VISIBLE LIGHT RESPONSE TiO2 PILLARED MMT POROUS PHOTOCATALYST
YU Zhan-jiang
(School of Chemistry and Chemical Engineering, Xianyang Normal University, Xianyang 712000,China)
0 Introduction
The issues of indoor air quality have received people′s immense attention on a global scale in today′s society. Some studies showed that the level of pollutants, especially, volatile organic compounds (VOCs) in indoor environment was actually higher than in outdoor environment. In addition, people generally spend more than 80% of their time in indoors, which results in a higher risk from inhalation of pollutants than outdoors. Hence, indoor air pollution treatment has already attracted widespread attention.
Photocatalytic degradation (TDP) of VOCs over TiO2semiconductor catalyst has showed to be a promising and effective method for pollution control. And TiO2has been applied by many researchers to remove a variety of pollutants photochemically[1,2].Unlike traditional pollution control method such as active carbon adsorption that merely transfers pollutant from gaseous phase to solid phase, TDP actually oxidizes pollutants into CO2and H2O. However, the shortcomings of TiO2such as big band gap (3.2eV), lower photocatalytic oxidation efficiency and small specific surface area etc. limit its application. Thus the development of TiO2photocatalyst with high activity and photocatalytic degradation efficiency under ultraviolet light (UV) and visible light (Vis) irradiation is needed. For this purpose, doping modification of TiO2with nonmetallic elements[3-5]and photocatalyst′s preparation of TiO2pillared layered silicates[6,7]have been investigated.
In 1955, Barrer[8]introduced the four-alkyl amine into the gallery of MMT layers, becoming the first man on pillared clay materials. Such pillared materials possesses strong selective absorption, higher catalytic activity and larger pore diameter, is suitable for heavy oil catalytic cracking. In 1977, Brindley[9]prepared alumina dioxide pillared MMT porous materials using polyhydroxy aluminium cations as an intercalation agent. In 1986, Sterte[10]prepared titanium pillared MMT porous materials via TiCl4hydrolyzation method. These catalysts pillared with metal or metallic oxide were also very effective on oil cracking, and overcame the weakness of poor thermal stability the catalyst pillared with organic cations possessed, thus the study and application on pillared porous materials had entered a new stage.
Although many researchers have done a lot of work on photocatalyst doping modification and pillared porous materials separately, rarely researchers combined with these two research works together to prepare a porous photocatalyst with extended specific surface and high visible light response photocatalytic activity. In this paper, taking tetrabutyl titanate as initial titanium source, methionine as a dopant, we prepared TiO2pillared MMT photocatalyst coupling-doped with S and N elements by acid-catalyzed sol and two-step methods.
1 Experimental
1.1 Materials and instruments
Tetrabutyl titanate (CR grade) and glacial acetic acid (AR grade) ware all obtained from Tianjin Chemical Reagent Co. Ltd., China and the doping reagent of methionine (BR) was from Fushan Biochemical Reagent Plant, China. The Na-based MMT with cation exchange capacity (CEC) 114 mmol/100 g was from Zhejiang Linan Montmorillonite Co., Ltd., China. XPS of photocatalyst was obtained on a photoelectron spectrometer (PE Co., Ltd., USA, PHI-5400). The diffuse reflectance spectrum (DRS) of a photocatalyst was measured using an UV-Vis spectrophotometer (Varian Co., Ltd., USA,Varian Carry 500). The specific surface area was determined from the amount of nitrogen adsorption at 77.3 K using BET method (Quantachrome Co., Ltd., Japan, NOVA 4200E). The gaseous formaldehyde concentration was recorded on a formaldemeter (PPM Technology Ltd., England, PPM-400). X-ray pattern (XRD) was obtained on a wide-angle goniometer (D/Max-3c, Rikagu, Japan) with a Cu Ka (λ=0.154 06 nm) source.
1.2 Preparation of TiO2 pillared MMT porous materials
7 mL tetrabutyl titanate was added to 20 mL mixed solution of 10 mL glacial acetic acid and 10 mL distilled water in the mechanical force stirring conditions at room temperature followed by addition of 40 mL distilled water drop by drop. The TiO2sol formed after 3 h continuously stir was added to 50 mL MMT (adsorbent) aqueous suspension (2wt%) in a flask. The mixed system of TiO2sol and MMT aqueous suspension was nonstop stirred for 6 h at room temperature followed by aging treatment for 3 h at room temperature. The grey white slurry formed was centrifuged and washed with distilled water for several times to remove the excess TiO2sol. The wet separation solid was dried under reduced pressure at temperature 80 ℃ for 24 h and then was ground into fine powder. Finally, the TiO2pillared MMT porous materials (TiO2-MMT) were obtained by sintering the fine powder at 400 ℃ for 2 h in open air.
1.3 Preparation of TiO2 pillared MMT porous photocatalyst
Equilibrium sorption experiment was carried out by taking TiO2-MMT powder 1.0 g (adsorbent) into 95 mL methionine aqueous solution(1.0 g/100 mL) and rough stirred by an agitator at temperature 30 ℃ for 6.0 h followed by centrifugal separation and distilled-water-washing to remove excess methionine. The TiO2pillared MMT photocatalyst coupling-doped with S and N(TiO2-MMT-P) was prepared via drying the separation solid under reduced pressure at 80 °C for 24 h followed by sintering treatment at 600 °C for 3.0 h in open air.
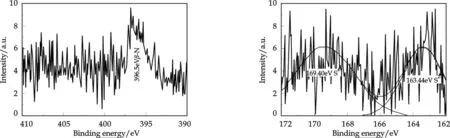
Fig.1 XPS energy spectrum of N1s Fig.2 XPS energy spectrum of S2p
1.4 Evaluation of photocatalytic activity
Visible light photocatalytic activity of the TiO2-MMT-P photocatalyst was evaluated by measuring the concentration of gaseous formaldehyde in a photo reactor (0.6 m×0.6 m×0.6 m) using the formaldemeter. A piece of 0.1 m×0.12 m glass plate coated with the TiO2-MMT-P was inserted into bracket in the photo reactor.A 100 W tungsten lamp (λ>450 nm) was used as a visible light source.
2 Results and Discussion
2.1 XPS analysis
The XPS energy spectra of TiO2-MMT-P photocatalyst are presented in Fig.1 and Fig.2. The N1s peak of 396.5 eV corresponds toβ-N atom[3]that substitutes the O atom in TiO2. The S2p peaks of 169.40 and 163.44 eV attribute to S4+and S2-respectively,which the S4+replaces the Ti4+while the S2-might substitutes for the O atom in TiO2[11]. So we conclude that the photocatalyst possesses the unique characteristic of S cation and N anion coupling-doping.
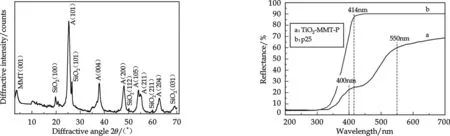
Fig.3 XRD pattern of prepared photocatalyst Fig.4 Diffuse reflectance spectra of TiO2-MMT-P
2.2 TiO2 crystal type
The XRD pattern of prepared photocatalyst is showed in Fig.3. All peaks attribute to anatase crystal of TiO2except for several weak crystal plane peaks of SiO2. In another word, there appear no other peaks like rutile crystal, which demonstrate that the S cation and N anion coupling-doping to TiO2restrain or retard the transform of TiO2from anatase to rutile crystal[12].
2.3 Optical properties
The DRS of the TiO2-MMT-P and p25 photocatalyst are compared in Fig.4. The TiO2-MMT-P photocatalyst obviously shifts red and exhibits strong absorption in the visible light region while the p25 merely features absorption in UV light region, which indicates that the S and/or N are introduced into TiO2crystal lattices, producing visible light adsorption.
The absorption edge of the photocatalyst could be calculated by the following equation[13]:
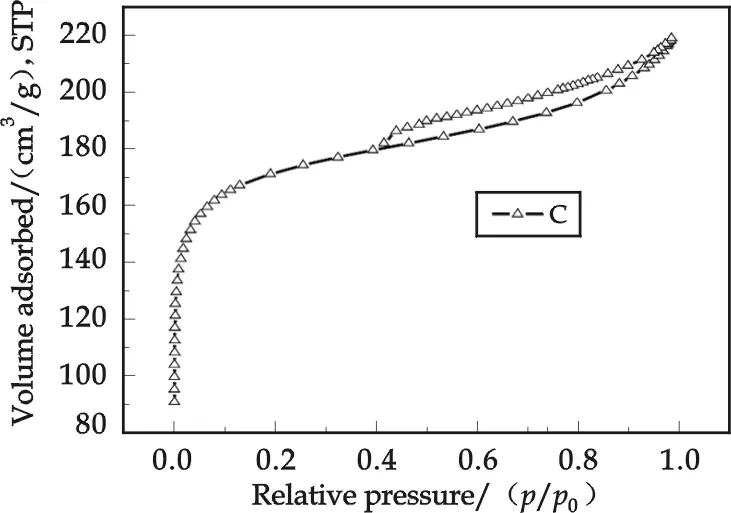
Fig.5 Nitrogen adsorption/desorption
WhereEgis the band gap (eV) of the photocatalyst andλ(nm) is the wavelength of the onset of the spectrum. The p25 photocatalyst shows an absorption edge at 414 nm corresponding to the band gap of 2.99 eV (Fig. 4(b)). Yet, the TiO2-MMT-P photocatalyst shows two absorption edges at 400 nm (3.10 eV) and 550 nm (2.25 eV). The first edge is the same as for the original titania, while the second edge seems to indicate the formation of a new S2p-based band which is located above the O2p-based valence band[14,15]. The DRS reveals the band gap could be greatly narrowed by co-doping of TiO2with N and S elements.
2.4 Nitrogen adsorption/desorption isotherm
The nitrogen adsorption/desorption experiment is usually used to research in a solid porosity.The large adsorption generally reflects a large porosity. The adsorption/desorption isotherm of TiO2-MMT-P photocatalyst (sintering temperature 600 °C) are presented in Fig.5.Using BET and Barrett-Joyner-Halenda (BJH) methods, the specific surface area, pore structure information and pore distribution etc. can be derived from the nitrogen adsorption/desorption isotherm, as shown in Tab.1.It can be seen from the results in the Tab.1, that the photocatalyst exhibits extended specific area, micropore area and pore volume, which is favored for surface adsorption, and is of significantly important for photocatalytic reaction because adsorption of any contamination on catalyst surface is precondition of a photocatalytic reaction.

Tab.1 The BET surface area, pore volume and mean diameter of the prepared photocatalyst
2.5 Photocatalytic activity
To evaluate the photocatalytic activity of prepared photocatalyst,the photocatalytic degradation test was performed by measuring the concentration of gaseous formaldehyde under visible light irradiation, in which the initial concentration of gaseous formaldehyde was set at 45 mg/m3, system temperature 25 °C, relative humidity 30% and dosage of photocatalyst 200 mg.Fig.6 illustrates the result of degradation for gaseous formaldehyde. The concentration of gaseous formaldehyde decreased with visible light irradiation time prolonging, and decreased to 6.77 mg/m3from the initial concentration of 45 mg/m3(degrading rate nearly 85%) after visible light irradiation for 300 min. So much higher degrading rate attributed to the synergistic effects of coupling-doping of S and N elements to TiO2, extensive specific surface area of MMT and quantum sized efficacy between layers of MMT.
3 Conclusions
The visible light response TiO2pillared MMT photocatalyst coupling-doped with S and N was prepared by two-step method. N2-and S4+substitute the O2-and Ti4+in TiO2respectively, making the photocatalyst with visible light response activity. The much higher visible light photocatalytic activity of the prepared photocatalyst for degrading the gaseous formaldehyde is due to the synergistic effects of coupling-doping of S and N elements, extensive specific surface area, finer micropore structure and quantum sized efficacy of the photocatalyst.
[1]Fujishima,A.,Cai,R.X.,Otsuki,J.,etal.Biochemical application of photoelectrochemistry: photokilling of malignant cells with TiO2powder[J]. Electrochim Acta,1993,38:153-157.
[2] Sopyan,I.,Watanabe,M.,Murasawa,S.,etal.An efficient TiO2thin-film photocatalyst:photocatalytic properties in gas-phase acetaldehyde degradation[J].J.Photochem. Photobiol., A: Chem.,1996,98:79-86.
[3] Asahi, R., Morikawa, T., Ohwaki, T.Visible-light photocatalysis in nitrogen-doped titanium oxides[J]. Science,2001,293:269-271.
[4] Umebayashi, T., Yamaki, T., Asai, K.Band gap narrowing of titanium dioxide by sulfur doping[J].Appl. Phys. Lett.,2002,81:454-456.
[5]Khan, S.U.M., Al-Shahry, M.,etal. Efficient photochemical water splitting by a chemically modifiedn-TiO2[J].Science,2002,297:2 243-2 245.
[6] Zhu, H.Y., Li, J.Y., Zhao, J.C..Photocatalysts prepared from layered clays and titanium hydrate for degradation of organic pollutants in water[J].Applied Clay Science, 2005,28:79-88.
[7] Ooka,C., Yoshida,H., Suzuki,K..Effect of surface hydrophobicity of TiO2-pillared clay on adsorption and photocatalysis of gaseous molecules in air[J]. Applied Catalysis A: General, 2004,260:47-53.
[8]Barrer, R.M., Macleod, D.M..Activation of montmorillonite by ion exchange and sorption complexes of tetra-alkyl ammonium montmorillonites[J].Trans. Faraday Soc., 1955,51:1 290-1 293.
[9] Brindley, G.W., Semples, R.E..Acidity and catalytic properties of aluminum pillared montomorillonite[J].Clay and Clay Miner,1977,12:229-237.
[10] Sterte, J.Synthesis and properties of titanium oxide cross-linked montmorillonite[J].Clay and Clay Minerals,1986,34(6):644~658.
[11] Ohno, T., Akiyoshi, M., Umebayashi, T.,etal.Preparation of S-doped TiO2photocatalysts and their photocatalytic activities under visible light[J].Applied Catalysis A: General,2004,265:115-121.
[12]Wang, Z.P., Xu, J.. Visible light induced photodegradation of organic pollutants on nitrogen and fluoride co-doped TiO2photocatalyst[J].Journal of Environmental Sciences,2005,17(1):76-80.
[13]Yin, S.. Synthesis of visible-light reactive TiO2-xNyphotocatalyst by mechanochemical doping[J].Solid State Sciences, 2005,7:1 479-1 485.
[14]Asahi, R., Morikawa, T., Ohwaki, T.,etal. Visible-Light photocatalysis in nitrogen-doped titanium oxides[J].Science, 2001,293(5 528) :269-271.
[15]Morikawa,T., Asahi, R., Ohwaki, T.,etal. Band-Gap narrowing of titanium dioxide by nitrogen doping[J].Jpn. J.Appl. Phys., Part 2,2001,40:561-562.

