CHARACTERIZATION OF IRON(Ⅲ) HYDROLYSIS PARTICLES IN PULP BLEACHING PROCESS
ZHANG Xue-jin, LI You-ming, LIU Ming-you
(State Key Laboratory of Pulp and Paper Engineering,National Engineering Research Center of Papermaking & Pollution Control,South China University of Technology,Guangzhou 510640,China)
0 Introduction
The presence of iron-containing colloids and particles in water is widely understood in many industry processes. In process water of pulping systems, iron(Ⅲ) is derived mainly from the wood used as raw material, refining, corrosion of process equipment, partly from recycle water, fresh water, and pulping and bleaching chemicals. Iron(Ⅲ) may be aging under pulping environment[1], and present as finely dispersed colloidal particles of hydrated oxides or hydroxide[2]. In fact, there are hardly any dissociative transition metal particles smaller than 0.45 μm[3], which implys hardly any free “fresh” iron(3) ions were left. In bleaching process, iron(Ⅲ) are present in different states, such as “nonequilibrium” suspended particles, oligomer dynamic equilibrium with carboxylic groups of fibre by means of ion exchange, and oligomer chelated with residual lignin by forming coordinate bond with phenolic groups[4,5]. Hydrolysis of iron(Ⅲ) plays an important role in the cellulose fiber adsorption and hydrogen peroxide catalytic decomposition, e.g. primary nucleation particles from hydrolysis of iron(Ⅲ) is adsorbed on fibers by ion exchange, while larger suspended particle from hydrolysis of iron(Ⅲ) is just trapped within the fiber lumens. In addition, amorphous hydrolysis products of iron(Ⅲ) are more activate to cantalytic decomposition of hydrogen peroxide. As a results, understanding the properties of iron(Ⅲ) colloids formed in pulping process may lead to better enrichment control and removement, as well as a reduction of invalid decomposition of hydrogen peroxide.
In pulping process, iron(Ⅲ) gives rise to two hazards. First, iron(Ⅲ) can adsorb or aggradate on fiber, and lead to brightness reversion. Second, iron(Ⅲ) accumulated in bleaching process can induce invalid decomposition of oxygen-based bleach, whether they are free or adsorbed on fiber. In fact,“fresh” iron(Ⅲ) and “aged” iron(Ⅲ) induce different influence on catalytic decomposition of hydrogen peroxide and adsorption on fiber in pulping process. In order to understand influence of iron(Ⅲ), certain physical properties of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) have modern significance, such as particle size distribution of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) particles with various pH, and transformation between hydroxide and hydrated oxides of iron(Ⅲ). The basic information for process flow is lacking in the literature, most likely due to the technical challenges in the measurement of these parameters for submicrometer colloids.
The formation of iron colloids is a complex issue in process flow, involving hydrolysis of the elemental iron through a series of steps to form primary nucleation particles (PNPs) and oxidation of PNPs with dissolved oxygen. The PNPs may dissolve, grow into some unstable colloids quickly, flocculate, and form some larger particles, “aged” iron(Ⅲ), with parts of-OH substituted by-O-bonds[6]. PNPs originate from “fresh” iron(Ⅲ) are typically in the submicrometer size range and require sophisticated analytical techniques for characterization, while oxide and flocculate, “aged” iron(Ⅲ), is much larger, visible to the unaided eye. The PNPs of “fresh” iron(Ⅲ) colloids can studied well with Photon Cross Correlation Spectroscopy(PCCS) and Photon Correlation Spectroscopy.(PCS) technique[7].
In order to control iron accumulation in process flow and reduce invalid decomposition of hydrogen peroxide, this work is to characterize iron(Ⅲ) hydrolysis particles , the particle size distribution of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) versus pH by Photon Cross Correlation Spectroscopy(PCCS) or Laser Light Diffraction technique(LLD), and the structure of the particles formed from “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) by Infra-red spectrum(IR), Scanning Electron Microscope-Energy Dispersive spectrum(SEM-EDS), and X-ray Diffraction(XRD).
1 Experimental
1.1 Chemical and materials
The chemicals used were of analytical reagent without purified. Ammonium ferric sulfate were supplied by Guangzhou Dongjiang Chemical Corporation Company (China). The water used was Milli-Q deionized water (18.2 MΩ·cm), and standard solutions of NaOH were prepared used for adjusting pH.
Stock standard solution of “fresh” iron(Ⅲ) solution was prepared by dissolving the appropriate Ammonium Ferric Sulfate in Milli-Q deionized water. “Aged” iron(Ⅲ) solution was prepared by dissolving the appropriate Ammonium Ferric Sulfate in Milli-Q deionized water at pH 6.5 and boiling for 3 h[8].
1.2 Methods
The amount of “fresh” solution was determined by Flame Atomic Absorption Spectroscopy(AAS) with WFX-1C Absorption Spectrograph. The particle size distribution with 20 ppm “fresh” iron was measured by Photon Cross Correlation Spectroscopy(PCCS) with Malvern ZEN3600 laser particle nano-size & potential analyzer, and the particle size distribution of “aged” iron(Ⅲ) solution was measured by Laser Light Diffraction technique(LLD) with Mastersizer 2000.
The samples of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) was concentrated by centrifugation and freeze-dried. And infrared spectra of the freeze-dried samples were recorded with IR with a SP2000(Pye Unicam Ltd) infrared spectrophotometer in the range 400 to 4 000 cm-1at room temperature. The samples of iron(Ⅲ) were also measured by SEM-EDS and XRD.
2 Results and Discussion
2.1 Determination of particle size and density and volume distribution of “fresh” iron(Ⅲ)
Tab.1Hydrodynamic diameter of“fresh”iron(Ⅲ)
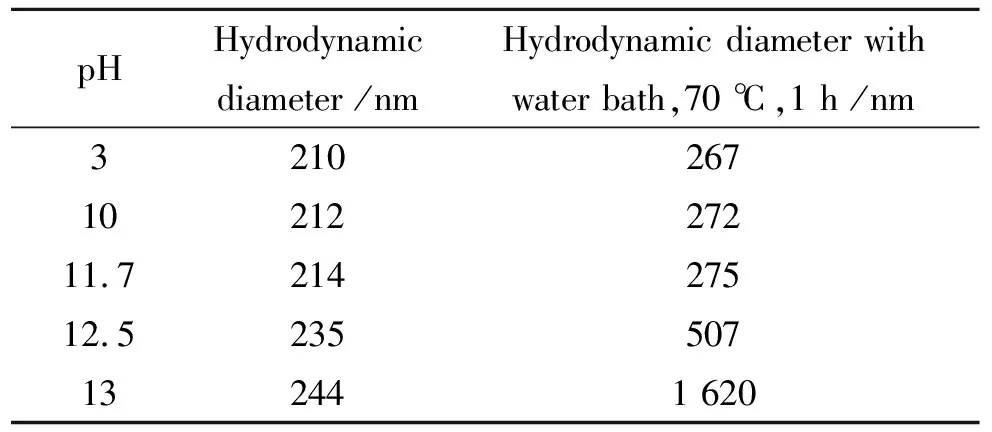
pHHydrodynamic diameter /nmHydrodynamic diameter with water bath,70 ℃,1 h /nm32102671021227211.721427512.5235507132441 620
To verify system performance, the particle size of 20 ppm “fresh” iron solution was measured with various pH. The reseults were contained in Tab.1. The particle size of “fresh” iron(Ⅲ) were similar under pH 11.7, about 210 nm to 220 nm. Above pH 12, a small increase in diameter was measured increasing with pH. The particle size of “fresh” iron(Ⅲ) with water bath at 70 ℃ for 1 hour increased by different degrees. Strong flocculation phenomena was generated, and the particle size incresed to 1 620 nm at pH 13.
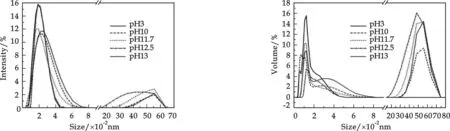
Fig.1Density distribution of “fresh” iron(Ⅲ) colloids
Fig.2Volume distribution of “fresh” iron(Ⅲ) colloids
Fig.1 presented the Photon Cross Correlation Spectroscopy density distribution for “fresh” iron. No high intensity signals were observed beyond 800 nm at pH 3. With alkali solution added, some high intensity signals were observed bwtween 2 000 nm to 7 000 nm above pH 10. Volume distribution of “fresh” iron(Ⅲ) colloids in correlation with pH were performed as described in Fig.2. As similar with density distribution, volume distribution was just between 0 to 800 nm at pH 3. A bimodal distribution was also shown, one in 0 to 800 nm and the others between 2 000 nm and 8 000 nm when the pH value was above 10. The results could be explained by coagulation process of iron(Ⅲ) PNPs with alkali added. An individual iron-hydroxide colloid may be described by
xFe3++yOH-
Colloids may be formed that have different totalxandy, and they may be composed of one of several ferric hydroxide species known in basic solutions[9]. A part of primary nucleation particles grow into some unstable colloids quickly, flocculate, and form some larger particles. As a result, the hydrodynamic diameter increased with pH increasing and a bimodal distribution was formed.
2.2 Distribution of particle size of “aged” iron(Ⅲ)
The particle size of colloidal particles in “aged” iron(Ⅲ) solutions was inert to pH, with volume weighted mean D~10 μm in Fig.3. Laser Light Diffraction technique(LLD) volume distribution of “aged” iron(Ⅲ) was uninterrupted with various pH. It indicated that “aged” iron(Ⅲ) had already hydrolyzed during aging process, and already formed larger iron(Ⅲ) colloidal particles, hydrated oxides/hydroxides suspension[6].
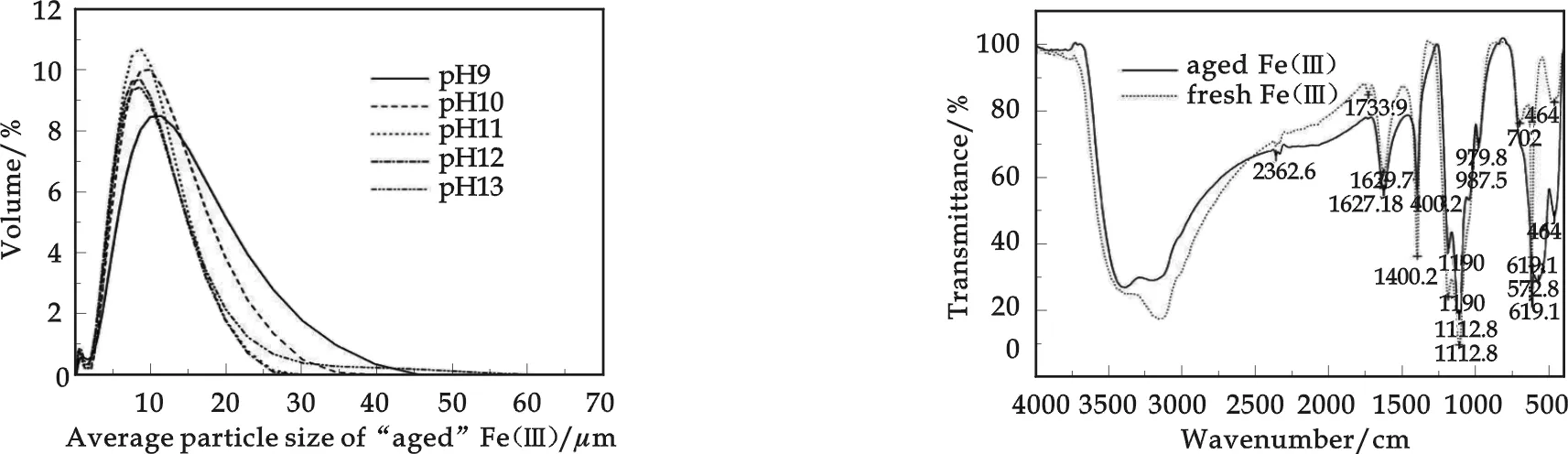
Fig.3Hydrodynamic diameter distribution of “aged” iron(Ⅲ) colloids
Fig.4FTIR spectra of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ)
2.3 FTIR of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ)
The FTIR spectra of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) displayed a number of absorption peaks,indicating a complex nature of the examined iron(Ⅲ) precipitates (Fig.4). The strong peak at 1 629 cm-1,1 401 cm-1was assigned to hydroxyl groups (-OH), or adsorbed water. The peaks at 464 cm-1,572 cm-1were assigned to Fe-O stretching vibrations, which impliedα-Fe2O3was formed[10,11]. The peak at 619 cm-1,702 cm-1, 1 114 cm-1represented Fe-OH bending vibrations. It is clearly shown in Fig.4 that a strong peak at 702 cm-1was just presented for “fresh” iron(Ⅲ) assigned to Fe-OH bending vibrations, while a strong peak at 572 cm-1was just presented Fe-O stretching vibrations assigned to Fe-O stretching vibrations. Therefore, one can conclude that parts of Fe-OH were substituted by -O- bonds for “aged” iron(Ⅲ) during aging process.
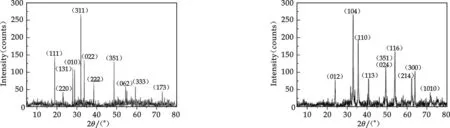
Fig.5 XRD of “fresh” iron(Ⅲ)
2.4 XRD of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ)
The results of XRD phase analysis of iron(Ⅲ) precipitates were given in Fig.5 and Fig.6. Both of the samples showed rather sharp or little broadened XRD lines. The phases present in the samples were identified according to the powder diffraction data contained in the PDF cards. XRD powder patterns of “fresh” iron(Ⅲ) was ascribed to Thenardite, PDF #37-1465. Hardly any strong line of iron(Ⅲ)-containing compounds were present in Fig.5. It indicated the “fresh” iron(Ⅲ) in hydrolytic process didn′t crystallize, and only sodium sulfate in solution crystallized. Iron(Ⅲ) was in the form of amorphous structure in the “fresh” iron(Ⅲ) precipitates. XRD powder patterns of “aged” iron(Ⅲ) was Ascribed to Hematite, PDF# 33-0664, shown in Fig.6. The result could be explained hydrolysis, aggregate, oxidize and crystallize of iron(Ⅲ) in aging process.
2.5 SEM-EDS of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ)
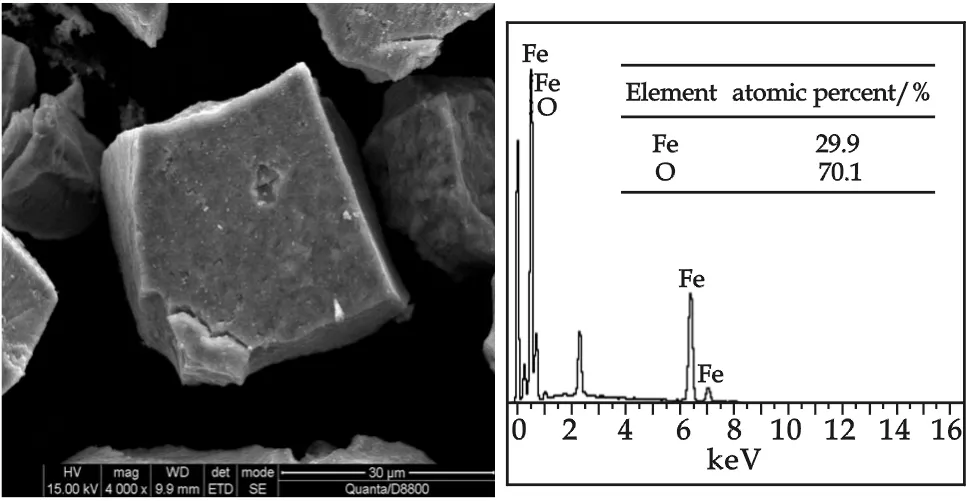
Fig.7 SEM images at ×4 000 magnification and EDX spectra of “fresh” iron(Ⅲ)
The SEM images and EDS spectra for iron(Ⅲ) precipitates surface were shown in Fig.7 and Fig.8. Iron(Ⅲ) precipitates were flocculated in the process of concentratation and centrifugation. Precipitates of “fresh” iron(Ⅲ) were in form of rectangle, and precipitates of “aged” iron(Ⅲ) showed an irregular surface. Although the size of the iron(Ⅲ) precipitates, “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) were similar, the EDS spectra gave an interesting finding. EDS is a very useful tool for identifying elements on the solid surface. As we know, iron(Ⅲ) precipitates mainly contained Fe, O, and H element.Fe/O atomic ratio of “fresh” iron(Ⅲ) precipitate was 29.9∶70.1, and macroscopic molecular formula can be taken as Fe29.1O17.2(OH)52.9. Fe/O atomic ratio of “aged” iron(Ⅲ) precipitate was 34.7∶65.3, and macroscopic molecular formula can be taken as Fe34.7O38.8(OH)26.5. As indicated by XRD spectra and IR of “aged” iron(Ⅲ), crystal Hematite was formed in “aged” iron(Ⅲ), as well as “fresh” iron(Ⅲ) hydroxides in amorphous form. Based on these findings, one could conclude that aging process of “fresh” iron(Ⅲ) was an oxidation and a phase inversion process.
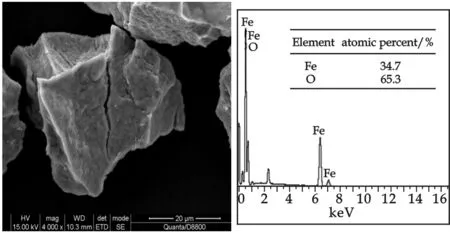
Fig.8 SEM images at ×4 000 magnification and EDX spectra of “aged” iron(Ⅲ)
3 Conclution
This study revealed the particle size and distribution with pH, phase composition, morphology, and structure of the particles precipitated of “fresh” iron(Ⅲ) and “aged” iron(Ⅲ) to simulate morphological diversity of iron(Ⅲ) in pulp bleaching process. By spectroscopic techniques involving Photon Cross Correlation Spectroscopy(PCCS) and Laser Light Diffraction technique(LLD), the particle size and density and volume distribution of “fresh” iron(Ⅲ), water bath at 70 ℃ for 1 h,and “fresh” iron(Ⅲ) were characterized. Density and volume distribution of “fresh” iron(Ⅲ) presented a bimodal distribution, while “aged” iron(Ⅲ) didn′t, which indicated that “aged” iron(Ⅲ) had already hydrolyzed during aging process, and already formed hydrated oxides/hydroxides suspension. The results of IR, XRD and SEM-EDS revealed that “Fresh” iron(Ⅲ) in hydrolytic process didn′t crystallize, and “aged” iron(Ⅲ) in hydrolytic process crystallized.
[1] J. L. Colodette, S Rothenberg, C. W. Dence. Factor affecting hydrogen peroxide stability in the brightening of mechanical and chemimechanical pulps. partⅠ: hydrogen peroxide stability in the absence of sodium silicate[J]. J. Pulp Paper Sci., 1988,14 (6): 126-132.
[2] Josef Gierer, Kerstin Jansbo, Torbj·Reitberger. Formation of hydroxyl radicals from hydrogen peroxide and their effection on bleaching of mechanical pulps[J]. J. Wood Chem.Tech., 1993,13(4): 561-581.
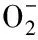
[4] P. S. Bryant. Metals Management in the Fiberline[C]. Submitted to 1996 Tappi Minimum Effluent Mill Symposium, Atlanta, Georgia: Tappi Press, 1996, 22-24.
[5] Li Youming, Chen Zhonghao,Liu Mingyou. Accumulation states of non-process metal elements in non(less)-pollution bleaching process[J]. China Pulp &Paper, 2004,23(4): 1-4.
[6] M. Taillefert, A.B. Bono, G.W. Luther. Reactivity of freshly formed Fe(Ⅲ) in synthetic solutions and (pore)waters: voltammetric evidence of an aging process[J]. Environmental Science & Technology.2000,34(11): 2 169-2 177.
[7] Von Gunten U., Schneider, W. J.. Primary products of the oxygenation of the iron(Ⅱ) at an oxic-anoxic boundary: nucleation, aggregation and aging[J]. Journal of Colloid and Interface Science, 1991,145(1): 127-139.
[8] J. L Colodette, S Rothenberg, C. W. Dence. Factor affecting hydrogen peroxide stability in the brightening of mechanical and chemimechanical pulps. partⅡ: hydrogen peroxide stability in the Presence of sodium silicate[J]. J. Pulp Paper Sci., 1989,15(1): 3-10.
[9] F. A. Cotton, G. Wilkinson. Advanced Inorganic Chemistry (5th ed.) [M]. New York :John Wiley and Sons, 1988.227-237.
[10] M. Gotic, S. Popovic, N. Ljubecic,etal. Structural properties of precipitates formed by hydrolysis of Fe3+ions in aqueous solutions containing and Cl-ions[J]. Journal of Materials Science. 1994,29:2 474-2 480.
[11] Mira Ristic, Svetozar Musiĉ, Matjaž Godec. Properties of γ-FeOOH, α-FeOOH and α-Fe2O3particles precipitated by hydrolysis of Fe3+ions in perchlorate containing aqueous solutions[J]. Journal of Alloys and Compounds, 2006,417(1-2): 29-299.

