基于量子化学分析的类黄酮抗氧化功能区划分
刘本国,杨继国,郭 烨,宁正祥,高建华
(1.河南科技学院食品学院,河南 新乡 453003;2.华南理工大学轻工与食品学院,广东 广州 510640)
基于量子化学分析的类黄酮抗氧化功能区划分
刘本国1,2,杨继国2,郭 烨1,宁正祥2,高建华2
(1.河南科技学院食品学院,河南 新乡 453003;2.华南理工大学轻工与食品学院,广东 广州 510640)
本研究采用量子化学方法对常见类黄酮的最高占有轨道组成、最活泼羟基位置与其抗氧化活性的关系进行研究。结果表明:类黄酮的分子结构可分为3个功能域:抗氧化能力决定域(B环)、抗氧化能力加强域(C环)、溶解性及亲和力调节域(A环)。 该功能域划分表明,可通过在类黄酮分子的A环上引入亲油或亲水性的基团来提高其在水相或油相中的溶解性,充分发挥其抗氧化作用。
类黄酮;抗氧化;分子修饰;量子化学;自由基
Theoretically, flavonoids have both hydrophilic and hydrophobic property due to the presence of hydrophobic benzene rings and hydrophilic hydroxyl groups. However, in fact, the solubility of flavonoids in either water- or oilphase is very low, which restricts the exhibition of their bioactivity. So, the purpose of molecular modification of flavonoids in recent reports was to improve the solubility of flavonoids[1-2]. But there was no corresponding theory to guide the selection of modification site.
With the development of computer technology and quantum chemistry, quantum chemistry calculation has been paid more and more attention. And many reports had used the quantum chemical parameters independently or combined other common parameters for quantitative structureactivity relationship (QSAR) study, The advantages of quantum chemical parameters were as following∶ 1) The molecular and sub-molecular structures could be displayed on the base of the whole molecular structure; 2) The chemical reaction could be directly explained by quantum chemical parameters[3-5].
In this paper, the quantum chemical parameters were used to describe the antioxidant activity of flavonoids, which was considered to be the bioactivity foundation of flavonoids[6-8]. The effects of molecular structures of fla-vonoid aglycones on the antioxidant activity were studied in order to conclude the method to improve the solubility of flavonoids with keeping their original antioxidant activity.
1 Materials and Methods
The optimized molecular structures of 14 flavonoids were obtained from computational calculations on the 3D molecular structure using the program Hyperchem 6.0 (HyperCube, Inc., Gainesville, FL, USA). The structures were drawn as 2D structures and the Molecular Mechanics force field (MM+) was selected for geometry optimization using the Polak-Ribiere algorithm. The obtained three-dimensional geometries were submitted to a final geometry optimization by using the AM1 method. Heat of formation of the parent molecules (Hfm) and the semi-quinone free radicals (Hff) were obtained to calculate the index∶ ΔHf= Hff-Hfm. To make a comparison, parent molecules and free radicals were all calculated in Unrestricted Hartree Fock (UHF) approximation. In the calculations, only the most stable conformations of flavonoids and their semi-quinone free radicals were taken into consideration. And the corresponding hydroxyl group donating H in the most stable semi-quinone free radical was determined as the most active hydroxyl group in a flavonoid molecule.
2 Results and Discussion
2.1 Choice of the calculation method and parameters of quantum chemistry
Many researches have already proved the close relationship between free radicals and many diseases, such as tumor, inflammation, atherosclerosis and physiological senescence[9-10]. Most of the free radicals have strong oxidant activity[12]. The excess free radicals could be very harmful to human health. Thus, it is practically significant to scavenge these excess free radicals. Flavonoids, widespread in fruits, vegetables, teas and medicinal plants, have received the greatest attention, and have been studied extensively, since they are highly effective antioxidants, less toxic than synthetic antioxidants such as butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT)[12]. Flavonoids as phenolic antioxidants play the antioxidant role by hydrogendonating and Electron-donating∶
1) Hydrogen-donating mechanism∶ the phenolic hydroxyl group can donate the hydrogen to other harmful free radicals so as to form the stable free radical with the form of semi-quinone to interrupt the chain reaction of harmful free radicals.
2) Electron-donating mechanism∶ Flavnoids can donate the electrons to harmful free radicals to interrupt the chain reaction[13-14].
The purpose of this study was to conclude the relationship between the antioxidant activity and the molecular structure of flavonoids by selecting the appropriate parameters. For the hydrogen-donating mechanism, the stability of semi-quinone free radical and its antioxidant activity were directly related. And the difference in heat of formation between the parent molecule and its semi-quinone free radical produced after H-abstraction (ΔHf), appeared to be a good index for measuring the scavenging activity of antioxidants[15]. The effectiveness of ΔHfcan be understood easily, as it represents the strength of the O-H bond. The lower strength of the O-H bond corresponds to a higher scavenging activity. As a result, the corresponding hydroxyl group donating H in the most stable free radical was determined as the most active hydroxyl group in a flavonoid molecule. On the other side, semi-empirical quantum chemistry method Austin Model 1 (AM1) was selected to calculate because it was better than other semi-empirical methods, such as Modified Neglect of Diatomic Differential Overlap (MNDO) and Parametric Method 3 (PM3), to calculate ΔHf. According to the previous research, the result of ΔHffrom AM1 method was similar to that from the calculation of Density Foundation Theory (DFT) on 6-31G (P)[16]. For electron-donating mechanism, the capacity of electron transfer is positively correlated to the capacity of electrondonating. So many parameters such as HOMO energy and relative adiabatic ionization potential were used to reflect the electron-donating capacity of antioxidant agent. According to Frontier Molecular Orbital Theory, HOMO was in charge of electron-donating, Zhang[17]thought that HOMO energy couldn t characterize the antioxidant activity of flavonoids. So this paper mainly focused on the main electron-donating active site of flavonoids, the composition of highest occupied molecular orbital was investigated.
2.2 The most active sites for H-donating of flavonoids
The ΔHfvalues and the most active hydroxyl groups of 14 flavonoids were shown in Table 1-3. Through the analysis of these data, it could be found that∶
1) In flavonoids with a C2-C3 double bond and a C3 hydroxyl group (such as kaempferol, quercetin and fisetin) , the most active hydroxyl groups for H-donating are the onesattached to C4' or C3.
2) In flavonoids with a C2-C3 double bond, but without a C3 hydroxyl group (such as luteolin, apigenin, genistein and daidzein), the most active hydroxyl groups for H-donating are the ones attached to C4'.
3) In flavonoids with a C2-C3 single bond and a hydroxyl group linked to C3 or not (such as naringenin, hesperetin, dihydromyricetin, taxifolin and eriodictyol), the most active H-donating hydroxyl groups are the ones attached to B ring.
4) In flavonoids with an ortho-dihydroxyl group on B ring (such as luteolin, quercetin, fisetin, dihydromyricetin, taxifolin and eriodictyol), the ΔHfvalues of these adjacent hydroxyl groups correspond to the values of the most active hydroxyl group for hydrogen-donating. This indicates that the substitution enhances radical scavenging ability.
5) The ΔHfvalue of the C5 or C7 hydroxyl group (such as chrysin and formononetin) is significantly higher than that of the hydroxyl group on B ring. This agrees with the conclusion that C ring deactivates A ring, reported by Zhang[18].
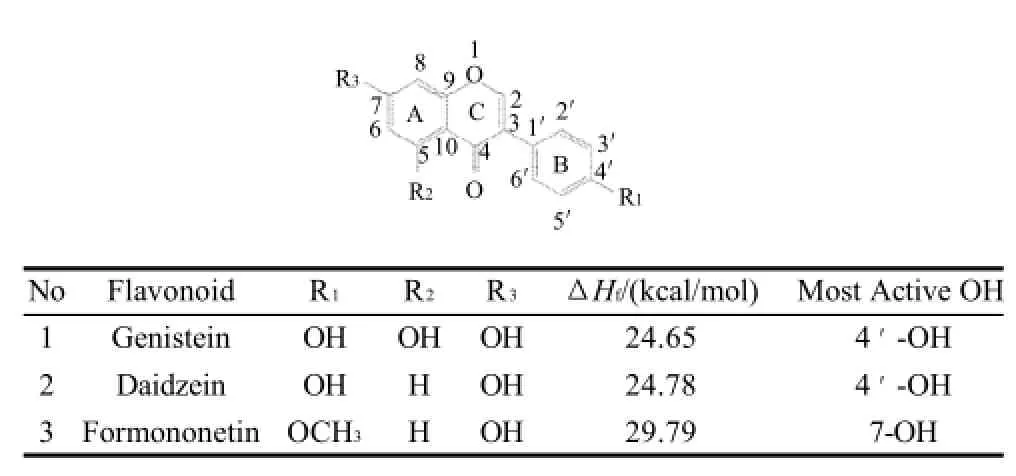
Table 3 Chemical structures and the most active OH sites of some isoflavones
The above analysis fully showed that considering from hydrogen-donating mechanism, the hydroxyl groups on B ring of flavonoids made a great contribution to the flavonoid antioxidant activity, in certain circumstances, the hydroxyl groups on C ring also contributed, which coincided with the result of his study[19].
2.3 HOMO of flavonoids
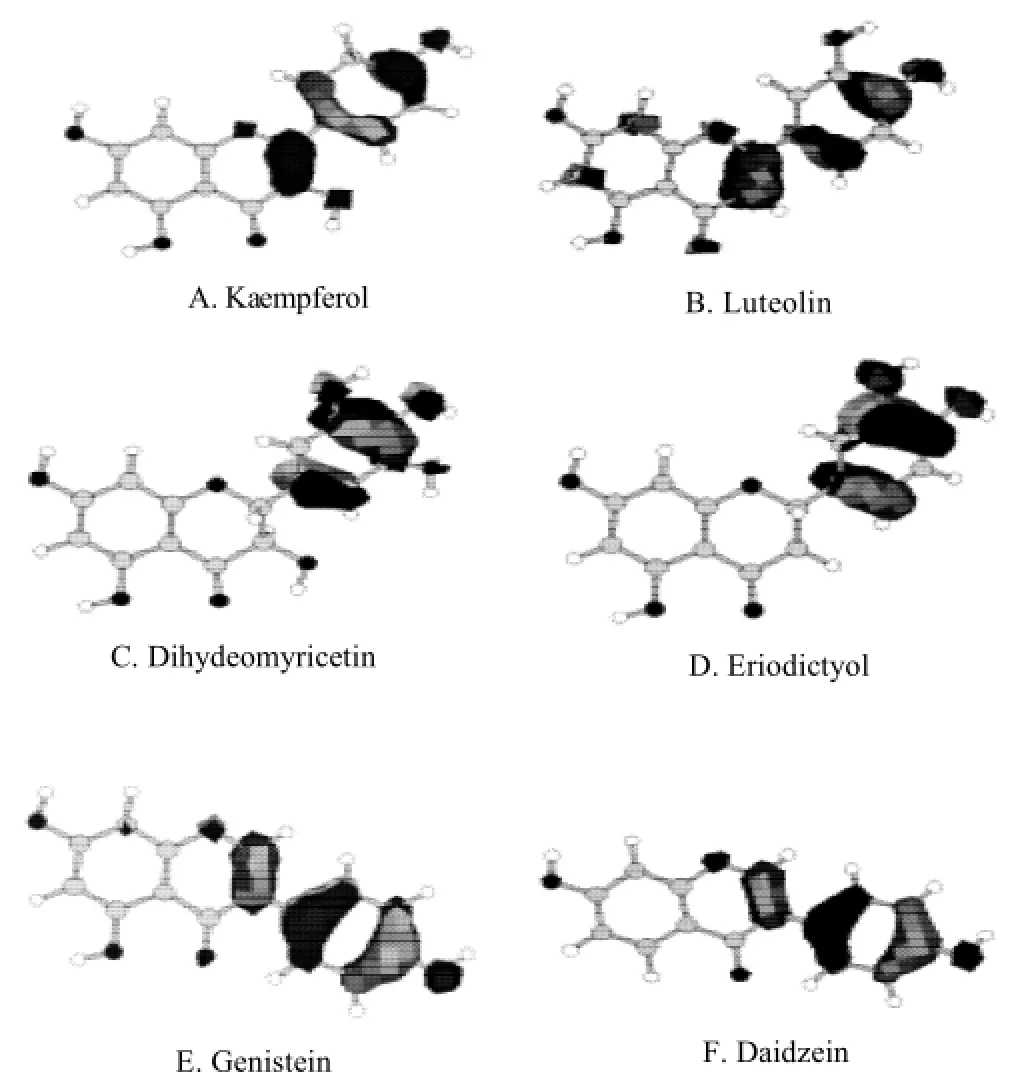
Fig.1 HOMO of some flavonoids (orbit contour value of 0.05)

Table 1 Chemical structures and the most active OH sites of some flavones and flavonols
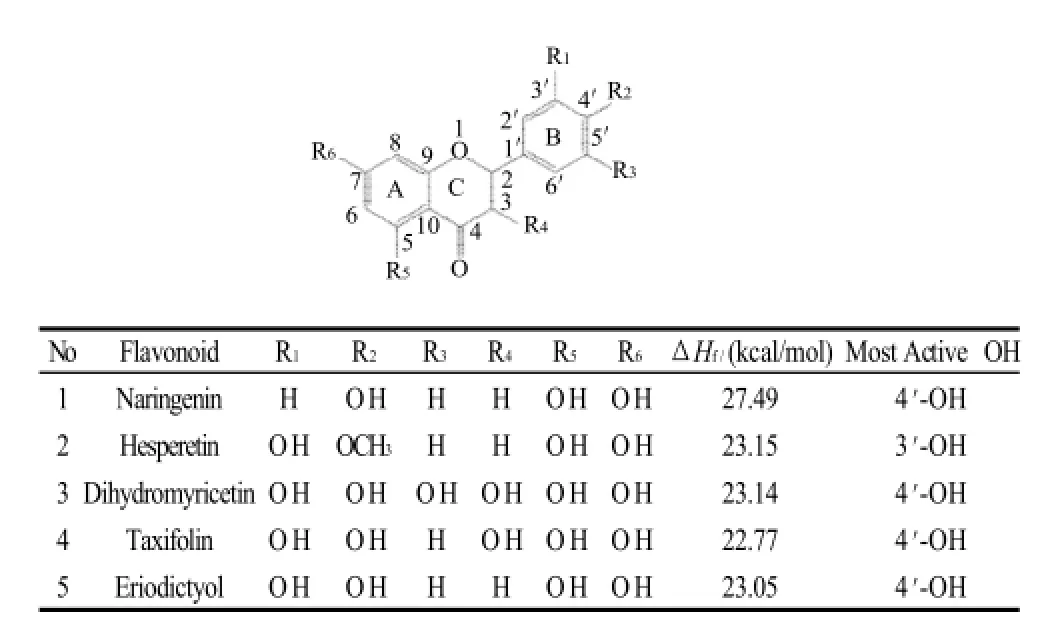
Table 2 Chemical structures and the most active OH sites of some flavanones and flavanonols
Frontier molecular orbit HOMO and LUMO were widely used in the discussion of molecular properties. According to Frontier Molecular Orbital Theory, the electron transfer is attributed to the interaction between HOMO and LUMO of reactant molecules. The frontier orbits play a decisive role in chemical reactions. The electron-donating is one of the antioxidant mechanisms of flavonoids, therefore, in this study, the semi-empirical quantum chemistry methods AM1 wasused to obtain the highest occupied orbit diagram (orbit contour value of 0.05) for 14 flavonoids, as shown in Figure 1.
It could be found through the analysis of these diagrams that∶ 1) In flavonoids with a C2-C3 double bond (such as kaempferol, luteolin, genistein and daidzein), HOMO mainly included B and C rings. 2) In flavonoids with a C2-C3 single bond (such as dihydromyricetin and eriodictyol), the HOMO mainly included B ring.
The above analysis fully showed that considering from electron-donating mechanism, B ring of flavonoids made a great contribution to antioxidant activity, and C ring contributed complementarily.
2.4 Division of antioxidant functional regions of flavonoids
Through the above discussion on the most active site for H-donating and HOMO of flavonoids, the relationships between flavonoid structures and antioxidant activities were shown below∶ 1) When flavonoids have hydroxyl groups on ring B, B ring will play a vital role in the flavonoid antioxidant activity. 2) When C2 and C3 are double bonded in the C ring, the C ring (particularly a hydroxyl group linked to C3) will further enhance the flavonoid antioxidant activity. 3) The antioxidant influence of ring A is less than that of ring B or C.
Moreover, flavonoids are usually glycosylated at ring A to form O-glucosides or C-glucosides. So, ring A plays an important role in adjusting its solubility and affinity. Therefore, the whole flavonoid structure can be divided into the three functional regions∶ 1) the crucial region of antioxidant activity (B ring); 2) The accessorial region of antioxidant activity (C ring); 3) the adjustable region of solubility and affinity (A ring). The division of the antioxidant functional regions suggests that by introducing hydrophobic or lipophilic groups to A ring, the solubility of flavonoids in either water- or oil-phase could be improved, which could make flavonoids fully play their antioxidant activity.
3 Conclusion
According to analysis of the most active sites for H-donating and HOMO of flavonoids, B ring and the hydroxyl groups on B ring made a great contribution to the antioxidant activity of flavonoids. And the molecular structure of flavonoids could be divided into three functional regions∶the crucial region of antioxidant activity (B ring), the accessorial region of antioxidant activity (C ring) and the adjustable region of solubility and affinity (A ring), which suggested that by introducing hydrophobic or hydrophilic groups to A ring, the solubility of flavonoids in either wateror oil-phase could be improved, which could make flavonoids fully play their antioxidant activity.
[1]LI Wei, ZHENG Cheng, NING Zhengxiang. The antioxidant activity of DMYL in lard system[J]. Food Science, 2005, 26(9)∶ 73-76.
[2]LI Wei, ZHENG Cheng, NING Zhengxiang, et al. Esterification of dihydromyricetin and their antioxidant activities[J]. Food Science and Technology, 2007, 32(5)∶ 198-201.
[3]JI Guodong, ZHAO Yuanhui, YUAN Xing. Quantum-chemical descriptors and their application in quantitative structure-activity and structureproperty relationship studies[J]. Journal of Northeast Normal University, 1998(4)∶ 47-53.
[4]KARELSON M, LOBANOV V S. Quantum-chemical descriptors in QSAR/QSPR studies[J]. Chemical Reviews, 1996, 96(3)∶ 1027-1043.
[5]WEBER K C, HONORIO K M, da SILVA S L, et al. Selection of quantum chemical descriptors by chemometric methods in the study of antioxidant activity of flavonoid compounds[J]. International Journal of Quantum Chemistry, 2005, 103(5)∶ 731-737.
[6]BURDA S, OLESZEK W. Antioxidant and antiradical activity of flavonoids[J]. Journal of Agricultural and Food Chemistry, 2001, 49(6)∶2774-2779.
[7]CAILLET S, YU H, LESSARD S, et al. Fenton reaction applied for screening natural antioxidant[J]. Food Chemistry, 2007, 100(2)∶ 542-552.
[8]HOLLMAN P C H, HERTOG M G L, KATAN M B. Analysis and health effects of flavonoids[J]. Food Chemistry, 1996, 57(1)∶ 43-46.
[9]YUAN Erdong, LIU Benguo, NING Zhengxiang. Preparation and antioxidant activity of camellianin A from Adinandra nitida leaves[J]. Journal of Food Processing and Preservation, 2008, 32(5)∶ 785-797.
[10]KOVATCHEVA E G, KOLEVA I I, ILIEVA M, et al. Antioxidant activity of extracts from Lavandula vera MM cell culture[J]. Food Chemistry, 2001, 72(3)∶ 1069-1077.
[11]LEFER D J, GRANDER D N. Oxidative stress and cardiac disease[J]. The American Journal of Medicine, 2000, 109(4)∶ 315-323.
[12]ZOU Yanping, LU Yanhua, WEI Dongzhi. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro[J]. Journal of Agricultural and Food Chemistry, 2004, 52(16)∶ 5032-5039.
[13]TEIXEIRA S, SIQUET C, ALVES C, et al. Structure-property studies on the antioxidant activity of flavonoids present in diet[J]. Free Radicals Biology & Medicine, 2005, 39(8)∶ 542-552.
[14]ZHANG Hongyu, CHEN Dezhan. Theoretical characterization and its application of free radical scavenging activity for phenolic antioxidants [J]. Acta Biophysica Sinica, 2000, 16(1)∶ 1-9.
[15]ZHAO Jihong, LIANG Yu, YAN Dayu. Structure-antioxidation relationship of falconoid antioxidants[J]. Journal of North China University of Technology, 2001, 13(1)∶ 36-44.
[16]ZHANG Hongyu, CHEN Dezhan. AM1 calculation of a parameter characterizing OH bond dissociation energy[J]. Chinese Journal of Organic Chemistry, 2001, 21(1)∶ 66-70.
[17]ZHANG Hongyu. Investigation on the effectiveness of HOMO to characterize antioxidant activity[J]. Journal of American Oil Chemist s Society, 1999, 76(9)∶ 1109-1110.
[18]ZHANG Hongyu. Theoretical methods used in elucidating activity differences of phenolic antioxidants[J]. Journal of American Oil Chemist s Society, 1999, 76(6)∶ 645-748.
[19]HE Jianbo, DU Jiaqi, YUAN Shengjie, et al. Electrochemical study on difference in antioxidant ability between luteolin and quercetin[J]. Food Science, 2009, 30(9)∶ 37-40.
Q623.54
A
1002-6630(2010)15-0167-04
2009-08-08
国家自然科学基金项目(20806029);河南省教育厅自然科学研究计划项目(2009A550005)
刘本国(1978—),男,副教授,博士,研究方向为食品化学与天然产物。E-mail:zzgclbg@126.com

