Ce掺杂In2O3纳米纤维的制备及其三乙胺气敏性能
王金兴 于连香 王浩铭 阮圣平 李佳静 吴凤清,*
(1吉林大学化学学院,长春 130012; 2长春汽车材料研究所,长春 130011; 3吉林大学电子科学与工程学院,长春 130021)
In2O3is widely used for various devices,such as transparent electrodes[1],gas sensors[2],photocatalysts[3],and solarcells[4],due to its excellentphysical andchemical properties.In particular,as a sensing material,In2O3with particular conformation can presentpromising gas-sensing performances[5].Recently,one-dimensional(1D)In2O3has gained increasing attention due to its large surface and high length-diameterratio[6].Forgas sensorapplication,these characteristics may provide more absorption sites for target gas molecules and make the electron transfer more effective,which results in the high sensitivity and fast reaction speed of these 1D In2O3sensing materials[7-8].
Triethylamine(TEA)isone ofthe mostimportantorganic amines,which is produced in the process of metabolism by animal organs orproteins.Itis also one of the toxic gases in the biology fieldandfoodstuff industry[9].Itwas reportedthatduring the process of fishes deterioration and afterthe death of seashells,some gaseous species,such as TEA,dimethylamine(DMA),trimethylamine(TMA),andammonia(NH3),are givenoff[9].Andthe concentrations and ratios of these gases are markedly dependent on the fish kind and freshness.Thus the sensors to these gases,especially with high sensitivity and good selectivity,will be useful inourdaily life.
In this paper,we report the TEA sensing properties of Cedoped In2O3nanofibers.Compared with the pure In2O3nanofibers,Ce-doped In2O3nanofibers exhibit improved and excellent TEA sensing properties,which indicate the potential application of Ce-doped In2O3nanofiber in fabricating high performance TEA sensors.
1 Experimental
1.1 Material preparation
All the chemicals(analytical grade reagents)were purchased from Beijing Chemicals Co.Ltd.and used as received without further purification.To prepare the pure and Ce-doped In2O3nanofibers,a certain amount of In(NO3)3·4.5H2O and Ce(NO3)3·6H2O powders were added to 8.8 g mixed solvent containing N, N-dimethylformamide(DMF)/EtOH with the mass ratio of 1:1 and stirred for2 h.Then 0.8 g polyvinylpyrrolidone(PVP)was added to the above solution with furtherstirring for6 h.The obtained solution was then loaded into a plastic syringe and connected to a high-voltage powersupply.20 kV was provided between the cathode(a flat aluminum foil)and the anode(syringe) at a distance of 25 cm.Finally,the In2O3nanofibers doped with different mass fractions of cerium(0%,2%,4%,and 6%(w, same as below)were obtained by calcining the as-spun composite fibers inairat600℃for4 h.
1.2 Characterization
The samples were characterized by X-ray diffractometer (XRD)(Shimadzu XD-3AX),scanning electron microscopy (SEM SHIMADZU SSX-550,Kyoto,Japan),transmission electron microscopy(TEM,HITACHI H-8100)with an acceleration voltage of 200 kV,and high-resolution transmission electron microscopy(HRTEMJEM-3010).
The gas sensing properties of the sensor were measured by a RQ2 intelligent test meter(Qingdao,China).The structure and fabrication of the sensor are presented in our previous report in detail[10].The sensitivity(S)is defined as S=Ra/Rg,where Rgand Radenote the sensor′s resistance in the presence and in the absence of the targetgases.
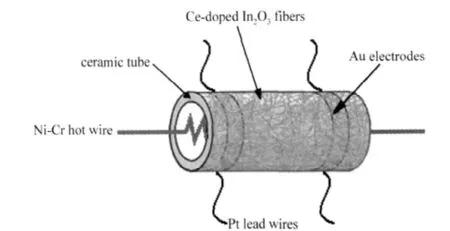
Fig.1 Schematic picture of sensor structure
1.3 Sensor fabrication
The as-synthesizedsample was mixed with deionized waterin a mass ratio of 100∶25 to form a paste.Then the paste was coated on a ceramic tube on which a pairof Au electrodes were previously printed.Pt lead wires attaching to these electrodes were used as electrical contacts.After the ceramic tube was sintered at 300℃for 2 h,a small Ni-Cr alloy wire(about 33 Ω)was placed through the tube as a heater,which provided the operating temperature.The thickness of the Ce-doped In2O3fiber film is about250 μm.The structure of the sensoris showninFig.1.
2 Results and discussion
2.1 Material characterization
Fig.2(a)shows the SEM image of the as-elecrospun PVP/ In(NO3)3·4.5H2O composite nanofibers.The nanofibers are randomly distributed to form a fibrous nonwoven,and have smooth anduniformsurface.Aftercalcinationat600℃for4 h(Fig.2(b)), the surface of these nanofibers shrinks and becomes bend and rough,indicating the formation of In2O3nanofibers.The length of the nanofibers can reach several tens of micrometers,and the average diameter is about 90 nm.A typical TEM image of 4% Ce-doped In2O3nanofiberin Fig.2(c)shows that the as-prepared nanofibers are composed of many interconnected grains of around 5 nm in size.High-resolution TEM(Fig.2(d))demonstrates the insight into the detailed atomic structure of Ce-doped In2O3nanofibers.The interplanerspacings(d)of0.29 and0.41 nm correspond to the[222]and[211]planes of cubic In2O3,respectively.
Fig.3 shows the XRD patterns of the pure and Ce-doped In2O3nanofibers.For pure In2O3,2%and 4%Ce-doped In2O3nanofibers,all the observed diffraction peaks can be indexed to cubic indium oxide(JCPDS file No.06-0416).For6%Ce-doped In2O3nanofibers,a peak corresponding to CeO2is observed at 28.5°.This suggests that some crystal CeO2may form on the surface of In2O3nanofibers.The similar phenomena have also beenfoundinotherpaper[11].
2.2 Gas sensitive properties of the samples

Fig.2 SEM images of electrospun PVP/In(NO3)3·4.5H2O composite nanofibers before(a)and after(b)calcination in air at 600℃,a typical TEM image of 4%(w)Ce-doped In2O3nanofibers(c)and the high-resolution TEM image of 4%(w)Ce-doped In2O3nanofibers(d)
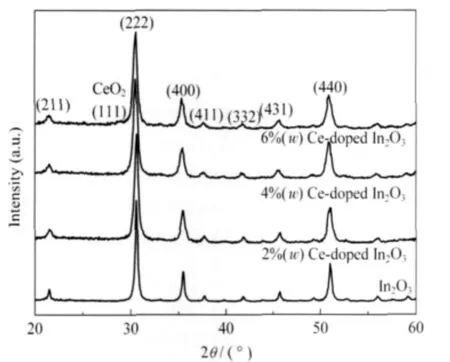
Fig.3 XRD patterns of the pure and Ce-doped In2O3nanofibers
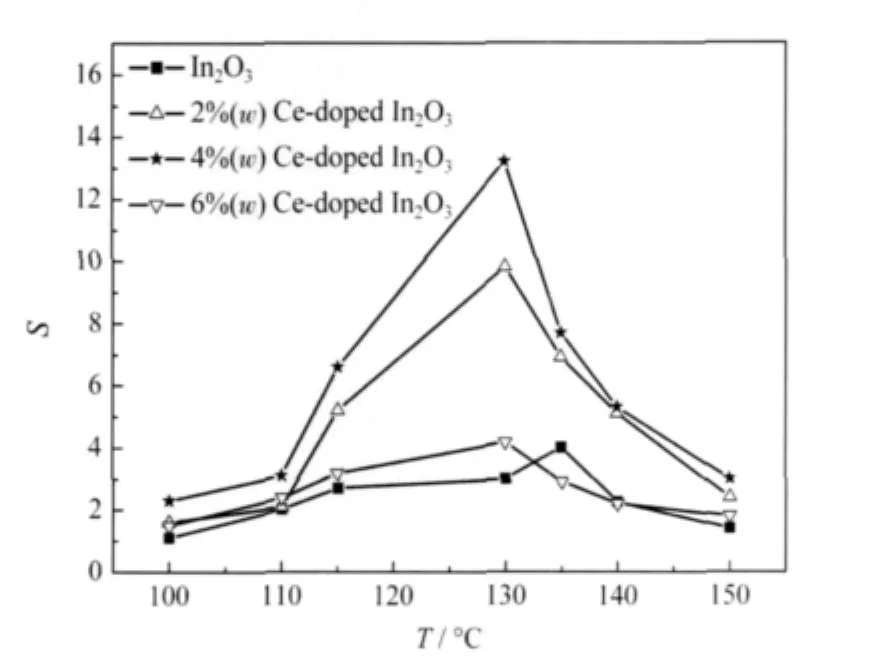
Fig.4 Sensitivities(S)of the pure and Ce-doped In2O3 nanofibers to 10 μL·L-1TEA at different operating temperatures
The sensitivities of the pure and Ce-doped In2O3nanofibers (n-type)to 10 μL·L-1TEA as a function of operating temperature are shown in Fig.4.Foreach sample,the sensitivity is found to increase with the increasing operating temperature,which attains the maximum at a certain value,and then decreases with a furtherrise of the operating temperature.Among all the samples, 4%Ce-doped In2O3nanofibers exhibit the highest sensitivity of 13.2 at 130℃,which is almost 4 times larger than that of the pure In2O3nanofibers.The 4%Ce-doped In2O3nanofibers also exhibit very high sensitivity and quick response/recovery speed to TEA as shown in Fig.5.As can be seen,we exposed the sensor to different concentrations of TEA continuously at 130℃. The sensitivities are about 2.6,5.5,13.2,28.7,39.4,56.8,61.9, and 72.2 to 3,6,10,30,50,100,200,and 300 μL·L-1TEA,respectively.And the response time and recovery time are about 5 and6 s,respectively.

Fig.5 Sensitivities vs measure time curves of 4%Ce-doped In2O3nanofibers to different concentrations(6-300 μL·L-1) of TEA at 130℃
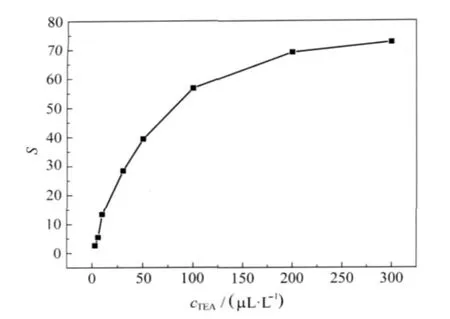
Fig.6 Sensitivities of 4%Ce-doped In2O3nanofibers to different concentrations of TEA at 130℃
The sensitivities of the 4%Ce-doped In2O3nanofibers to different concentrations of TEA at 130℃are shown in Fig.6.In the low concentrations(3-30 μL·L-1),the sensitivity increases rapidly with increasing the TEA concentrations,and then gradually slow(30-200 μL·L-1).Finally the fibers reach saturation at about300 μL·L-1.These results suggestthat4%Ce-doped In2O3nanofibers can be used to detectTEA atlow concentration level. In orderto furtherunderstand the practicability of ourfibers,the sensor was exposed to 10 μL·L-1TEA,CH3OH,H2S,TMA, C2H5OH,DMA,NH3,and H2O at 130℃,respectively.The selectivity shown in Fig.7 indicates that the sample can distinguish TEA from these interferential gases effectively.Thus 4%Cedoped In2O3nanofibers can be used to detect TEA at various atmospheres.
The sensing mechanism of In2O3based gas sensors was clarified in previous work[12].The most widely accepted model is that the resistance change of the In2O3gas sensors is primarily caused by the adsorption and desorption of the gas molecules on the surface of the In2O3film[5].When In2O3nanofibers are exposed to air,oxygen adsorbs on the exposed surface of the In2O3and is ionized O-orO2-,resulting in a decrease of the carrier concentration and electron mobility.When exposed to a reducing gas such as TEA,the reducing gas reacts with the adsorbed oxygen molecules andreleases the trapped electrons back to the conduction band,which increases the carrier concentration and carrier mobility of In2O3.Therefore the resistance change of the In2O3sensors can easily be found[7,12].The reaction between surface oxygenspecies andTEA canbe simply describedas[13]:
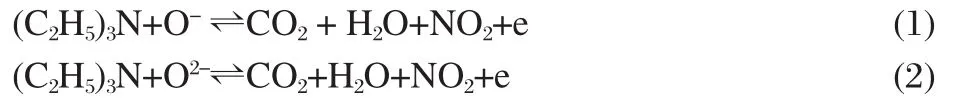
The high sensitivity and quick response/recovery speed of the sensorare attributed to the 1D structure of these nanofibers.It is widely accepted that 1D nanostructure with high surface-to-volume ratios(a highersurface area provides more sites foranalyte molecules adsorption)canfacilitate fastmass transferof the analyte molecules to and from the interaction region as well as require charge carriers to transverse the barriers introduced by molecularrecognitionalong the 1D nanostructures[14].

Fig.7 Selectivities of 4%Ce-doped In2O3nanofibers at different atmospheres
Ce is widely used to enhance the sensing performances of ZnO,TiO2,as well as In2O3inthis case[15-17].Formerpapers[18-20]reported that the existence of Ce in metal-oxide-semiconductors can improve the sensitivity prominently.In our case,Ce supports the catalytic conversionof TEA into its oxidation products. This may be due to spill-overof activated fragments to the semiconductorsurface to react with the adsorbed oxygen species and is called chemical sensitization[18-20].Anotherreason forthe sensing improvement is that Ce may play the same role as La orother rare earth elements in In2O3.They can increase the catalytic activities of In2O3effectively,which is a base of the high sensitivity[21-23].Moreover,Pure In2O3has only one kind of grains arranged uniformly,where in the case of activated films the grains are of different natures such as CeO2and In2O3.The modification causes the formationof heterogeneous intergrainboundaries ofCeO2-In2O3.Andthe increasedbarrierheights of the intergranularregions of activatedIn2O3will increase the sensorbase resistivity,and thereby improve the film sensing properties accordingly[24].The sensitivity decreases in the case of 6%Ce-doped In2O3nanofibers,which is based on that too many Ce clusters will formed on the surface of In2O3atthis situation,and the TEA molecules will be burned on the clustersurface without causing any electrical signal,leading to the decrease of sensitivity[25].
3 Conclusions
In summary,the TEA sensing properties of the pure and Cedoped In2O3nanofibers are investigated.Among all the samples, 4%Ce-doped In2O3nanofibers exhibit the highest sensitivity with quick response and recovery speed.Especially,these nanofibers can distinguish TEA from other interferential gases effectively.These results make Ce-doped In2O3nanofibers good candidates forfabricating TEA sensors.
1 Koida,T.;Fujiwara,H.;Kondo,M.Solar Energy Materials& Solar Cells,2009,93:851
2 Prim,A.;Pellicer,E.;Rossinyol,E.;Peiró,F.;Cornet,A.;Morante, J.R.Adv.Funct.Mater.,2007,17:2957
3 Chen,L.Y.;Liang,Y.;Zhang,Z.D.Eur.J.Inorg.Chem.,2009: 903
4 Sharma,R.;Mane,R.S.;Min,S.K.;Han,S.H.J.Alloy.Compd., 2009,479:840
5 Wang,X.Q.;Zhang,M.F.;Liu,J.Y.;Luo,T.;Qian,Y.T.Sens. Actuators B-Chem.,2009,137:103
6 Vomiero,A.;Bianchi,S.;Comini,E.;Faglia,G.;Ferroni,M.;Poli, N.;Sberveglieri,G.Thin Solid Films,2007,515:8356
7 Xu,P.C.;Cheng,Z.X.;Pan,Q.Y.;Xu,J.Q.;Xiang,Q.;Yu,W.J.; Chu,Y.L.Sens.Actuators B-Chem.,2008,130:802
8 Zheng,W.;Lu,X.F.;Wang,W.;Dong,B.;Zhang,H.N.;Wang,Z. J.;Xu,X.R.;Wang,C.J.Am.Ceram.Soc.,2010,93:15
9 Natale,C.D.;Brunink,J.A.J.;Bungaro,F.;Davide,F.;d′Amico, A.;Paolesse,R.;Boschi,T.;Faccio,M.;Ferri,G.Meas.Sci. Technol.,1996,7:1103
10 Wang,J.X.;Zou,B.;Ruan,S.P.;Zhao,J.;Wu,F.Q.Mater.Chem. Phys.,2009,117:489
11 Ge,C.Q.;Xie,C.S.;Cai,S.Z.Mater.Sci.Eng.B,2007,137:53
12 Barsan,N.;Koziej,D.;Weimar,U.Sens.Actuators B-Chem., 2007,121:18
13 Takao,Y.;Nakanishi,M.;Kawaguchi,T.;Shimizu,Y.;Egashira, M.Sens.Actuators B-Chem.,1995,24-25:375
14 Kolmakov,A.;Moskovits,M.Annu.Rev.Mater.Res.,2004,34: 151
15 Al-Kuhaili,M.F.;Durrani,S.M.A.;Bakhtiari,I.A.Appl.Surf. Sci.,2007,255:3033
16 Trinchi,A.;Li,Y.X.;Wlodarski,W.;Kaciulis,S.;Pandolfi,L.; Viticoli,S.;Comini,E.;Sberveglieri,G.Sens.Actuators B-Chem., 2003,95:145
17 Yamazoe,N.;Sakai,G.;Shimanoe,K.Catal.Surv.Asia,2003,7: 63
18 Fang,G.J.;Liu,Z.L.;Liu,C.Q.;Yao,K.L.Sens.Actuators BChem.,2000,66:46
19 Siemons,M.;Simon,U.Sens.Actuators B-Chem.,2006,120:110 20 Ruiz,A.M.;Cornet,A.;Morante,J.R.Sens.Actuators B-Chem., 2004,100:256
21 Kapse,V.D.;Ghosh,S.A.;Chaudhari,G.N.;Raghuwanshi,F.C.; Gulwade,D.D.Vacuum,2009,83:346
22 Xu,L.;Dong,B.;Wang,Y.;Bai,X.;Chen,J.;Liu,Q.;Song,H. J.Phys.Chem.C,2010,114:9089
23 Niu,X.;Zhong,H.;Wang,X.;Jiang,K.Sens.Actuators B-Chem., 2006,115:434
24 Patil,D.R.;Patil,L.A.;Patil,P.P.Sens.Actuators B-Chem., 2007,126:368
25 Belmonte,J.C.;Manzano,J.;Arbiol,J.;Cirera,A.;Puigcorbé,J.; Vilà,A.;Sabaté,N.;Gràcia,I.;Cané,C.;Morante,J.R.Sens. Actuators B-Chem.,2006,114:881

