甘油三酯体外抗肿瘤活性
傅伟昌,顾小红,俞惠新,汤 坚,*,蒋再良
(1.吉首大学食品研究所,湖南 吉首 416000;2.食品科学与技术国家重点实验室,江南大学,江苏 无锡 214122;3.江苏省原子医学研究所,江苏 无锡 214063; 4.江苏无锡市富仁生物科技有限公司,江苏 宜兴 214252)
甘油三酯体外抗肿瘤活性
傅伟昌1,2,顾小红2,俞惠新3,汤 坚2,*,蒋再良4
(1.吉首大学食品研究所,湖南 吉首 416000;2.食品科学与技术国家重点实验室,江南大学,江苏 无锡 214122;3.江苏省原子医学研究所,江苏 无锡 214063; 4.江苏无锡市富仁生物科技有限公司,江苏 宜兴 214252)
经非水反相高效液相色谱(NARP-HPLC)纯化,从癞葡萄籽油(MCVO)中分离得到了6个含共轭亚麻酸(CLns)的甘油三酯(TGs)组分。以纯度为96%的α-桐酸(α-ESA)为对照,考察甘油三酯组分对5个人体肿瘤细胞株(ECA-109、SGC-7901、Bel-7402、LS-174-T、KB)和1个人体正常细胞株(L02)的生长抑制效果。结果显示含CLn 的TG组分对人体肿瘤细胞的抑制作用远强于α-ESA,而且由3个CLns 组成的TG是TG组分中效果最弱的。结果表明:TG的脂肪酸组成和TG的分子结构(可使CLn稳定性提高)对含CLn 的TG组分抑制人体肿瘤细胞的生长具有重要影响。
癞葡萄籽油;甘油三酯;共轭亚麻酸;α-桐酸
Momordica Charantia L. Var. abbreviata Ser. (MCV) is identified as a species of Momordica Charantia. by Service Center for Products of Quality in Jiangsu Province, China. Triacylglycerols (TGs) are major components of the seed oil of MCV (MCVO). All of these TGs contain conjugated trienoic acids, mainlyα-eleostearic acid [α-ESA, 9c,11t,13toctadecatrienoic acid, 18∶3, a kind of conjugated linolenic acids (CLn)], which accounts for approximately 55% of the total fatty acids of MCVO[1]. TGs containing CLn were termed as CTGs in this paper. CLn were hydrolysates of CTGs.
Igarashi and Miyazawa investigated the cytotoxic effects of conjugated trienoic acids on various human tumorcell lines and reported that the cytotoxic action of the fatty acids (FAs) against human tumor cells was attributable to the conjugated trienoic structure[2]. The inhibitory effects of dietary administration of bitter melon oil (BMO), a mixture of CTGs from the seed oil of Momordica charantia L., on the development of colonic neoplasms was investigated using an animal colon carcinogenesis model initiated with a colon carcinogen azoxymethane (AOM), and the results from this investigation suggested that BMO could suppress AOM-induced colon carcinogenesis[3]. The modifying effects of dietary feeding of CLn isolated from BMO on the development of AOM-induced colonic aberrant crypt foci (ACF) had also been investigated in the male F344 rat, which suggested a possible chemopreventive action of CLn during the early phase of colon tumorigenesis that occurs through modulation of cryptal cell proliferation activity and/or apoptosis[4].
It was generally accepted that TGs must be degraded to release free fatty acids (FFA) to pass into the mucosal cells. The inhibitory effect of lipids on tumor cell lines was ascribed to the action of special FAs, which are the principal component of lipids. Therefore, the antitumor effects of TGs in the intact form have been completely neglected.
Since the mixture of CTGs and the hydrolysate of CTGs both exhibited cytotoxic effects on tumor cell lines, this investigation aimed to determine whether an individual CTG, which is in its intact form, could be cytotoxic against human tumor cells, and whether CTGs with special FA composition and structure could display strong inhibitory effects on human tumor cells.
If the inhibitory effects of CTGs on human tumor cell lines were confirmed to be stronger than that of the FFA form of CLn at the same equivalent concentration of CLn, the contributions of the component of non-conjugated FAs in CTGs and the stereospecific structure of CTGs to cytotoxic effect should be further considered for treatment purposes. Such an effect would be similar to structural modifications of medicine, such as penicillium, to protect or enhance the bioactive center of medicine.
The molecular structure of TGs, including the distribution of FAs in the various stereospecific positions of glycerol backbone, was generally accepted to affect the nutritional, biochemical, and physical properties of lipids[5]. The functions of an individual CTG might be determined by the FAs’composition and positions of three glycerol backbones that FAs occupied. Furthermore, TGs are not required to be completely enzymatically cleaved to FFAs for absorption, which is not in accordance with general concept[6].
TGs in MCVO were separated from MCVO by nonaqueous reversed-phase high-performance liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry (NARP-HPLC/APCI-MS). Structures of TGs in MCVO were identified according to the relative abundances of specific fragment ions of diacylglycerols (DGs) from TGs[7-8]. The cytotoxicity of CTGs, which were isolated from MCVO, was investigated in the present study.
1 Materials and Methods
1.1 Materials, reagents and instruments
The MCV seeds were collected from ripened fruits, supplied by Wuxi FuRen Biologic Science and Technology CO. Ltd., China, and simply air-dried by atmospheric air in a shaded area. The tung seeds were gathered from a suburb of Jishou city, China. Human tumor cell lines, which included ECA-109 (esophageal carcinoma), SGC-7901 (carcinoma ventriculi), Bel-7402 (hepatoma), LS-174-T (colon carcinoma), and KB (mouth epidermal carcinoma), as well as the human normal cell L02 (liver cell) were obtained from the Cell Bank of Shanghai Institutes for Biological Sciences (Shanghai, China). These tumor cell lines were cultured by the Jiangsu Institute of Nuclear Medicine (Wuxi, China).
RPMI 1640 were obtained from Gibicol (Grand Island, N. Y., USA). Sulforhodamine B (SRB) was a product of Sigma (St. Louis, USA). Newborn calf serum was supplied by Shanghai Shisheng Biologic Science and Technology Co. Ltd., (Shanghai, China). Other solvents and reagents were of AR or HPLC grade, purchased from Sinopharm Chemical Reagent Co. Ltd.(Shanghai, China). 96-well plates(NVNC,Roskilde,Denmark).
WaterTM600 HPLC system (Waters, Milford, MA, USA). C18column at 30 ℃ (25 cm×10.0 mm, 10μm particle size, Hanbang Science and Technology Co. Ltd., Huai’an, China). Microplate ReaderTM(TMBio-Rad, Model 3550, Hercules, USA).
1.2 Methods
1.2.1 Extraction and purification of MCVO
Total lipids were extracted, according to the method of Bligh and Dyer[9], and purified, according to the AOCS Official Method[10], in order to obtain a TGs mixture.
1.2.2 Preparation and identification of α-ESA
α-ESA, a typical CLn, had been used as a control in many studies of the cytotoxic effects of lipids containingCLn in tumor cell lines[2,4]. For this reason, α-ESA, which was isolated from tung oil, was used as a control in the present study. Tung oil was saponified, according to the method of Cao et al[11], to obtain total FFAs. α-ESA was purified from the total FFAs by recrystallization from 90% ethanol at -20℃and identified by RP-HPLC/Electrospray Ionization-MS.
1.2.3 Preparation and identification of TGs of MCVO
TGs were separated by NARP-HPLC from TGs mixture of MCVO. For NARP-HPLC experiments, a WaterTM600 HPLC system with a UV detector at 270 nm was used. Samples were dissolved in 2-propanol∶hexane (5∶4, V/V) at a concentration of 100 mg/mL and were injected into a Lichrospher C18column at 30 ℃ . The mobile phase consisted of 60% acetonitrile and 40% 2-propanol-hexane (5∶4, V/V). The flow rate was 3 mL/min. Structural identification of TGs in MCVO was performed according to References[8].
1.2.4 Cell cultures
After separation by NARP-HPLC from TGs mixture,α-ESA and TG fractions of MCVO were purged with nitrogen gas to remove the solvent and then dissolved in ethanol or tetrahydrofuran (THF) to a concentration of 20 mmol/L to prepare stock solutions. Samples were prepared from the stock solutions and diluted to the final concentrations of 25-500 μmol/L with RPMI-1640 medium. Dimethylsulfoxide (DMSO) was a commonly used solvent in the past cytotoxic experiment; however, DMSO was not an appropriate solvent in the present experiment, since samples would be layered when the stock solutions prepared with DMSO were diluted to the final concentrations of 25-500μmol/L. The final concentration of ethanol or THF to concentrations of 2.5% (V/V) did not affect experimental results (data not shown).
All cell types were pre-cultured in RPMI-1640 medium supplemented with 10% (V/V) newborn calf serum, 100 units/mL penicillin, and 100μg/mL streptomycin. Cultures were incubated at 37 ℃ in a humidified atmosphere of 95% air and 5% CO2.
Cells were seeded in 96-well plates with (0.6-1)×104cells per well, and each parallel well was 6 wells. After incubation for 24 h, different doses of samples were added. Pure RPMI-1640 medium was added as the negative control. After incubation for 72 h, the number of viable cells was determined by the Sulforhodamine B (SRB) method, according to the methods of Skehan et al[12]. The absorbance at 531nm of each solution per well was measured using a Microplate ReaderTM.
1.2.5 Statistical analysis
Statistical analysis was performed using a one-way ANOVA followed by the Tukey-test at P<0.05.
2 Results and Discussion
2.1 Identification of the purified TGs of MCVO
TG fractions were separated by NARP-HPLC from the TG mixture of MCVO, and a total of six fractions were obtained. Structures of these fractions were identified by APCI-MS[8]. TGs in Fraction I were identified as CLnCLnCLn, TGs in Fraction II were identified as CLnCLnL and CLnLCLn, and Fraction III was coeluted-compounds consisting of four kinds of TGs with a partition number (PN) of 40, which could not be separated by NARP-HPLC. TGs in Fraction III were identified as LCLnL, OCLnCLn, CLnOCLn, and PCLnCLn. TGs in Fraction IV were identified as CLnCLnS, while Fraction V was coeluted-compounds consisting of eight kinds of TGs with PN of 44. TGs in Fraction V were identified as CLnOO, OCLnO, LCLnS, LSCLn, CLnLS, CLnPO, CLnOP, and PCLnO. TGs in Fraction VI were identified as SCLnS. Abbreviations for fatty acyl residues were as follows∶ P, palmitic acid (C16∶0); S, stearic acid (C18∶0); O, oleic acid (C18∶1); L, linoleic acid (C18∶2); CLn, conjugated linolenic acid (C18∶3). The middle fatty acyl residues occupied sn-2 positions.
2.2 Cytotoxicity to human cells of the MCVO TGs
As shown in Table 1 (for Bel-7402 cells), Table 2 (for SGC-7901 cells), Table 3 (for KB cells), Table 4 (for L02 cells), Table 5 (for ECA-109 cells), and Table 6 (for LS-174-T cells), the numbers of viable cells for the six human cell lines decreased in a dose-dependent manner (P<0.05).Note∶ Compared with α-ESA,the effects were considered significant at P<0.05(*) and P<0.01(**). Hereinafter the same .
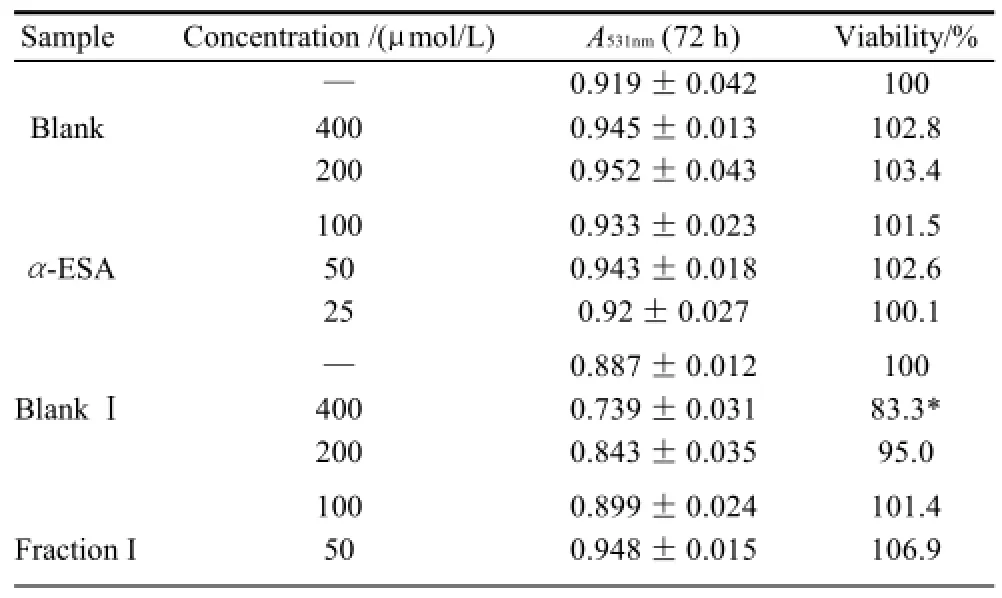
Table 1 Inhibitory effect of triacylglycerols of MCVO on the proliferation of Bel-7402 cells (x±s, n=6)
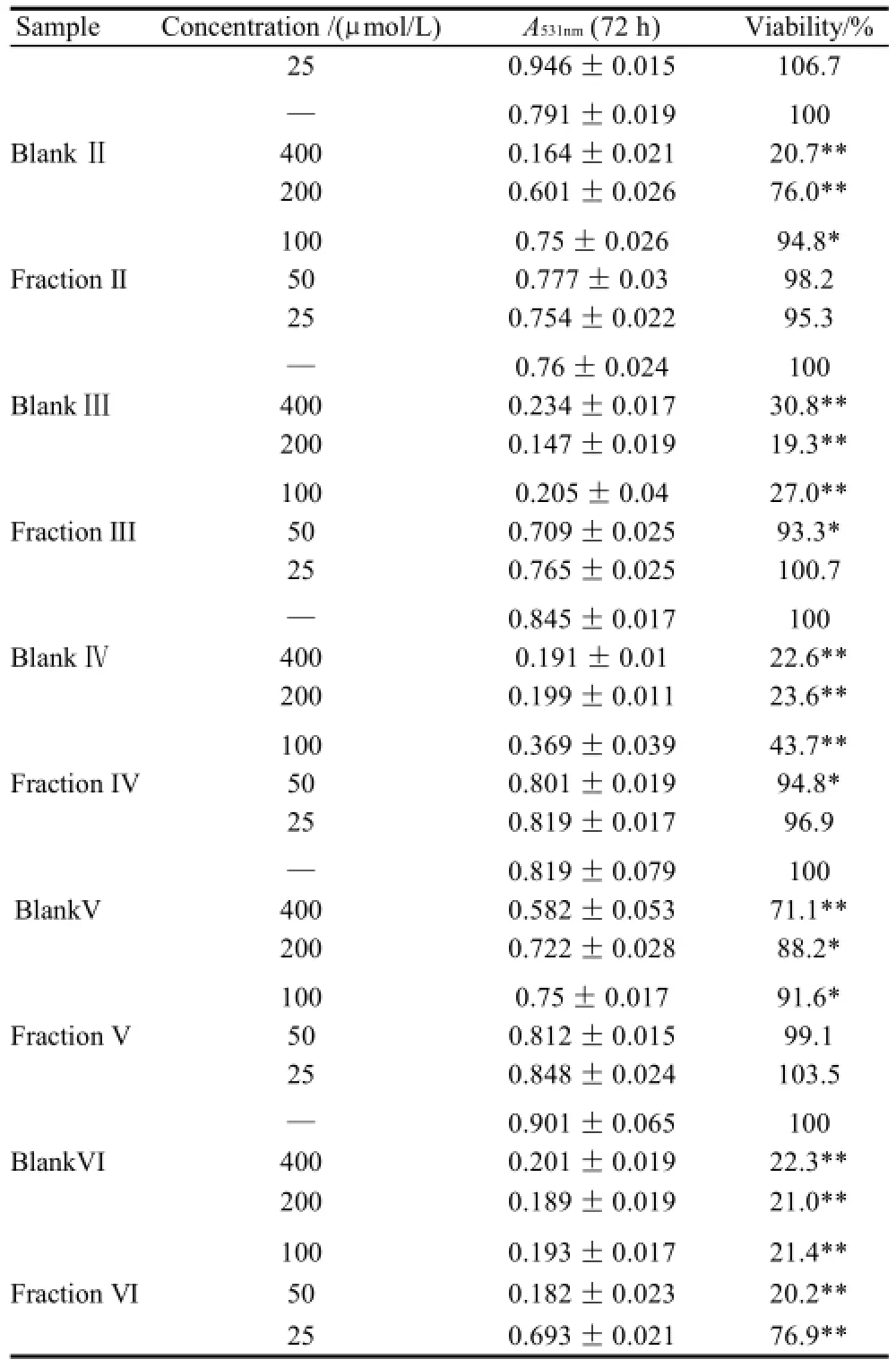
续表1
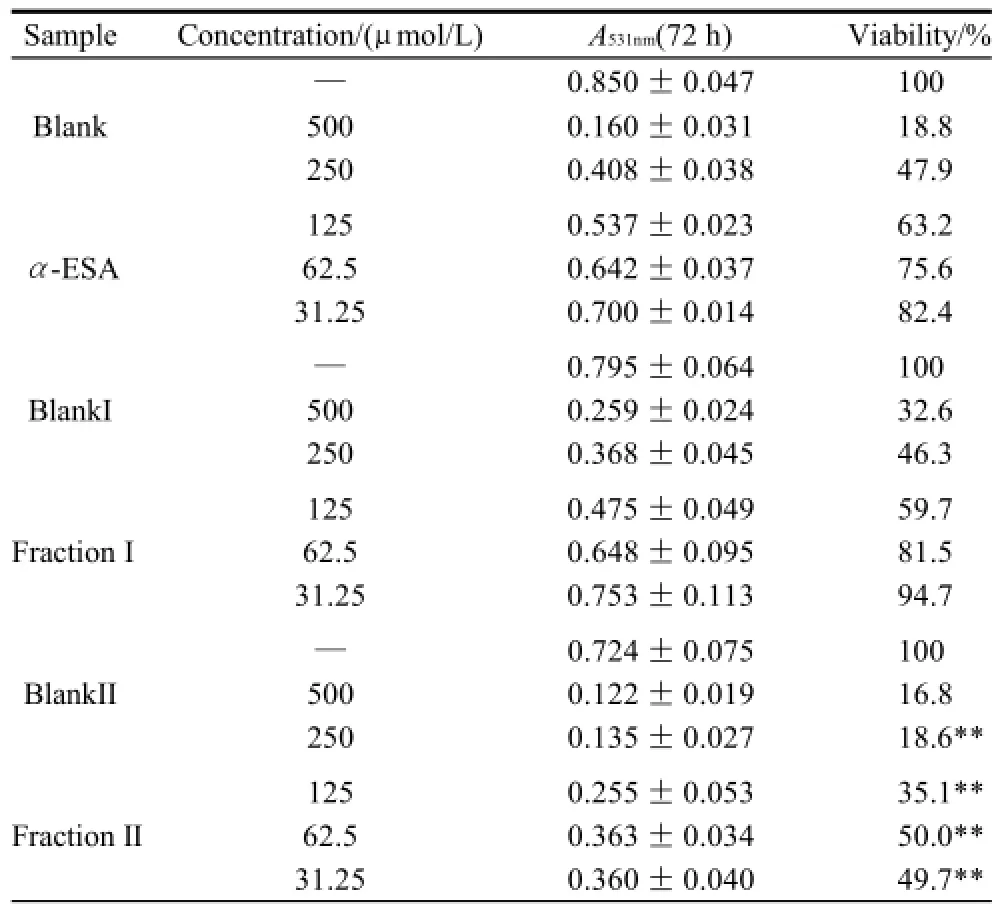
Table 2 Inhibitory effect of triacylglycerols of MCVO on the proliferation of SGC-7901 cells (x±s, n=6)
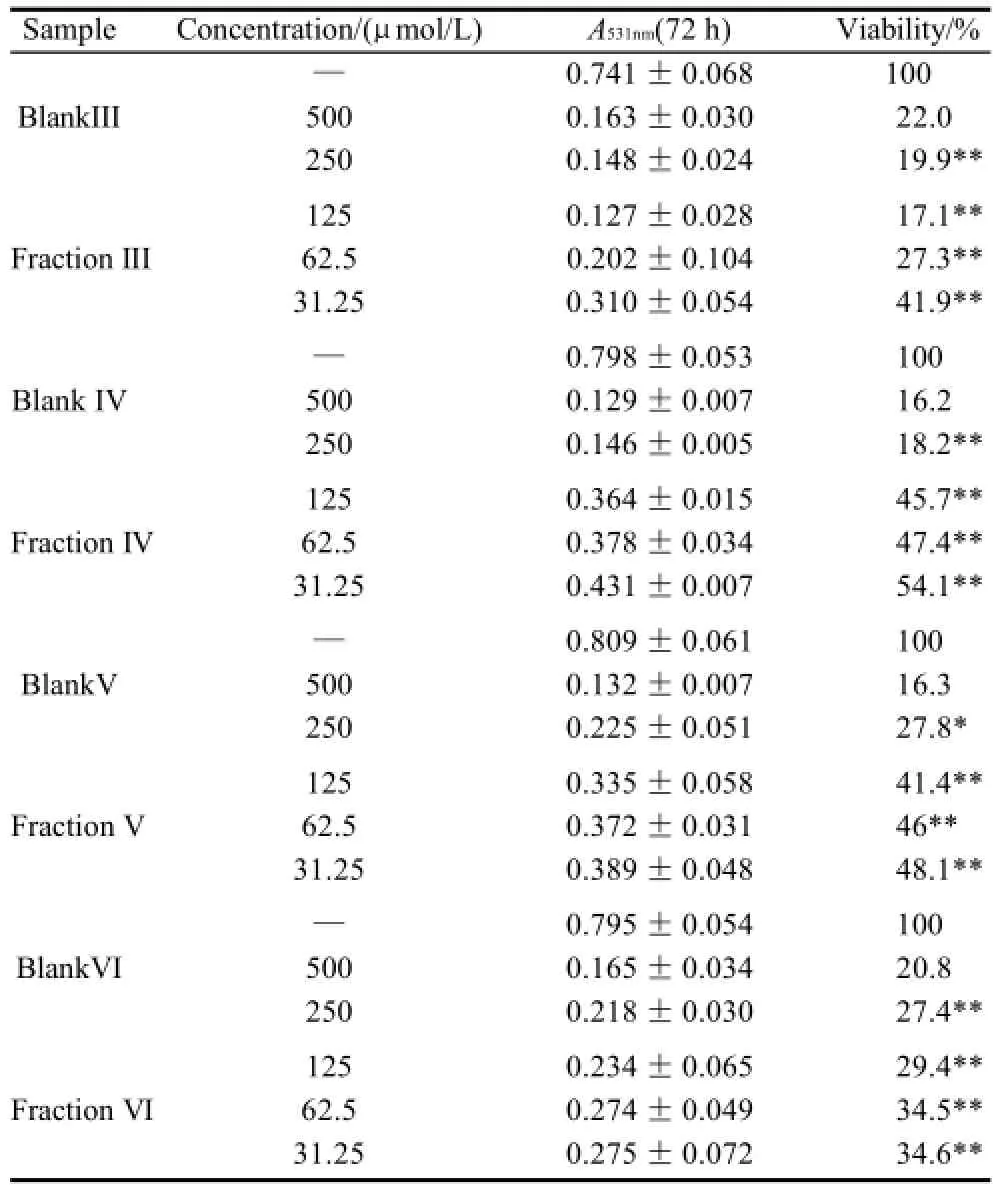
续表2
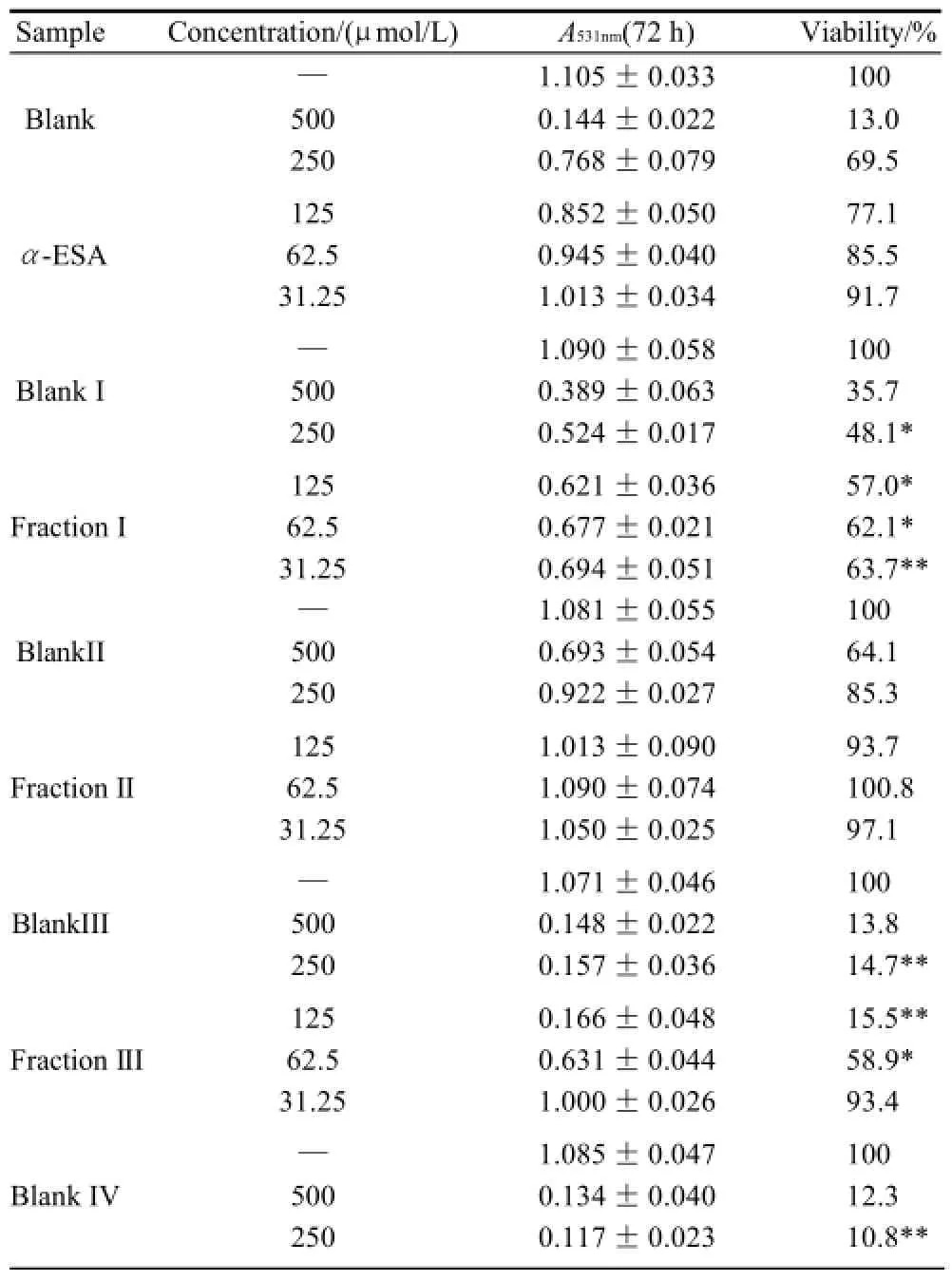
Table 3 Inhibitory effect of triacylglycerols of MCVO on the proliferation of KB cells (x±s, n=6)
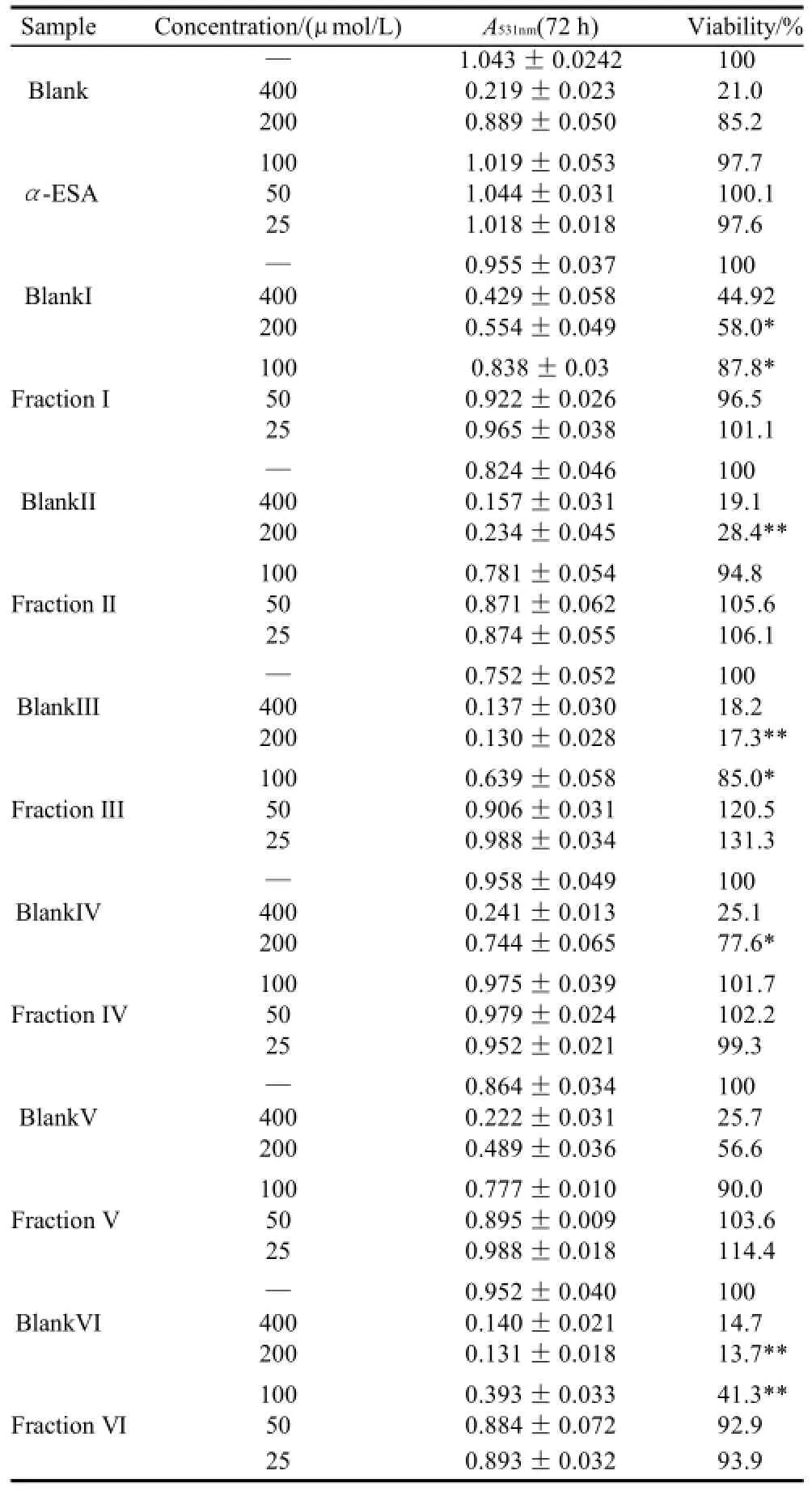
Table 4 Inhibitory effect of triacylglycerols of MCVO on the proliferation of L02 Cells (x±s, n=6)
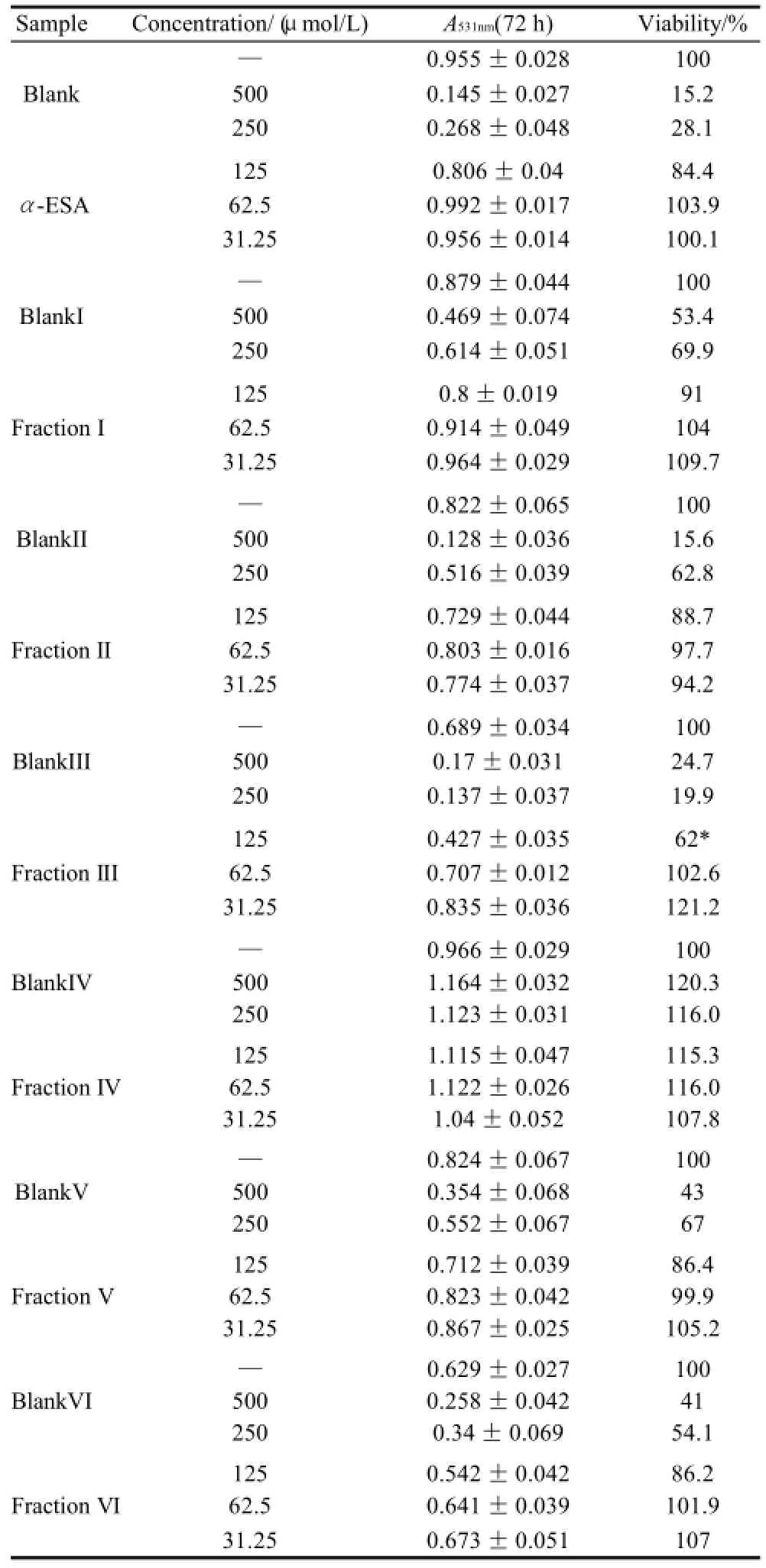
Table 5 Inhibitory effect of triacylglycerols of MCVO on the proliferation of ECA-109 cells (x±s,n=6)
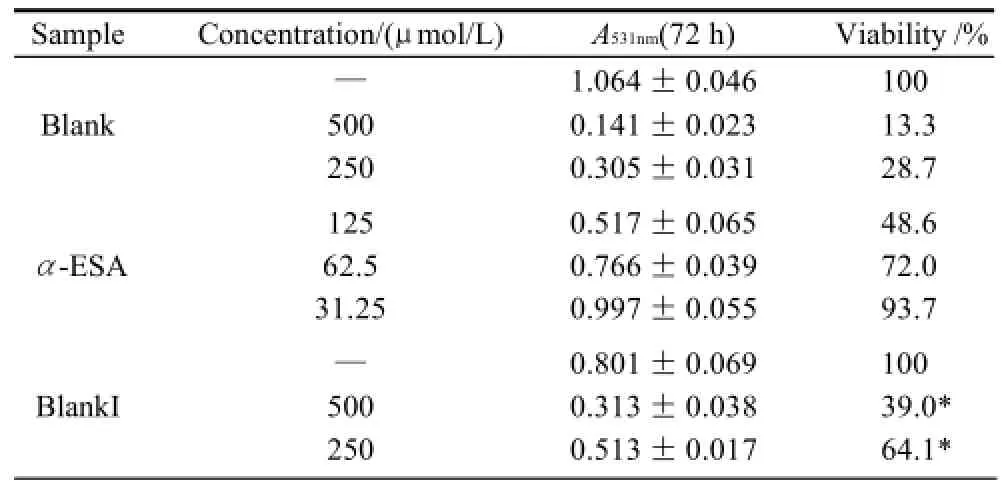
Table 6 Inhibitory effect of triacylglycerols of MCVO on the proliferation of LS-174T cells (x±s,n=6)
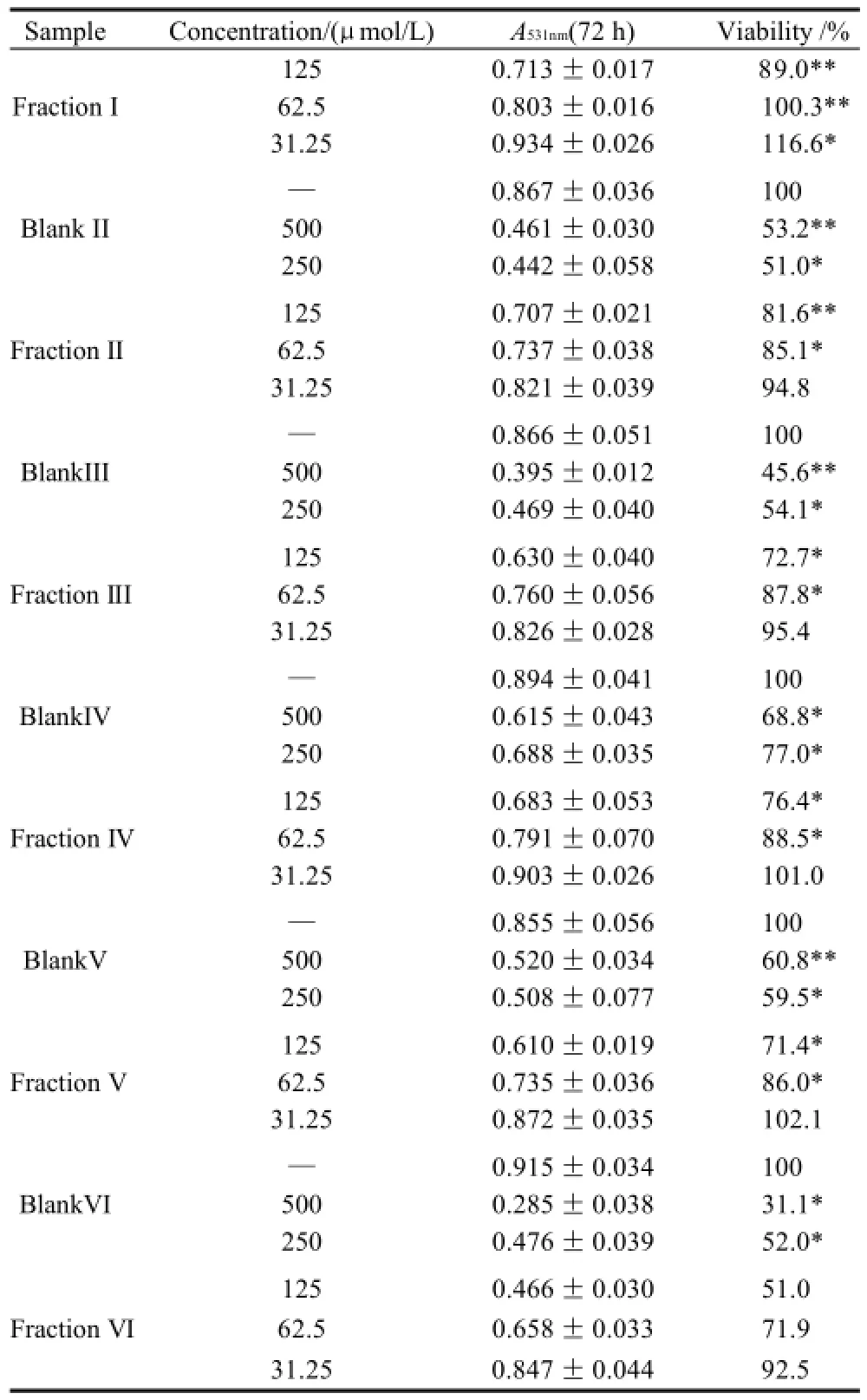
续表6
As a control, α-ESA displayed weaker cytotoxicity than that of the MCVO TGs on five cell lines, except for the cell line LS-174-T. Fraction I (CLnCLnCLn) displayed the weakest cytotoxicity against most of tumor cell lines in six TGs fractions, while Fraction VI (SCLnS) exhibited the strongest cytotoxicity.
The inhibitory effects of CLn on tumor cell growth have already been reported, demonstrating that CLn displayed intensive cytotoxicity[2-4,13]. Suzuki et al.[14]studied the cytotoxic effects of various CLn isomers on mouse tumor cells and human monocytic leukemia cells. These previous results suggested that the cis/trans configuration of the 9,11, 13-CLn isomers has an insignificant influence on the cytotoxic effects of CLn, and 9,11,13-CLn isomers were more cytotoxic than 8,10,12-CLn isomers on tumor cells. Igarashi et al.[15]reported that all-trans CLns exhibited the strongest growth-inhibitory effect among the CLn isomers. However, all-trans CLns were known to be rare components in natural seed oils[16], resulting in the limited effects of the all-trans CLns. Our preliminary work indicated that the MCVO TGs contained three CLn isomers that included α-ESA, which is the principal component of FAs of MCVO, and two rare isomers, which were both 9,11,13-CLn with different configuration[1]. Though the CLn isomers in the MCVO TGs were not identified for cis or trans configurations in the present investigation, according to the results of Suzuki et al.[14], there may be weak influences of the MVCO TGs containing the rare CLn isomers on the experimental results. In the present study, α-ESA displayed intensive cytotoxicity against cell line LS-174-T, which is a colon carcinoma (Table 6). This result was in accordance with the References[2,4], although the observed viability did not reach the previously obtained level. However, α-ESA exhibited weaker cytotoxicity against the five other cell lines than the six TG fractions of MCVO (Tables 1-5). These results suggested that α-ESA might have a selective cytotoxic or cytostatic activity on tumor cell lines at a certain concentration. Kitamura et al.[17]also determined that α-ESA did not exert clear modification effects on 7,12-dimethylbenz[a]anthracene and 1,2-dimethylhydrazineinduced mammary and colon carcinogenesis in female Sprague-Dawley rats. Another implication of these results was that the cytotoxicity of α-ESA was really weaker than the CTGs in MCVO.
Tsuzuki et al.[18-19] determined that tumor growth was suppressed by α-ESA via lipid peroxidation and that α-ESA was easily oxidized in the FFA form, indicating that the use of α-ESA in the FFA form in food or medicine would result in oxidation of these compounds before absorption and prevent beneficial bioactivities. Oxidation of CLn could be attenuated by triacylglycerol esterification[19]. The cytotoxic action of α-ESA and CTGs against human tumor cells might be attributable to conjugated trienoic structures[2], but the stability of CLn might also play an important role.
The results presented herein demonstrated that the inhibitory effects of α-ESA in the FFA form on tumor cell growth were weaker than those of CTGs. α-ESAs in MCVO TGs were all of the natural triacylglycerol esterification form, which may indicate the underlying mechanism for the stronger inhibitory effects of CTGs rather than α-ESA on most human tumor cell, especially at low concentrations.
The results also indicated that the FA compositions and the molecular structures of CTGs were critical for the apparent cytotoxicity to human tumor cell lines, which might indicate the cause for the different effects on tumor cell linesamong the six fractions of CTGs. For example, Fraction I (CLnCLnCLn) displayed weak effects. The equivalent number of CLn of Fraction I was three times that of Fraction VI (SCLnS). The effects of Fraction VI were hypothesized to be weaker than those of Fraction I; however, contradictory results were obtained.
TGs at high concentration were suggested to aggregate on the membrane surfaces of tumor cells and block the tumor cells, resulting in tumor cell death, which would not involve the action of FAs. However, this mechanism was not verified, since different TGs at the same concentration level did not exhibit the same inhibitory effects on all tumor cell lines. In the present study, different TGs containing CLn at a certain concentration displayed selective cytotoxic or cytostatic activities on tumor cell lines.
3 Conclusions
The inhibitory effects of the TGs containing CLn on human tumor cell lines were more potent than that of α-ESA, and the effect of TG with three CLns was the weakest. These results suggest that the fatty acid compositions and molecular structures of the TG with more intense stability, which contain CLn, might affect the displayed cytotoxicity to human tumor cell lines.
[1]FU Weichang, PAN Saikun, GU Xiaohong, et al. Analysis of fatty acids in the seed oil of Momordica charantia L. var. abbreviata Ser. by GCMS[J]. China Oils Fats, 2007, 32(12)∶ 79-82.
[2]IGARASHI M, MIYAZAWA T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells[J]. Cancer Lett, 2000, 148∶ 173-179.
[3]KOHNO H, YASUI Y, SUZUKI R, et al. Dietary seed oil rich in conjugated linolenic acid from bitter melon inhibits azoxymethane-induced rat colon carcinogenesis through elevation of colonic PPARγ expression and alteration of lipid composition[J]. Int J Cancer, 2004, 110∶ 896-901.
[4]KOHNO H, SUZUKI R, NOGUCHI R, et al. Dietary conjugated linolenic acid inhibits azoxymethane-induced colonic aberrant crypt foci in rats[J]. Cancer Sci, 2002, 93(2)∶ 133-142.
[5]FAUCONNOT L, HAU J, AESCHLIMANN J M, et al. Quantitative analysis of triacylglycerol regioisomers in fats and oils using reversedphase high-performance liquid chromatography and atmospheric pressure chemical ionization mass spectrometry[J]. RCM, 2004, 18∶ 218-224.
[6]BRACCO U. Effect of triglyceride structure on fat absorption[J]. Am J Clin Nutri, 1994, 60(Suppl 6)∶ 1002S-1009S.
[7]HOLCAPEK M, JANDERA P, FISCHER J, et al. Analytical monitoring of the production of biodiesel by high-performance liquid chromatography with various detection methods[J]. J Chromatogr A, 1999, 858∶13-31
[8]FU Weichang, GU Xiaohong, TAO Guanjun, et al. Structure identification of triacylglycerols in the seed oil of Momordica charantia L. var. abbreviata Ser[J]. J Am Oil Chem Soc, 2009, 86(1)∶ 33-39.
[9]BLIGH E G , DYER W J. A rapid method of total lipid extraction and purification[J]. Can J Biochem Physiol, 1959, 37(8)∶ 911-917.
[10]AOCS. Official methods and recommended practices of the american oil chemists society[M]. 5th ed. American Oil Chemists Society, Champaign, Illinois, USA. 1998.
[11]CAO Ying, YANG Lin, GAO Hongli, et al. Re-characterization of three conjugated linolenic acid isomers by GC-MS and NMR[J]. CPL, 2007, 145∶ 128-133.
[12]SKEHAN P, STORENG R, SCUDIERO D, et al. New colorimetric cytotoxicity assay for anti-cancer-drug screening[J]. J Natl Cancer Inst, 1990, 82∶ 1107-1112.
[13]YASUI Y, HOSOKAWA M, SAHARA T, et al. Bitter gourd seed fatty acid rich in 9c,11t,13t-conjugated linolenic acid induces apoptosis and up-regulates the GADD45, p53 and PPARgamma in human colon cancer Caco-2 cells[J]. Prostaglandins Leukot & Essent Fatty Acids, 2005, 73 (2)∶ 113-119.
[14]SUZUKI R, NOGUCHI R, OTA T, et al. Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells[J]. Lipids, 2001, 36(5)∶ 477-482.
[15]IGARASHI M, MIYAZAWA T. Preparation and fractionation of conjugated trienes from linolenic acid and their growth-inhibitory effects on human tumor cells and fibroblasts[J]. Lipids, 2005, 40(1)∶ 109-113.
[16]TAKAGI T, ITABASHI Y. Occurrence of mixtures of geometrical isomers of conjugated octadecatrienoic acids in some seed oils∶ analysis by open-tubular gas liquid chromatography and high performance liquid chromatography[J]. Lipids, 1981, 16(7)∶ 546-551.
[17]KITAMURA Y, YAMAGISHI M, OKAZAKI K, et al. Lack of chemopreventive effects of α-eleostearic acid on 7, 12-dimethylbenz[a] anthracene (DMBA) and 1,2-dimethylhydrazine (DMH)-induced mammary and colon carcinogenesis in female Sprague-dawley rats[J]. Food Chem Toxicol, 2006, 44∶ 271-277.
[18]TSUZUKI T, TOKUYAMA Y, IGARASHI M, et al. Tumor growth suppression byα-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation[J]. Carcinogenesis, 2004, 25 (8)∶ 1417-1425.
[19]TSUZUKI T, IGARASHI M, IWATA T, et al. Oxidation rate of conjugated linoleic acid and conjugated linolenic acid is slowed by triacylglycerol esterification andα-tocopherol[J]. Lipids, 2004, 39(5)∶475-480.
TS225.1
A
1002-6630(2010)15-0271-07
2009-11-02
傅伟昌(1969—),男,副教授,博士,研究方向为食品分析,食品功能性因子检测。E-mail:fuwch@163.com
* 通信作者:汤坚(1945—),男,教授,研究方向为食品风味、食品分析,食品功能性因子检测。E-mail:jiantang06@yahoo.com.cn

