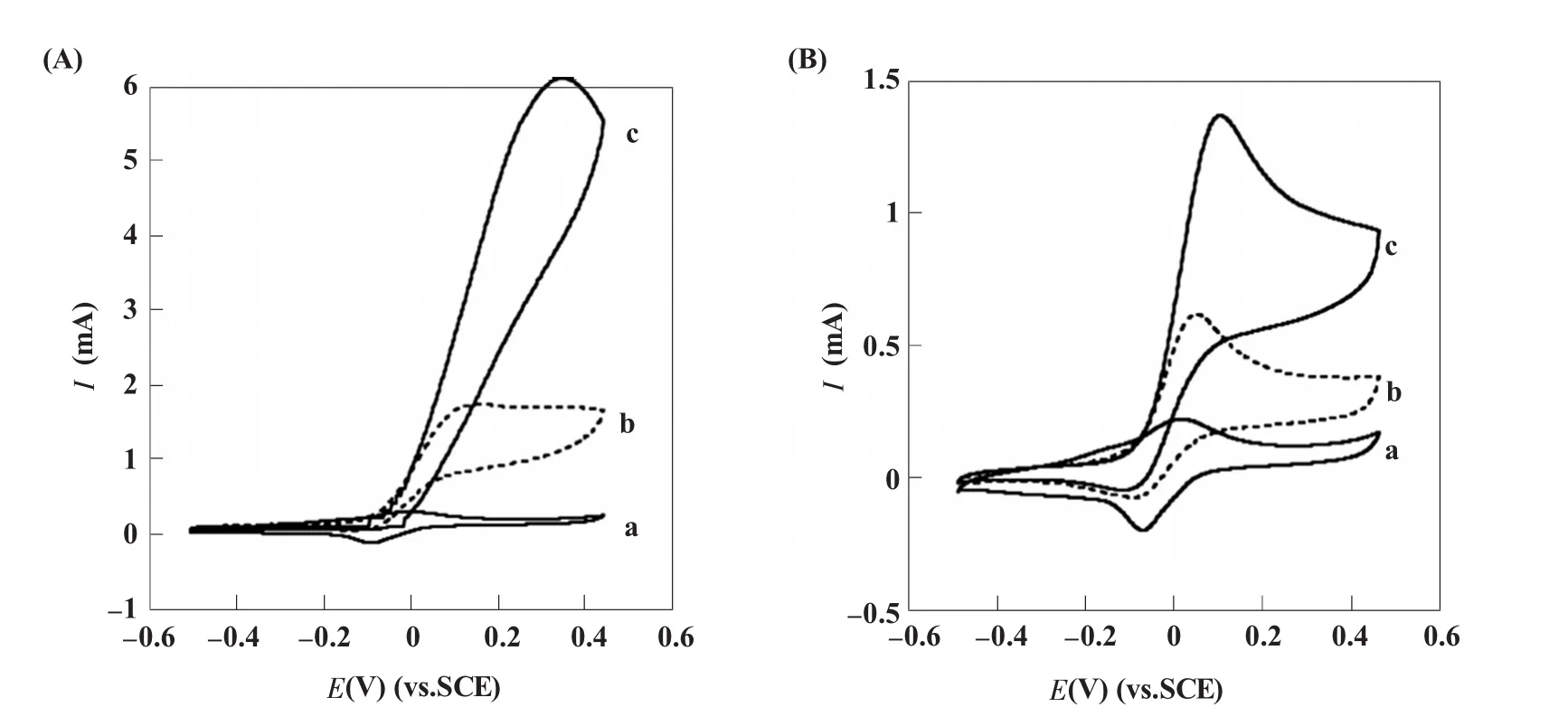
图2 葡萄糖氧化酶存在下通过微杆介导的葡萄糖的电化学催化氧化[4](A)聚苯胺-纳米金微杆介导的不同浓度葡萄糖氧化的循环伏安图;(B)聚苯胺-聚苯乙烯磺酸盐微杆介导的不同浓度葡萄糖氧化的循环伏安图。a:0 mmol/L;b:10 mmol/L;c:60 mmol/LFig.2 Bioelectrocatalytic oxidation of glucose by solubilized GOx,1 mg mL-1,and mediated by the microstructured redox-active rod assemblies[4](A) Cyclicvoltammogramsrecorded in thepresenceof the microstructured assemblies composed of PAn/Au-NPs and different concentrations of glucose; (B)Cyclic voltammograms recorded in the presence of the microstructured assemblies composed of PAn/PSS and different concentrations of glucose.a:0 mmol/L;b:10 mmol/L;c:60 mmol/L
除了基于GOD的葡萄糖传感器外,在基于辣根过氧化物酶 (horseradish peroxidase,HRP)的H2O2传感器[17~28]、基于次黄嘌呤氧化酶 (xanthine oxidase,XOD)的次黄嘌呤/黄嘌呤传感器[29~31]、基于乙醇脱氢酶 (alcohol dehydrogenase,ADH)的乙醇传感器[32]、基于乳酸脱氢酶(lactate dehydrogenase,LDH)的乳酸传感器[33]、基于乙酰胆碱酯酶的含磷杀虫剂传感器[34,35]、基于胆固醇氧化酶的胆固醇传感器[36],以及基于酪氨酸酶的酚类传感器[37,38]等设计中,纳米金的应用同样取得了很好的效果。此外,生理条件下并不作为酶的一些氧化还原蛋白,比如血红蛋白 (hemoglobin,Hb)、肌红蛋白 (myoglobin,Mb)以及细胞色素c(cytochrome c,cyt c),经过与纳米金在电极表面共修饰组装后也可表现出一定的酶活性,因而可用于构建检测H2O2、NO等底物的生物传感器[39~45]。
纳米金的另一个主要应用是参与构建基于抗原-抗体识别的电化学免疫传感器。图3显示的是一个典型的纳米金参与的免疫传感器的示意图。相比经典的酶联免疫传感器——ELISA,纳米金更为稳定,在复杂的检测环境不易降解和变性。更重要的是,通过与其他纳米材料联用,可以实现多靶标的检测。基于纳米金的这些优良特性,近年来,涌现出众多基于纳米金的免疫传感器[46~64]。比如,蒋健晖等[65]以人免疫球蛋白G(h IgG)为模型分析物,使固定化抗体与分析目标物h IgG及纳米金标记的h IgG抗体结合于电极表面,通过金沉积使电极表面的纳米金颗粒直径增大,并通过金增强表面吸附伏安分析实现了h IgG的高灵敏定量免疫检测。袁若[66]等通过LBL组装将多层辣根过氧化物酶/纳米金及L-半胱氨酸等材料组装到电极表面,实现对甲胎蛋白抗体的固定,研制出用于检测甲胎蛋白抗原(alpha-fetoprotein,AFP)的免疫传感器。信号标记对于免疫传感器而言是烦琐的一步,Yu等[67]使用纳米金发展了无标记的IgE检测,并有望成为通用的免疫传感设计。
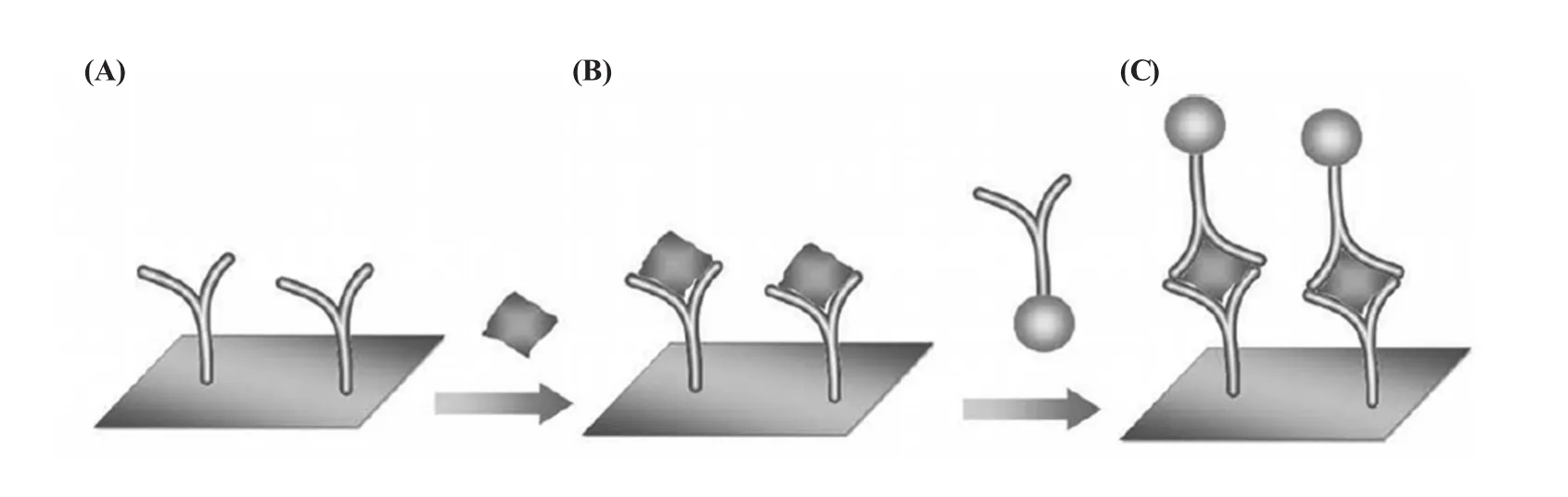
图3 (A)一抗通过静电作用或共价连接固定到碳电极表面;(B)目标蛋白(抗原)被固定的一抗识别;(C)金属纳米颗粒标记的二抗结合抗原形成“三明治”结构,从而可被电化学手段定量检测到[46]Fig.3 (A)Primary antibodies are immobilized on the carbon-electrode surfaces through electrostatic attraction or covalent-binding reactions;(B)Target protein(antigen)is recognized by the primary antibody immobilized on the surface;(C)Metal nanoparticle-labeled secondary antibody binds to the antigen through its unique epitope,forming a sandwich-type complex on the surface.The accumulation of metal nanoparticles on the surface can be quantified using electrochemical-measurement techniques[46]
综合来看,纳米金用于构建以蛋白质为识别元件的电化学生物传感器主要具备以下几个优势:1)作为金属材料,纳米金是电子的良导体,可以最大限度地促进电子传递,从而实现低电位下的电分析,以避免高电位中的噪音和干扰。2)作为纳米材料,其大的比表面积十分有利于蛋白质以及电化学信号探针等分子在电极表面的大量固定,从而有效地提高信号强度并实现信号放大。3)纳米金具有良好的生物相容性,即使进行在体实验也未发现对细胞有明显的毒副作用;在分子层面,这种良好的生物相容性还可为蛋白质提供良好的微环境,从而保持蛋白质的生物活性。4)纳米金可以很方便的通过静电作用或共价作用进行表面的化学修饰,从而为多样化的蛋白质表面固定方案打下基础。
纳米银和纳米铂是另外两种在电化学生物传感器构建中较为常用的金属纳米材料[68~75]。由于与纳米金的性质相似,基于这两种纳米材料的传感器设计与基于纳米金的传感器设计有异曲同工之妙,在此不一一赘述。
2 碳纳米管
自从1991年首次被报道以来,碳纳米管 (carbon nanotubes,CNTs)可以说是被研究得最多的纳米材料。对其理化性质的深入了解,以及对其功能的不断开拓,为其在生物传感器构建中的广泛应用打下了基础。与纳米金一样, CNTs同样也具备极好的电子传递能力、蛋白质的高负载能力以及良好的生物相容性,而且,由于CNTs本身的物质基础就是碳,因此其功能化将更为方便和多样。此外,由于CNTs为一维纳米材料,意味着CNTs在电极表面的组装将呈现网络状,因此,非常有利于蛋白质的固定。
Davis等[76]精细地描绘了GOD在单壁碳纳米管 (single-walled nanotubes,SWNTs)上的组装 (图4)。他们通过氧化SWNTs,使之表面带上充裕的活性基团,一方面有助于SWNTs在溶液中的分散,另一方面也给GOD的组装提供了便利条件。结果显示,即使不加入EDC、NHS等共价连接的活化剂,GOD也能在SWNTs表面很强地吸附并保持活性。他们将此吸附有GOD的SWNTS组装到玻碳电极上,得到了良好的GOD响应,其信号强度是没有SWNTs参与时的10倍,显示出SWCNTs在酶负载和能量转换上都具有良好的效果。
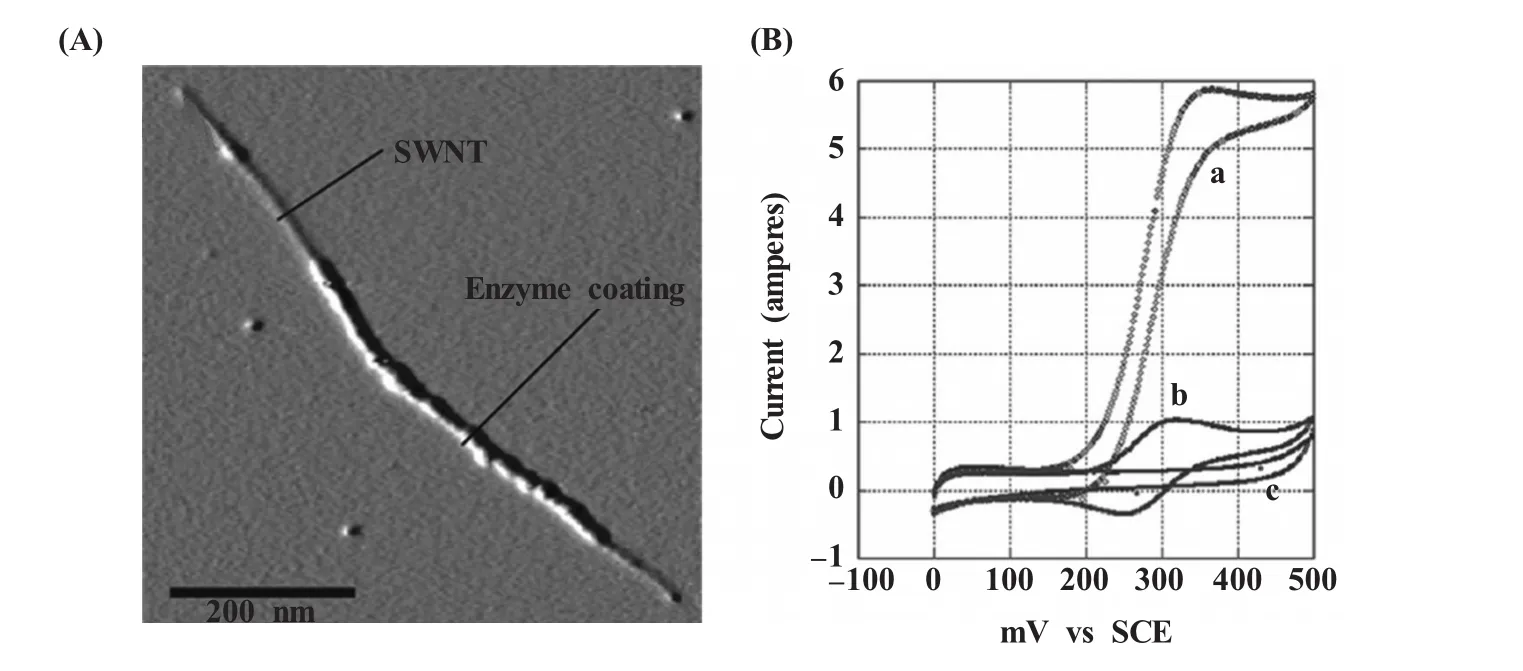
图4 (A)葡萄糖氧化酶修饰的单壁碳纳米管的原子力显微镜照片;(B)葡萄糖氧化酶-单壁碳纳米管共修饰的玻碳电极在无(曲线c)或有(曲线b)二茂铁一元酸,以及加入50 mmol/L葡萄糖(曲线a)后的循环伏安图[76]Fig.4 (A)Amplitude AFM image of a glucose oxidase-modified SWNT.Scale bar=200 nm.(B)Voltammetric response of a GOX-SWNT-modified GC electrode in the absence(curve c)and presence(curve b)of 0.5 mmol/L ferrocenemonocarboxylicacid (FMCA). Thecatalyticresponse(curvea) isobserved on theaddition of 50 mmol/L glucose[76]
除了在电极表面的平铺组装外,CNTs还可以通过化学气相沉积 (chemical vapor deposition,CVD)直接在电极表面垂直生长。Fang等[77]运用这种方法在电极表面生长CNTs,并利用 (4-羧苯基)重氮盐在CNTs表面的电化学还原对CNTs进行了功能化,从而共价连接Hb,结果发现Hb具有良好的电化学响应,并可用于构建H2O2传感器(图5)。Wisitsoraat等[78]也利用这种方法在金覆盖的SiO2/Si基底上生长了CNTs,然后通过电化学聚合在CNTs表面固定聚苯胺 (polyaniline,PANI)和胆固醇氧化酶,利用循环伏安法对底物胆固醇进行了检测,取得了良好的结果。
目前,基于以上两种方式,利用CNTs构建酶传感器[79~97]和免疫传感器[98~106]的报道数量众多,在此不一一列举。需要提出的是,由于CNTs难以具备纳米金那样良好的形态分布,因此对有序的表面组装提出了挑战。另外,大多数蛋白质的尺寸都属于零维的纳米级,因此在一维的CNTs表面组装相对而言缺少灵活性。出于这些考虑,将CNTs与零维的纳米颗粒,如纳米金、纳米铂等联合运用,在一定程度上可以克服两者在某些方面的缺陷,因而也是传感器构建中的良好策略[107~113]。
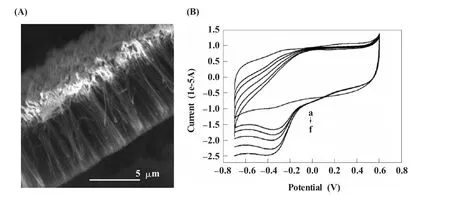
图5 (A)碳纳米管阵列的扫描电子显微镜照片;(B)血红蛋白-碳纳米管阵列修饰电极在加入不同浓度过氧化氢前后的循环伏安图[77]Fig.5 (A)SEM images of ACNTs;(B)Cyclic voltammograms of the Hb-ACNTs electrode in PBS(pH 7.0)before(a)and after the addition of 160,360,560,760,960 mmol/L H2O2(curve b to f).Scan rate:40 mV/s[77]
3 纳米氧化物
除了具备纳米材料共有的一些性质外,纳米氧化物还依材料的不同具备一些特殊的效应,比如纳米Fe3O4的磁效应、纳米TiO2的光电效应等。而这些效应在新型生物传感器的构建中可以产生一些意想不到的效果。在这一部分,我们将着重就纳米氧化物特殊效应的应用进行总结和回顾。
磁性纳米颗粒现在已被广泛用于分离富集、靶向运输等方面的研究,但在生物传感领域,磁效应的应用还不广泛。Willner等[114]利用纳米Fe3O4首次实现了电化学催化的磁控制,如图6所示,他们首先合成了能够稳定分散于有机溶剂中的磁性纳米Fe3O4,并构建了一个双层的电解液,上层为有机溶剂甲苯,下层为水溶液。当磁场处于上方时,Fe3O4分散在甲苯中,下方的GOD可以催化葡萄糖的氧化并得到电信号;但当磁场转移到电极下方,Fe3O4将被吸引下来覆盖住电极表面,从而阻碍GOD与电极的电子传递,失去了电驱动的GOD便无法进行催化,由此,通过转换磁场方向即可非常方便地控制酶催化反应进程。
纳米TiO2是另一种具有特殊效应 (光电效应)的纳米材料,由于具有极强的紫外线屏蔽能力和很高的表面活性,纳米TiO2已经被大量用于污水处理、消毒杀菌,以及在化妆品和涂料中防紫外线侵蚀。作者所在实验室曾利用纳米TiO2这种特有的性质构建了高灵敏的H2O2传感器 (图7)[115]。一般来讲,蛋白质在紫外线照射下很容易变性,但是我们利用纳米TiO2与Hb共修饰后发现,一定时间的紫外线照射后,Hb不仅没有失活,其催化活性反而大大提高,电化学检测H2O2的灵敏度提高了3倍,检测限降低了两个数量级。这一方面得益于TiO2对紫外线的屏蔽保护了Hb,另一方面,TiO2在紫外线激发下产生的激发态活性物质促进了Hb催化性能的提高。作者所在实验室在后续的研究中发现纳米ZnO也具有类似的效用[116]。
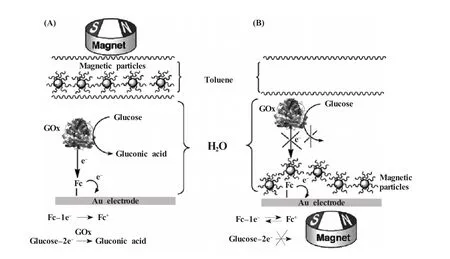
图6 (A)磁性纳米颗粒远离电极从而激活二茂铁介导的葡萄糖的电化学催化氧化;(B)电极表面被疏水的磁性纳米颗粒覆盖从而阻碍了电化学催化的物质扩散过程[114]Fig.6(A)The magnetic nanoparticles are retracted from the electrode surface,which is activated toward the ferrocene-mediated bioelectrocatalytic oxidation of glucose;(B)The electrode surface is blocked by the hydrophobic magnetic nanoparticles toward the diffusional bioelectrocatalytic process,while the surface-confined ferrocene is electrochemically active[114]
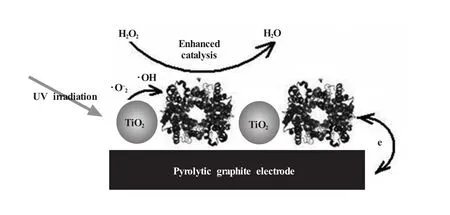
图7 二氧化钛纳米颗粒与血红蛋白共修饰电极的示意图[115]Fig.7 Scheme of TiO2nanoparticle/Hb co-modified electrode[115]
4 量子点
量子点作为荧光标记物,已经被广泛用于荧光示踪。在电化学生物传感器领域,量子点同样有着特殊的用途。以金属硫/硒/碲化物 (Zn/Cd/Pb-S/Se/Te)等为代表的量子点,一方面是很好的生物标记材料,另一方面,其中的金属离子Zn2+、Cd2+、Pb2+可用于阳极溶出伏安法检测,从而提供电化学信号。
Wang等[117]利用三种量子点 (ZnS、CdS以及PbS)分别标记三种抗体,并利用磁性纳米颗粒进行分离富集,通过溶出伏安法检测Zn2+、Cd2+、Pb2+在不同电位下的峰电流,可以同时检测三种抗原的存在 (图8)。其他一些课题组也利用量子点的这种性质进行了一些传感设计[118~120]。

图8 基于不同无机纳米颗粒的多蛋白的电检测原理[117](A)引入抗体修饰的磁珠;(B)抗原结合到磁珠表面的抗体上;(C)纳米颗粒标记的二抗被磁珠捕获;(D)对纳米颗粒进行电化学溶出分析Fig.8 Multi-protein electrical-detection protocol based on different inorganic nanoparticle(NP)tracers[117](A)Introduction of antibody-modified magnetic beads;(B)Binding of antigens to antibodies on the magnetic beads;(C)Capture of the NP-labeled secondary antibodies;(D)Dissolution of NPs and electrochemical stripping detection
总的来说,基于量子点的电化学传感设计具有以下一些优点:1)由于近年来量子点相关技术的日趋成熟,用于电化学检测的量子点的制备以及功能化比较方便,有商品化的产品可供选择;2)不同的量子点可以提供不同的电化学信号,这为多组分的分析提供了条件;3)溶出伏安法是基于金属离子的氧化还原分析,其理论检测限可达到飞摩尔水平,这为痕量检测打下了基础。
5 复合纳米材料
不同的纳米材料各自具备一定的特性,在电化学生物传感器的设计中使用单一的材料难以充分发挥纳米材料的性能,因此,同时使用多种纳米材料成为一个解决方案。一种思路是首先合成两种或多种纳米材料,然后在传感器的构建中同时或在不同阶段分别运用,比如图6所介绍的量子点与磁性纳米颗粒的运用;另一种思路则是在纳米材料的合成阶段将不同的材料进行组装,即合成复合纳米材料,将不同纳米材料的特性整合到一个纳米复合体中。一个很好的例子是CNTs与金属纳米颗粒复合的材料,另一个例子则是合成核/壳结构的纳米颗粒,而且这种做法目前更为常见。
Yu等[121]合成了MgFe2O4@SiO2核/壳纳米颗粒,由SiO2外壳提供良好的酪氨酸酶组装界面,由磁性的MgFe2O4内核实现蛋白在电极表面的磁控富集。由此构建的电化学生物传感体系可以对酶底物苯酚实现良好的检测。这种核/壳纳米颗粒一方面保持了磁性纳米颗粒的磁效应,另一方面又为蛋白质提供了良好的组装界面和微环境,因而成功地综合了两种纳米材料的优良特性。Zhu等[122]则充分利用AgCl和PANI的特性合成了AgCl@PANI纳米颗粒,一方面AgCl外壳克服了PANI在水相中难以分散的缺点,并为后续的电化学信号获取提供了良好的电子传递界面,另一方面,PANI为传感器在碱性条件下的使用提供了条件,并有效地防止了抗坏血酸的氧化,他们利用此复合纳米材料构建了用于检测多巴胺的电化学生物传感器。此外, Calvo、Zeng等课题组[123~127]也开展了一系列基于核/壳纳米颗粒的传感器研究工作。
6 展 望
纳米科技与电化学技术的结合和相互渗透,为基于蛋白质构建生物传感器提供了重大的创新机遇和诱人的市场前景,各种具有优良性能的纳米材料在生物传感器构建中的应用,不仅大大提升了生物传感器的性能,还拓宽了生物传感器的适用范围,为其在临床检测、食品安全、环境监测、医疗卫生等领域的应用开辟了新的道路。当然,新技术出现的同时也面临着新的挑战,比如如何实现原位分析、如何构建稳定专一的电化学生物传感芯片、如何建立健全的评价纳米材料效用及安全性的标准体系,等等。随着蛋白质科学,以及纳米技术、电化学技术、分子识别技术、表面固定技术等相关技术的不断发展,我们相信这些问题会逐步得到解决,电化学生物传感器也会获得更大的发展,展示更为广阔的应用前景。
1.张先恩.生物传感技术原理与应用.长春:吉林科学技术出版社,1991 Zhang XE. The principle andapplication ofbiosensing technology. Changchun:Jilin Science and Technology Publishing House,1991
2.张学记,鞠熀先,王·约瑟夫.电化学与生物传感器.北京:化学工业出版社,2009 Zhang XJ,Ju HX,Wang J. Electrochemistry and biosensors.Beijing:Chemical Industry Press,2009
3. Xiao Y,Patolsky F,Katz E,Hainfeld JF,Willner I."Plugging into enzymes":Nanowiring of redox-enzymes by a gold nanoparticle.Science,2003,299:1877~1881
4. GranotE,KatzE,BasnerB,WillnerI. Enhanced bioelectrocatalysis using Au-nanoparticle/polyaniline hybrid systems in thin films and microstructured rods assembled on electrodes.Chem Mater,2005,17:4600~4609
5.Zhang S,Wang N,Yu H,Niu Y,Sun C.Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles foruse as glucose biosensor.Bioelectrochemistry,2005,67:15~22
6. Yang W,Wang J,Zhao S,Sun Y,Sun C.Multilayered construction of glucose oxidase and gold nanoparticles on Au electrodes based on layer-by-layer covalent attachment.Electrochem Commun,2006,8(4):665~672
7. Liu SQ,Ju HX.Reagentless glucose biosensor based on direct electron transfer of glucose oxidase immobilized on colloidalgoldmodified carbon paste electrode. Biosens Bioelectron,2003,19:1 77~183
8. Luo XL,Xu JJ,Du Y,Chen HY.A glucose biosensor based on chitosan-glucose oxidase-gold nanoparticles biocomposite formed by one-step electrodeposition. Anal Biochem,2004,334:284~289
9.MansoJ,MenaML,Yanez-SedenoP,PingarronJ.Electrochemical biosensors based on colloidal gold-carbon nanotubescomposite electrodes. J ElectroanalChem,2007,603(1):1~7
10.Wu BY,Hou SH,Yin F,Li J,Zhao ZX,Huang JD,Chen Q.Amperometric glucose biosensor based on layer-by-layer assembly of multilayer films composed of chitosan,gold nanoparticles and glucose oxidase modified Pt electrode.Biosens Bioelectron,2007,22:838~844
11.Crespilho FN,Ghica ME,Florescu M,Nart FC,Oliveira ON,Brett CMA.A strategy for enzyme immobilization on layer-by-layer dendrimer-gold nanoparticle electrocatalytic membrane incorporating redox mediator. Electrochem Commun,2006,8(10):1665~1670
12.Sun Y,Bai Y,Yang W,Sun C.Controlled multilayer films ofsulfonate-capped gold nanoparticles/thionine used for construction of a reagentless bienzymatic glucose biosensor.Electrochim Acta,2007,52(25):7352~7361
13.Liu Y,Wu S,Ju HX,Xu L.Amperometric glucose biosensing ofgold nanoparticles and carbon nanotube multilayer membranes.Electroanalysis,2007,19(9):986~992
14.Zhang S,Yang W,Niu Y,Li Y,Zhang M,Sun C.Construction of glucose biosensor based on sorption of glucose oxidase onto multilayers of polyelectrolyte/nanoparticles.Anal Bioanal Chem,2006,384(3):736~741
15.Zhang FH,Cho SS,Yang SH,Seo SS,Cha GS,Nam H.Gold nanoparticle-based mediatorlessbiosensorprepared on microporous electrode.Electroanalysis,2006,18(3):217~222
16.Xue MH,Xu Q,Zhou M,Zhu JJ.In situ immobilization of glucose oxidase in chitosan-gold nanoparticle hybrid film on prussian blue modified electrode for high-sensitivity glucose detection.Electrochem Commun,2006,8:1468~1474
17.Lin J,Qu W,Zhang S.Disposable biosensor based on enzyme immobilized on Au-chitosan-modified indium tin oxide electrode with flow injection amperometric analysis.Anal Biochem,2007,360:288~293
18.XuSY,PengB,HanXZ. A third-generationH2O2biosensorbased on horseradish peroxidase-labeled Au nanoparticles self-assembled to hollow porouspolymeric nanopheres.Biosens Bioelectron,2007,22(8):1807~1810
19.Feng H,Wang H,Zhang Y,Yan BN,Shen GL,Yu RQ.A direct electrochemical biosensing platform constructed by incorporating carbon nanotubes and gold nanoparticles onto redox poly(thionine)film.Anal Sci,2007,23(2):235~239
20.Gao FX,Yuan R,Chai YQ,Chen SH,Cao SR,Tang MY.Amperometric hydrogen peroxide biosensor based on the immobilization of HRP on nano-Au/Thi/poly (paminobenzene sulfonic acid)-modified glassy carbon electrode.J Biochem Biopharm Meth,2007,70:407~413
21.LuoXL,XuJJ,ZhangQ,YangGJ,ChenHY.Electrochemically deposited chitosan hydrogel for horseradish peroxidase immobilization through gold nanoparticles self-assembly.Biosens Bioelectron,2005,21:190~196
22.Lin JH,Zhang LJ,Zhang SS.Amperometric biosensor based on coentrapment of enzyme and mediator by gold nanoparticles on indium–tin oxide electrode.Anal Biochem,2007,370:180~185
23.Tangkuaram T,Ponchio C,Kangkasomboon T,Katikawong P,Veerasai W.Design and development of a highly stable hydrogen peroxide biosensoron screen printed carbon electrode based on horseradish peroxidase bound with gold nanoparticles in the matrix of chitosan.Biosens Bioelectron,2007,22:2071~2078
24.Jia JB,Wang BQ,Wu AG,Cheng GJ,Li Z,Dong SJ.A method to construct a third-generation horseradish peroxidase biosensor:Self-assembling gold nanoparticles to three-dimensional sol?gel network.Anal Chem,2002,74(9):2217~2223
25.Zhu Q,Yuan R,Chai YQ,Zhuo Y,Zhang Y,Li XL,Wang N.A novel amperometric biosensor for determination of hydrogen peroxide based on Nafion and polythionine as well as gold nanoparticles and gelatin as matrixes.Anal Lett,2006,39(3):483~494
26.Chen SH,Yuan R,Chai YQ,Xu L,Wang N,Li XN,Zhang LY. Amperometric hydrogen peroxide biosensor based on the immobilization ofhorseradish peroxidase(HRP)on the layer-by-layer assembly films of gold colloidal nanoparticles and toluidine blue. Electroanalysis,2006,18(5):471~477
27.Wang L,Wang EK.A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized on colloidal Au modified ITO electrode.Electrochem Commun,2004,6(2):225~229
28.Li XL,Wu J,Gao N,Shen GL,Yu RQ.Electrochemical performance of l-cysteine-goldparticle nanocomposite electrode interface as applied to preparation of mediator-free enzymatic biosensors. Sens Actuators B Chem,2006,117:35~42
29. AgüíL,Manso J,Yáñez-Sedeño P,PingarrónJM.Amperometric biosensor for hypoxanthine based on immobilized xanthine oxidase on nanocrystalgold-carbon paste electrodes.Sens Actuators B Chem,2006,113:272~280
30. Çubukçu M,Timur S,Anik U.Examination of performance ofglassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection.Talanta,2007,74:434~439
31.Liu YJ,Nie LH,Tao WY,Yao SZ.Amperometric study of Au-colloid function on xanthine biosensor based on xanthine oxidase immobilized in polypyrrole layer.Electroanalysis,2004,16:1271~1278
32.Liu YJ,Yin F,Long YM,Zhang ZH,Yao SZ.Study of the immobilization ofalcoholdehydrogenase on Au-colloid modified gold electrode by piezoelectric quartz crystal sensor,cyclic voltammetry,and electrochemical impedance techniques.J Colloids Interf Sci,2003,258:75~81
33.Jena BK,Raj CR.Electrochemical biosensor based on integrated assembly of dehydrogenase enzymes and gold nanoparticles.Anal Chem,2006,78(18):6332~6339
34.Shulga O,Kirchhoff JR.An acetylcholinesterase enzyme electrode stabilized by an electrodeposited gold nanoparticle layer.Electrochem Commun,2007,9:935~940
35.Du D,Chen SZ,Cai J,Zhang AD.Electrochemical pesticide sensitivity test using acetylcholinesterase biosensorbased on colloidalgold nanoparticle modified sol-gel interface.Talanta,2008,74:766~772
36.Zhou ND,Wang J,Chen T,Yu ZG,Li GX.Enlargement ofgold nanoparticles on the surface ofself-assembled monolayer modified electrode:A new mode in biosensor design.Anal Chem,2006,78:5227~5230
37 Carralero V,Mena ML,Gonzalez-Cortés A,Yáñez-Sedeño P,Pingarrón JM.Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes: Application to the measurement of a bioelectrochemical polyphenols index in wines.Anal Chim Acta,2005,528:1~8
38.Carralero V,Mena ML,Gonzalez-Cortés A,Yáñez-Sedeño P,Pingarrón JM. Developmentofa high analytical performance-tyrosinasebiosensorbasedonacomposite graphite-Teflon electrode modified with gold nanoparticles.Biosens Bioelectron,2006,22:730~736
39.Zhang L,Jiang X,Wang EK,Dong SJ.Attachment of gold nanoparticles to glassy carbon electrode and its application for the direct electrochemistry and electrocatalytic behavior of hemoglobin.Biosens Bioelectron,2005,21:337~345
40.Fang B,Wang GF,Yang XH,Zha QQ,Zhang WZ,Kan XW.Electrochemistry of hemoglobin on a gold colloid-1,4-benzenedimethanethiol modified electrode and electrocatalyte detection of hydrogen peroxide.Anal Lett,2004,37(14):2911~2924
41.Chen SH,Yuan R,Chai YQ,Zhang LY,Wang N,Li XL.Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles.Biosens Bioelectron,2007,22:1268~1274
42.Yang WW,Li YC,Bai Y,Sun CQ.Hydrogen peroxide biosensor based on myoglobin/colloidal gold nanoparticles immobilized on glassy carbon electrode by a Nafion film.Sens Actuators B Chem,2006,115:42~48
43.Zhang H,Hu NF.Conductive effect of gold nanoparticles encapsulated inside polyamidoamine(PAMAM)dendrimers on electrochemistry of myoglobin(Mb)in{PAMAM-Au/Mb}n layer-by-layer films. J PhysChem B,2007,111(35):10583~10590
44.Zhang H,Lu HY,Hu NF.Fabrication of electroactive layer-by-layer films of myoglobin with gold nanoparticles of different sizes.J Phys Chem B,2006,110(5):2171~2179
45.Sun W,Qin P,Zhao RJ,Jiao K.Direct electrochemistry and electrocatalysisofhemoglobin on gold nanoparticle decorated carbon ionic liquid electrode.Talanta,2010,80:2177~2181
46.Kerman K,Saito M,Yamamura S,Takamura Y,Tamiya E.Nanomaterial-based electrochemical biosensors for medical applications.Trends Anal Chem,2008,27(7):585~592
47.Zhuo Y,Yuan R,Chai YQ,Zhang Y,Li XL,Zhu Q,Wang N.An amperometric immunosensor based on immobilization of hepatitis B surface antibody on gold electrode modified gold nanoparticles and horseradish peroxidase.Anal Chim Acta,2005,548:205~210
48. Tang DP,Ren JJ. Directand rapid detection of diphtherotoxin via potentiometric immunosensor based on nanoparticlesmixture and polyvinylbutyralasmatrixes.Electroanalysis,2005,17(24):2208~2216
49.Zhuo Y,Yuan R,Chai YQ,Zhang Y,Li XL,Wang N,Zhu Q.Amperometric enzyme immunosensors based on layerby-layer assembly of gold nanoparticles and thionine on Nafion modified electrode surface for α-1-fetoprotein determinations. SensActuatorsB Chem,2006,114:631~639
50.Xu YY,Bian C,Chen SF,Xia SH.A microelectronic technology based amperometric immunosensor for α-fetoprotein using mixed self-assembled monolayers and gold nanoparticles.Anal Chim Acta,2006,561:48~54
51.Mao X,Jiang JH,Huang Y,Shen GL,Yu RQ.Gold nanoparticle accumulation using magnetic particles:A new strategy for electrochemicalimmunoassay basedon the reversible reaction between dethiobiotin and avidin.Sens Actuators B Chem,2007,123:198~203
52.Chen ZP,Peng ZF,Zhang P,Jin XF,Jiang JH,Zhang XB,Shen GL,Yu RQ.A sensitive immunosensor using colloidal gold as electrochemical label.Talanta,2007,72:1800~1804
53.Chen H,Jiang JH,Huang Y,Deng T,Li JS,Shen GL,Yu RQ. Anelectrochemicalimpedanceimmunosensorwith signal amplification based on Au-colloid labeled antibody complex.Sens Actuators B Chem,2006,117:211~218
54.ChuX,FuX,ChenK,ShenGL,YuRQ.An electrochemicalstripping metalloimmunoassay based on silver-enhanced gold nanoparticle label.Biosens Bioelectron,2005,20:1805~1812
55.Liu Y,Qin ZH,Wu XF,Jiang H.Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on micro-comb electrode.Biochem Eng J,2006,32:211~217
56.Tang DP,Yuan R,Chai YQ.Electrochemical immunobioanalysis for carcinoma antigen 125 based on thionine and gold nanoparticles-modified carbon paste interface.Anal Chim Acta,2006,564:158~165
57. Wu L,Chen J,Du D,Ju HX. Electrochemical immunoassay forCA125 based on cellulose acetate stabilized antigen/colloidalgold nanoparticles membrane.Electrochim Acta,2006,51:1208~1214
58.Chen J,Tang JH,Yan F,Ju HX.A gold nanoparticles/solgel composite architecture for encapsulation of immunoconjugate for reagentless electrochemical immunoassay.Biomaterials,2006,27:2313~2321
59.Huang HZ,Ran PX,Liu ZG.Impedance sensing of allergen-antibody interaction on glassy carbon electrode modified by gold electrodeposition. Bioelectrochemistry,2007,70:257~262
60.Du DN,Xu XX,Wang SF,Zhang AD.Reagentless amperometric carbohydrate antigen 19-9 immunosensor based on direct electrochemistry of immobilized horseradish peroxidase.Talanta,2007,71:1257~1262
61.Fu XH.Electrochemical immunoassay for carbohydrate antigen-125 based on polythionine and gold hollow microspheres modified glassy carbon electrodes.Electroanalysis,2007,19:1831~1839
62.Li XL,Yuan R,Chai YQ,Zhang LY,Zhuo Y,Zhang Y.Amperometric immunosensor based on toluidine blue/nano-Au through electrostatic interaction for determination of carcinoembryonic antigen.J Biotechnol,2006,123:356~366
63.An HZ,Yuan R,Tang DP,Chai YQ,Li N.Dualamplification of antigen-antibody interactions via backfilling gold nanoparticles on(3-mercaptopropyl) trimethoxysilane sol-gel functionalized interface.Electroanalysis,2007,19:479~486
64.Liu Y.Electrochemical detection of prostate-specific antigen based on gold colloids/alumina derived sol-gel film.Thin Solid Film,2008,516:1803~1808
65.王潇蕤,周敬良,张艳丽,蒋健晖.基于纳米金增强吸附伏安分析的电化学免疫传感技术用于蛋白分子检测.化学传感器,2009,29:18~24 Wang XR,Zhou JL,Zhang YL,Jiang JH.Nano-based payments adsorptive voltammetric strengthen the analysis ofthe immune electrochemicalsensing technology for detection of protein molecules.Chemical Sensors,2009,29:18~24
66.黎雪莲,袁 若,柴雅琴,朱 强,张凌燕,王 娜.基于多层酶/纳米金固定甲胎蛋白免疫传感器的研究.化学学报,2006,64:325~330 Li XL,Yuan R,Chai YQ,Zhu Q,Zhang LY,Wang N.Reagentless amperometric immunosensor for α-1-Fetoprotein based on multilayers of horseradish peroxidase/nano Au. Acta Chimica Sinica,2006,64:325~330
67.Chen CR,Liu DY,Wu ZS,Luo QM,Shen GL,Yu RQ.Sensitive label-free electrochemical immunoassay by electrocatalytic amplification.Electrochem Commun,2009,11:1869~1872
68.Wang FC,Yuan R,Chai YQ,Tang DP.Probing traces of hydrogen peroxide byused ofa biosensorbased on mediator-free DNA and horseradish peroxidase immobilized on silver nanoparticles.Anal Bioanal Chem,2007,387(2):709~717
69.Cao Y,Wang J,Xu YY,Li GX.Sensing purine nucleoside phosphorylase activity by using silver nanoparticles.Biosens Bioelectron,2010,25:1032~1036
70.Ren ML,Meng XW,Chen D,Tang FQ,Jiao J.Using silver nanoparticle to enhance current response of biosensor.Biosens Bioelectron,2005,21:433~437
71.Gan X,Liu T,Zhu XL,Li GX.An electrochemical biosensor for nitric oxide based on silver nanoparticles and hemoglobin.Anal Sci,2004,20:1271~1275
72.Gan X,Liu T,Zhong J,Liu XJ,Li GX.Effect of silver nanoparticles on the electron transfer reactivity and the catalytic activity of myoglobin.ChemBioChem,2004,5:1686~1691
73.Xu JZ,Zhang Y,Li GX,Zhu JJ.An electrochemical biosensor constructed by nanosized silver particles doped sol-gel film.Mat Sci Eng C,2004,24:833~836
74.Shang FJ,Glennon JD,Luong JHT.Glucose oxidase entrapment in an electropolymerized poly(tyramine)film with sulfobutylether-β-cyclodextrin on platinum nanoparticle modified boron-doped diamond electrode.J Phys Chem C,2008,112:20258~20263
75.Cao ZX,Zou YJ,Xiang CL,Sun LX,Xu F.Amperometric glucose biosensor based on ultrafine platinum nanoparticles.Anal Lett,2007,40:2116~2127
76.Azamian BR,Davis JJ,Coleman KS,Bagshaw CB,Green MLH.Bioelectrochemical single-walled carbon nanotubes.J Am Chem Soc,2002,124(43):12664~12665
77.Yang J,Xu Y,Zhang RY,Wang YZ,He PG,Fang YZ.Direct electrochemistry and electrocatalysis of the hemoglobin immobilized on diazonium-functionalized aligned carbon nanotubes electrode. Electroanalysis,2009,21:1672~1677
78.Wisitsoraat A,Karuwan C,Wang EK,Phokharatkul D,Sritongkham P,Tuantranont A. High sensitivity electrochemical cholesterol sensor utilizing a vertically aligned carbon nanotube electrode with electropolymerized enzyme immobilization.Sensors,2009,9:8658~8668
79.Esplandiu MJ,Pacios M,Cyganek L,Bartroli J,Valle M.Enhancing the electrochemical response of myoglobin with carbon nanotube electrodes.Nanotechnology,2009,20(35):355502
80.Wang SF,Xie F,Liu GD.Direct electrochemistry and electrocatalysis ofheme proteins on SWCNTs-CTAB modified electrodes.Talanta,2009,77:1343~1350
81.Nagaraju DH,Pandey RK,Lakshminarayanan V.Electrocatalytic studies of cytochromecfunctionalized single walled carbon nanotubes on self-assembled monolayer of 4-ATP on gold.J Electroanal Chem,2009,627:63~68
82.Chen SH,Yuan R,Chai YQ,Yin B,Xu Y.Multilayer assembly of hemoglobin and colloidal gold nanoparticles on multiwall carbon nanotubes/chitosan composite for detecting hydrogen peroxide.Electroanalysis,2008,20:2141~2147
83.Wang ZG,Wan Y,Xu H,LiG,XuZK.Carbon nanotube-filled nanofibrous membranes electrospun from poly(acrylonitrile-co-acrylic acid) for glucose biosensor. JPhys Chem C,2009,113:2955~2960
84.Ivnitski D,Artyushkova K,Rincon RA,Atanassov P,Luckarift HR,Johnson GR.Entrapment of enzymes and carbon nanotubes in biologically synthesized silica:Glucose oxidase-catalyzed direct electron transfer.Small,2008,4:357~364
85.Wu XE,Zhao F,Varcoe JR,Thumser AE,Avignone-Rossa C,Slade RCT.Direct electron transfer of glucose oxidase immobilized in an ionic liquid reconstituted cellulose-carbon nanotube matrix.Bioelectrochemistry,2009,77:64~68
86.Zou Y,Xiang C,Sun LX,Xu F.Glucose biosensor based on electrodeposition of platinum nanoparticles onto carbon nanotubes and immobilizing enzyme with chitosan-SiO2sol-gel.Biosens Bioelectron,2008,23:1010~1016
87.Liu XQ,Shi LH,Niu WX,Li HJ,Xu GB.Amperometric glucose biosensor based on single-walled carbon nanohorns.Biosens Bioelectron,2008,23:1887~1890
88.Deng SY,Jian GQ,Lei JP,Hu Z,Ju HX.A glucose biosensorbased on directelectrochemistry ofglucose oxidase immobilized on nitrogen-doped carbon nanotubes.Biosens Bioelectron,2009,25:373~377
89. Gao RF,Zheng JB. Amine-terminated ionic liquid functionalized carbon nanotube-gold nanoparticles for investigating the direct electron transfer of glucose oxidase.Electrochem Commun,2009,11:608~611
90.Lee JY,Park EJ,Lee CJ,Kim SW,Pak JJ,Min NK.Flexible electrochemical biosensors based on O2plasma functionalized MWCNT. Thin Solid Films,2009,517:3883~3887
91.Deng L,Wang Y,Shang L,Wen D,Wang F,Dong S.A sensitive NADH and glucose biosensor tuned by visible light based on thionine bridged carbon nanotubes and gold nanoparticles multilayer. Biosens Bioelectron,2008,24:951~957
92.Yao YL,Shiu KK.A mediator-free bienzyme amperometric biosensor based on horseradish peroxidase and glucose oxidase immobilized on carbon nanotube modified electrode.Electroanalysis,2008,20:2090~2095
93.Rakhi RB,Sethupathi K,Ramaprabhu S.A glucose biosensor based on deposition ofglucose oxidase onto crystalline gold nanoparticle modified carbon nanotube electrode.J Phys Chem B,2009,113:3190~3194
94. Gouveia-Caridade C,Pauliukaite R,BrettCMA.Development of electrochemical oxidase biosensors based on carbon nanotube-modified carbon film electrodesfor glucose and ethanol. Electrochim Acta,2008,53:6732~6739
95.Zhang W,Wang LL,Zhang N,Wang GF,Fang B.Functionalization ofsingle-walled carbon nanotubes with cubic prussian blue and its application for amperometric sensing.Electroanalysis,2009,21:2325~2330
96.Yan YM,Baravik I,Yehezkeli O,Willner I.Integrated electrically contacted glucose oxidase/carbon nanotube electrodes for the bioelectrocatalyzed detection of glucose.J Phys Chem C,2008,112:17883~17888
97.Cosnier S,Ionescu RE,Holzinger M.Aqueous dispersions of SWCNTs using pyrrolic surfactants for the electro-generation of homogeneous nanotube composites.Application to the design of an amperometric biosensor.J Mater Chem,2008,18:5129~5133
98.Fernandez-Sanchez C,Pellicer E,Orozco J,Jimenez-Jorquera C,Lechuga LM,Mendoza E.Plasma-activated multi-walled carbon nanotube-polystyrene composite substrates for biosensing.Nanotechnology,2009,20(33):335501
99.Kim JP,Lee BY,Lee J,Hong S,Sim SJ.Enhancement of sensitivity and specificity by surface modification of carbon nanotubes in diagnosis of prostate cancer based on carbon nanotube field effect transistors.Biosens Bioelectron,2009,24:3372~3378
100.Viswanathan S,Rani C,Anand AV,Ho JAA.Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode.Biosens Bioelectron,2009,24:1984~1989
101.Munge BS,Krause CE,Malhotra R,Patel V,Gutkind JS,Rusling JF.Electrochemical immunosensors for interleukin-6. Comparison ofcarbon nanotube forestand gold nanoparticle platforms.Electrochem Commun,2009,11:1009~1012
102. LinJH,HeCY,ZhangLJ,ZhangSS. Sensitive amperometricimmunosensorforα-fetoprotein based on carbon nanotube/gold nanoparticle doped chitosan film.Anal Biochem,2009,384:130~135
103.Su HL,Yuan R,Chai YQ,Zhuo Y,Hong CL,Liu ZY,Yang X.Multilayer structured amperometric immunosensor built by self-assembly of a redox multi-wallcarbon nanotube composite. Electrochim Acta,2009,54:4149~4154
104.Buch M,Rishpon J.An electrochemical immunosensor for C-reactive protein based on multi-walled carbon nanotube-modified electrodes. Electroanalysis,2008,20:2592~2594
105. MahmoudKA,HrapovicS,LuongJHT. Picomolar detection ofprotease using peptide/single walled carbon nanotube/gold nanoparticle-modified electrode.ACS Nano,2008,2:1051~1057
106. MahmoudKA,LuongJHT. Impedancemethodfor detecting HIV-1 protease and screening for its inhibitors using ferrocene-peptide conjugate/Au nanoparticle/singlewalled carbon nanotube modified electrode.Anal Chem,2008,80:7056~7062
107.Yang J,Zhang RY,Xu Y,He PG,Fang YZ.Direct electrochemistry study of glucose oxidase on Pt nanoparticle-modified aligned carbon nanotubeselectrode by the assistance of chitosan-CdS and its biosensoring for glucose.Electrochem Commun,2008,10:1889~1892
108.Claussen JC,Franklin AD,Haque A,Porterfield DM,Fisher TS. Electrochemical biosensor of nanocubeaugmented carbon nanotube networks.ACS Nano,2009,3:37~44
109.Wen ZH,Ci SQ,Li JH.Pt nanoparticles inserting in carbon nanotube arrays: Nanocomposites forglucose biosensors.J Phys Chem C,2009,113:13482~13487
110.Zou YJ,Xiang CL,Sun LX,Xu F.Glucose biosensor based on electrodeposition of platinum nanoparticles onto carbon nanotubes and immobilizing enzyme with chitosan-SiO2sol-gel. Biosens Bioelectron,2008,23:1010~1016
111.Zhao K,Zhuang SQ,Chang Z,Songm H,Dai LM,He PG,Fang YZ.Amperometric glucose biosensor based on platinum nanoparticles combined aligned carbon nanotubes electrode.Electroanalysis,2007,19:1069~1074
112.Tsai MC,Tsai YC.Adsorption of glucose oxidase at platinum-multiwalled carbon nanotube-alumina-coated silica nanocomposite for amperometric glucose biosensor.Sens Actuators B Chem,2009,141:592~598
113.Wang YT,Yu L,Zhu ZQ,Zhang J,Zhu JZ,Fan CH.Improved enzyme immobilization for enhanced bioelectrocatalytic activity of glucose sensor.Sens Actuators B Chem,2009,136:332~337
114.Katz E,Baron R,Willner I.Magnetoswitchable electrochemistry gated by alkyl-chain-functionalized magnetic nanoparticles:Control of diffusional and surface-confined electrochemical processes.J Am Chem Soc,2005,127:4060~4070
115.Zhou H,Gan X,Wang J,Zhu XL,Li GX.Hemoglobinbased hydrogen peroxide biosensor tuned by the photovoltaic effect of nano titanium dioxide.Anal Chem,2005,77:6102~6104
116.Zhu XL,Yuri I,Gan X,Suzuki I,Li GX.Electrochemical study of the effect of nano-zinc oxide on microperoxidase and its application to more sensitive hydrogen peroxide biosensorpreparation. Biosens Bioelectron,2007,22:1600~1604
117.LiuGD,WangJ,Kim J,JanMR,CollinsGE.Electrochemicalcodingformultiplexed immunoassaysof proteins.Anal Chem,2004,76(23):7126~7130
118.Liu GD,Lin YY,Wang J,Wu H,Wai CM,Lin YE.Disposable electrochemical immunosensor diagnosis device based on nanoparticle probe and immunochromatographic strip.Anal Chem,2007,79(20):7644~7653
119.Wu H,Liu G,Wang J,Lin Y.Quantum-dots based electrochemical immunoassay of interleukin-1α.Electrochem Commun,2007,9(7):1573~1577
120.Cui RJ,Pan HC,Zhu JJ,Chen HY.Versatile immunosensor using CdTe quantum dots as electrochemical and fluorescent labels.Anal Chem,2007,79(22):8494~8501
121.Liu ZM,Liu YL,Yang HF,Yang Y,Shen GL,Yu RQ.A phenolbiosensorbased on immobilizing tyrosinase to modified core-shell magnetic nanoparticles supported at a carbon paste electrode.Anal Chim Acta,2005,533:3~9
122.Yan W,Feng XM,Chen XJ,Li XH,Zhu JJ.A selective dopamine biosensor based on AgCl@polyaniline core-shell nanocomposites.Bioelectrochemistry,2008,72(1):21~27
123.Scodeller P,Flexer V,Szamocki R,Calvo EJ,Tognalli N,TroianiH,Fainstein A. Wired-enzyme core-shellAu nanoparticlebiosensor.JAm Chem Soc,2008,130:12690~12697
124.Tang L,Zeng GM,Liu JX,Xu XM,Zhang Y,Shen GL,LiYP,Liu C. Catecholdetermination in compost bioremediation using a laccase sensor and artificial neural networks.Anal Bioanal Chem,2008,391(2):679~685
125.Wang Y,Qian WP,Tan Y,Ding SH,Zhang HQ.Direct electrochemistry and electroanalysis of hemoglobin adsorbed in self-assembled films ofgold nanoshells.Talanta,2007,72(3):1134~1140
126.Zhang Y,Zeng GM,Tang L,Huang DL,Jiang XY,Chen YN.A hydroquinone biosensor using modified core-shell magnetic nanoparticles supported on carbon paste electrode.Biosens Bioelectron,2007,22(9-10):2121~2126
127.Qiu JD,Peng HP,Liang RP.Ferrocene-modified Fe3O4@SiO2magnetic nanoparticles as building blocks for construction ofreagentless enzyme-based biosensors. Electrochem Commun,2007,9(11):2734~2738
This work was supported by grants from The National Science Fund for Distinguished Young Scholars(20925520)and Shanghai Science and Technology Committee(09DZ2271800)
Progress of Electrochemical Biosensors Fabricated with Nanomaterials
CHEN Guifang1,LIANG Zhiqiang2,LI Genxi1,2
1.Department of Biochemistry and National Key Laboratory of Pharmaceutical Biotechnology,Nanjing University,Nanjing 210093,China;
2.Laboratory of Biosensing Technology,School of Life Science,Shanghai University,Shanghai 200444,China
Feb 2,2010 Accepted:Apr 28,2010
LI Genxi,Tel:+86(25)83593596,E-mail:genxili@nju.edu.cn
Biosensorshave
more and more research interestsdue to the rapidlyincreased requirement for measurements in life sciences.Protein-based electrochemical biosensors,which are particular focused because of practical advantages,have been widely used in clinic,industry,environment,and agriculture.Due to the rapid development of nano Sci-Tech,many kinds of novel biosensors are fabricated.In this paper,we summarize the progress of protein-based electrochemical biosensors,with the emphasis on the discussion ofthe application ofnanomaterials to the developmentofelectrochemicalbiosensors fabricated with protein and nanomaterials.
Protein;Nanomaterial;Electrochemical biosensor;Enzyme biosensor;Immunosensor
2010-02-02;接受日期:2010-04-28
国家杰出青年科学基金项目(20925520),上海市科学技术委员会项目(09DZ2271800)
李根喜,电话:(025)83593596,E-mail:genxili@nju.edu.cn
O657.1

