Proton-exchange Sulfonated Poly(ether ether ketone)/SulfonatedPhenolphthalein Poly(ether sulfone) Blend Membranes in DMFCs*
GAO Qijun (高启君), WANG Yuxin (王宇新), XU Li (许莉), WANG Zhitao (王志涛) and WEI Guoqiang (卫国强)
Proton-exchange Sulfonated Poly(ether ether ketone)/SulfonatedPhenolphthalein Poly(ether sulfone) Blend Membranes in DMFCs*
GAO Qijun (高启君)1,2, WANG Yuxin (王宇新)1,2, XU Li (许莉)1,**, WANG Zhitao (王志涛)1,2and WEI Guoqiang (卫国强)1,2
1School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China2State Key Laboratory of Chemical Engineering, Tianjin 300072, China
sulfonated poly(ether ether ketone), sulfonated phenolphthalein poly(ether sulfone), blend membrane, direct methanol fuel cell
1 INTRODUCTION
Direct methanol fuel cells (DMFCs) have attracted considerable attention as electrical power sources, especially for portable or mobile power, owing to their high efficiency, low emissions, and easy fuel carriage [1]. Development and research on the cells have been an active area since the 1990s [2, 3]. DMFC technology has made significant progress, but two obstacles need to be surmounted before commercialization [4, 5]. First, the anode catalyst is inactive and unstable, causing a high overpotential loss of the anode. Second, severe methanol crossover from anode to cathode in the commercially available perfluorosulfonic acid (PFSA) proton-exchange membranes (PEM) (.., Nafion®) reduces fuel efficiency and increases the mixed electrode potential of the cathode, resulting in low cell performance [4, 5]. Moreover, the production of the commercially available PFSA polymer membranes is costly. Therefore, it is urgent to develop PEM with improved properties, including high proton conductivity, low methanol permeability, and low cost.
Much effort has been made in recent years to develop alternative fluorine-free polymer membranes[6-12] and to modify PFSA polymer membranes [13-15]. It is widely recognized that sulfonated poly(ether ether ketone) (SPEEK) polymers are promising materials for membranes in DMFCs [6]. SPEEK polymers, in theory, have higher ion-exchange capacity (IEC) than PFSA polymers, which may compensate for the demerit of weaker acidity of their own sulfonic groups (SO3H). SPEEK membranes also exhibit lower methanol crossover and are less costly to produce than PFSA membranes [7]. The demand for high proton conductivity calls for SPEEK membranes to have a high degree of sulfonation (DS) and to function at high temperatures. However, highly sulfonated SPEEK membranes tend to swell excessively or even dissolve at high temperatures. Several attempts have been made to overcome the excessive swelling while maintaining high proton conductivity, for example, by synthesizing SPEEK with various ratios of hydrophobic block to hydrophilic block [16], by introducing cross-links between some of the sulfonic groups in the SPEEK membrane [17], and by adding inorganic particles (.., ZrO2) into the SPEEK matrix [18].
Blend of polymers with different properties is also an important approach in PEM research. A sulfonated polymer is blended with a non-conductive or relatively poorly conductive engineering thermoplastic. The sulfonated polymer provides high proton conductivity, and the engineering thermoplastic maintains the mechanical integrity. This approach aims to combine the desired properties and features of each component. SPEEK was blended with a variety of nonconductive polymers, including poly(ether imide) (PEI) [19, 20], poly(ether sulfone) (PES) [21], polyaniline (PANI) [22], polybenzimidazole (PBI) [23] and poly(vinylidene fluoride) (PVDF) [24]. These blend membranes showed, to different extents, improved mechanical stability and decreased methanol crossover, but at the cost of decreased proton conductivity.
Zaidi. [25] doped with inorganic acids to enhance the proton conductivity of SPEEK/PEI blend membranes, but the initially high conductivity decreased because of gradual leaking of acid from the membranes. Blend membranes comprising two different sulfonated polymers were studied (.., SPS/SPPO, SPEKK/SPEKK) [26, 27].
In this study, sulfonated phenolphthalein poly(ether sulfone) (SPES-C) is blended with the SPEEK matrix to prepare SPEEK/SPES-C blend membranes. Phenolphthalein poly(ether sulfone) (PES-C) is a novel thermoplastic engineering material and can be sulfonated easily in concentrated sulfuric acid [28]. The SPES-C membrane has low methanol permeability and high resistance to swelling, even at a DS of 65%, owing to the rigidity of the main chains and the steric hindrance effect of phenolphthalein side chains. It is expected that the blending of SPES-C into the SPEEK matrix leads to more thermostable membranes with high proton conductivity, low methanol permeability, and low swelling degree.
2 EXPERIMENTAL
2.1 Materials

2.2 Sulfonation of PEEK and PES-C
PEEK (10 g) was added gradually to 100 ml of concentrated sulfuric acid in a three-necked flask with vigorous stirring at 60ºC. At a prescribed time point, the acid polymer solution was added to a large excessive ice-cold water with continuous agitation. The SPEEK precipitate was rinsed repeatedly with deionized water until the water reached pH 7. Then the SPEEK was dried at room temperature for 2 days followed by drying at 60ºC for 24 h under vacuum. The PES-C was sulfonated as described in a previous study [29]. The IEC and DS of the sulfonated polymers were determined by a classical back-titration method [29].
2.3 Membrane preparation
Membranes were prepared by solution casting. SPEEK and SPES-C in prescribed amount were dissolved separately in DMF (10%, by mass) and then the two solutions were mixed and stirred for 6 h. The mixed solution was cast onto a glass plate and dried overnight at 60ºC in a vacuum oven, followed by annealing at 100ºC for 4 h. After cooling to room temperature, the membrane was peeled from the glass plate with deionized water. Finally, the membrane was treated with 1 mol·L-1sulfuric acid at room temperature for 24 h and subsequently rinsed with deionized water several times to remove acid completely. Membranes were kept in deionized water before testing. The thickness of the dried membranes was 80-90 μm.
2.4 Membrane characterization
Fourier transform infrared spectroscopy (FT-IR) was measured in absorbance mode by using an FT-IR spectrometer (Bio-RAD FTS 6000) in the range of wave numbers 4000-600 cm-1to compare the position of IR bands and to check the presence of functional groups and their interaction in composite membranes. Prior to the measurement, the samples were dried at 80°C for 24 h.
Thermogravimetric analysis (TGA) was used to estimate the thermal stability of the blend membranes. We used a TGA thermogravimetric analyzer (TA-50 Instrument Shimadzu TGA) at a heating rate of 10 K·min-1in nitrogen gas in the temperature range 30-800°C. All specimens were dried overnight at 90°C under vacuum before measurements.
The glass transition temperature (g) was determined with a differential scanning calorimeter (DSC) (TA-50 Instrument Shimadzu DSC). Measurements were performed over the temperature range 30-400ºC at a heating rate of 5 K·min-1in nitrogen gas.
The morphology of the cross-section of samples was examined with an environment-scanning electron microscope (PHILIPS XL30 ESEM). The samples were cryo-fractured in liquid nitrogen to obtain fresh cross-sections, which were coated with gold before measurements.
The swelling degree (SD) of the specimens was obtained by measuring the area difference between the dry and the wet states as described in Ref. [23]. The membranes were cut into 3 cm × 4 cm rectangles and dried overnight at 90°C under vacuum before measuring the area (d). The dried membranes were immersed in 1 mol·L-1methanol for 48 h to reach equilibrium at the desired temperature. The wet membranes were wiped dry with tissue paper and the area was measured again (w). SD was calculated (in area percent) as follows:

wheredandware the areas of dry and corresponding wet membrane sheets, respectively. Three sheets of each membrane composition were measured, and the average was calculated.
Figure 11H-NMR spectra of SPEEK samples

Figure 21H-NMR spectra of PES-C and SPES-C samples

The proton conductivity of samples in the lateral direction was measured with a measurement cell and a frequency response analyzer (FRA) (Autolab PG-STAT20). Two stainless steel electrodes connected to the FRA were pressed against the membrane to be tested. The measurement temperature was controlled from room temperature to 160°C. The conductivity,, was calculated from the impedance data with the relation/(×), whereandare the distance between the electrodes and the cross-sectional area of the membrane, respectively, andwas derived from the low intersection of the high-frequency semi circle on a complex impedance plane with the() axis. For membranes that dissolved below 160°C, their dissolution was determined by measuring the mass before and after the proton conductivity measurement.
3 RESULTS AND DISCUSSION
3.1 1H-NMR study
The1H-NMR spectra of the PEEK and PES-C were obtained with a Varian Unity-plus 400 NMR spectrometer, to determine the specific sites of sulfonation reaction in the polymer chains as well as their DS. Polymers (2.5%, by mass) in deuterated dimethyl sulfoxide (DMSO-d6) were prepared as1H-NMR samples. The chemical shift of tetramethylsilane was used as the internal standard reference. The spectral data were obtained at 30ºC in the resonance frequency range of 3.99-95 MHz.


The intensity of the distinct hydrogen signal at position g for SPEEK and that at position k for SPES-C are used to determine the Hgand Hkcontents, respectively. Noticing that the contents of Hgand Hkare equivalent to that of sulfonic acid groups, DS can be calculated by the following equation:
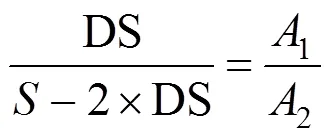
whereis the total number of hydrogen atoms per repeat unit of the polymer before sulfonation, which is 12 for PEEK and 20 for PES-C,1is the peak area of the Hgsignal for SPEEK or that of the Hksignal for SPES-C, and2is the sum of the peak areas of all the signals except Hgfor SPEEK or except Hkfor SPES-C. The DS values obtained from NMR data and the back-titration method are given in Table 1. The DS values obtained from the two methods are in reasonably good agreement, confirming the validity of the back-titration method.

Table 1 DS values of SPEEK and SPES-C obtained fromdifferent methods
3.2 FT-IR spectra

3.3 Thermogravimetric analysis




3.4 Glass transition temperature and scanning electron microscope images


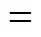
The introduction of sulfonic groups increases the intermolecular interaction and the molecular size of the polymer, leading to an increase in glass transition temperature of the polymer. As shown in Fig. 5, the glass transition temperatures of PES-C and PEEK are around 260ºC and 143ºC, respectively, while those of SPES-C and SPEEK are around 276ºC and 205ºC, respectively.
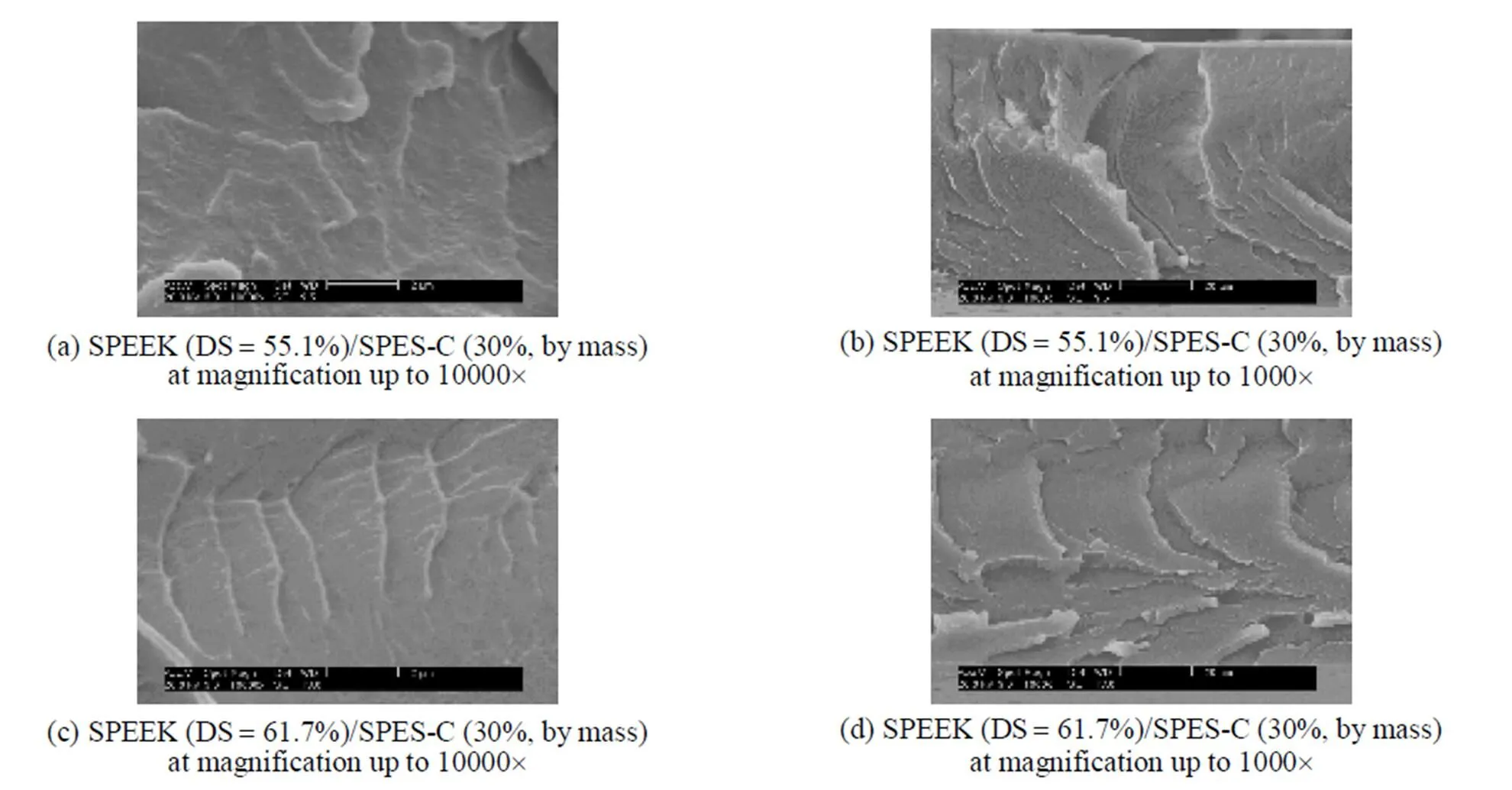
Figure 5 shows that the SPEEK/SPES-C blend membrane has only one glass transition temperature, instead of two for each of the components, indicating that SPEEK and SPES-C are perfectly mixed in the membrane [30]. Phase separation is not observed from the scanning electron microscope (SEM) images of the SPEEK/SPES-C blend membranes at magnification up to 10000´(Fig. 6), confirming the homogeneity of the membrane as concluded from the DSC measurement.

3.5 Swelling behavior

3.6 Methanol permeability


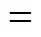
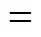

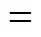
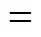
The difference in methanol permeability between NafionÒ115 and the SPEEK membrane can be explained qualitatively by the differences in their microstructures and the acidity of their sulfonic acid functional groups [6]. Nafion®115 macromolecules consist of very hydrophobic perfluorinated backbones and very hydrophilic side chains with sulfonic acid functional groups. It lead to relatively large microphase separation in NafionÒ115, resulting in the low resistance to methanol permeation. The situation for SPEEK polymer is rather different. The carbon-hydrogen main chains with ether links, phenyl rings, and carbonyl groups in SPEEK make it less hydrophobic and more rigid compared with NafionÒ115. Furthermore, the acidity of sulfonic acid functional groups of the SPEEK polymer is weaker than that of NafionÒ115. Therefore, the microphase separation in the SPEEK membrane is not obvious and the hydrophilic ion channels are narrower, resulting in low methanol permeability. As shown in Fig. 8, the methanol permeability of the SPEEK/SPES-C blend membranes is even lower and decreases with the increase of SPES-C content in the membrane. The addition of SPES-C inhibits effectively the swelling of SPEEK matrix (Fig. 7) and thus imposes higher resistance to methanol crossover.
3.7 Proton conductivity
The relation between the conductivity in 100% RH and the reciprocal of the temperature for all the SPEEK and SPEEK/SPES-C (10%-40%, by mass) membranes, before they dissolve, can be described by the Arrhenius equation and exhibits straight lines in a semilogarithmic graph (Fig. 9). The apparent activation energy of proton transfer is obtained from the slope of the lines. It is noteworthy that the apparent activation energy of the SPEEK/SPES-C (30%, by mass) blend membrane has approximately 39 kJ×mol-1, in contrast to 38 kJ×mol-1for the SPEEK membrane and 9 kJ×mol-1for Nafion®115. The high apparent activation energy of SPEEK/SPES-C membranes is due to their low swelling degree and small variation with temperature (as shown in Fig. 9). Narrow ion channels and rich branches with dead-end “pockets” in the membrane will contribute to the high barrier to proton transfer [6].
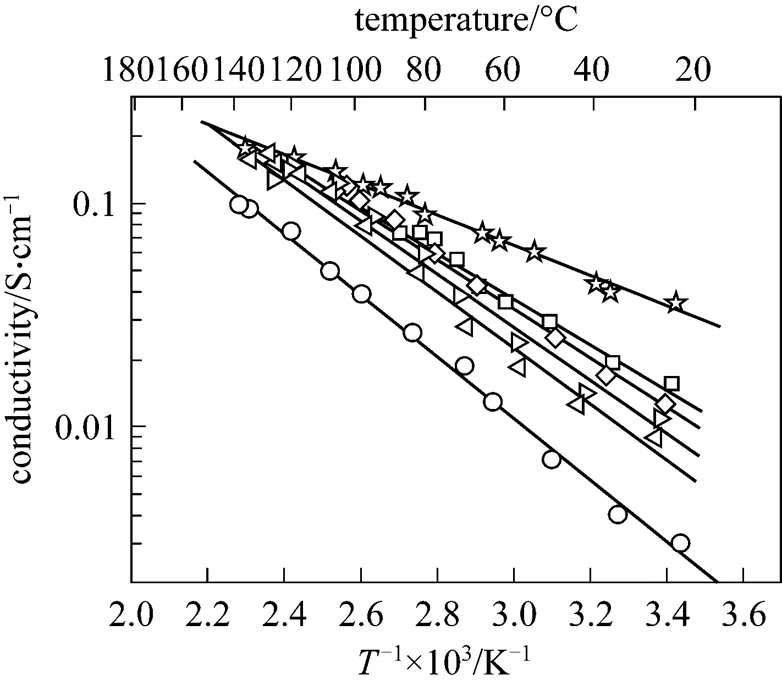


SPEEK and SPES-C materials are weakly acidic compared with Nafion®115. The dissociation of sulfonic acid functional groups in these polymers increases at high temperatures [6, 29], while that of Nafion®115 approaches 100% at room temperature. Therefore, the elevation of temperature increases the proton mobility and proton content in the SPEEK and SPEEK/SPES-C membranes, which results in a much faster increase of proton conductivity with temperature.
3.8 Applicable temperature

Figure 10 shows*as a function of the mass content of SPES-C in the blend membrane, which is markedly increased with the mass content of SPES-C polymer. As discussed in Section 3.5, the blending of SPES-C with SPEEK inhibits effectively the swelling and dissolution of the membranes. The increase of*with the increased content of SPES-C in the blend membrane is attributed to the depressed swelling and dissolution of the blend at high temperatures.

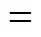
The extension of the applicable temperature of the membrane is beneficial for DMFCs in many aspects. Working at higher temperatures, the membrane exhibits higher proton conductivity, lowering ohmic loss in DMFCs. The electrocatalysts are also more active and more tolerant to carbon monoxide poisoning at high temperatures [33]. When methanol fuel is fed in the form of gas at high temperature, the methanol crossover and its negative effects can be reduced markedly. Operating at high temperature also simplifies the management of water and heat in the DMFC systems [33].
4 CONCLUSIONS
SPEEK/SPES-C blend membranes with high levels of miscibility and thermal stability were prepared. Differential scanning calorimeter reveals acceptable miscibility of the two polymers. The methanol permeability of the membranes is about one order of magnitude lower than that of Nafion®115, and decreases with the increase of SPES-C content. The blending of SPES-C into the membranes also inhibits effectively their swelling, so that the membranes can be used at higher temperatures, presenting higher proton conductivity. The results of this study indicate a strong potential of these blend membranes for use in DMFCs.
1 Hogarth, M.P., Hards, G.A., “Direct methanol fuel cells, Technological advances and further requirements”,.., 40, 150-159 (1996).
2 Surampudi, S., Narayanan, S.R., Vamous, E., Frank, H., Halpert, G., Lconti, A., Kosek, J., Prakash, G.K., Olah, G.A., “Advances in direct oxidation methanol fuel cells”,., 47, 377-385 (1994).
3 Kuver, A., Kamloth, K.P., “Comparative study of methanol crossover across electropolymerized and commercial proton exchange membrane electrolytes for the acid direct methanol fuel cell”,., 43, 2527-2535 (1998).
4 Ren, X., Zawadzinski, T.A., Uribe, F., Dai, H., “Methanol cross-over in direct methanol fuel cells”,..., 95, 284-289 (1995).
5 Heinzel, A., Barragan, V.M., “A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells”,., 84, 70-74 (1999).
6 Kreuer, K.D., “On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells”,..., 185, 29-39 (2001).
7 Yang, B., Manthiram, A., “Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells”,.-6, A229-A231 (2003).
8 Wycisk, R., Lee, J.K., Pintauro, P.N., “Sulfonated polyphosphazene-polybenzimidazole membranes for DMFCs”,..., 152, A892-A898 (2005).
9 Gu, S., He, G.H., Wu, X.M., Guo, Y.J., Liu, H.J., Peng, L., Xiao, G.k., “Preparation and characteristics of crosslinked sulfonated poly(phthalazinone ether sulfone ketone) with poly(vinyl alcohol) for proton exchange membrane”,..., 312, 48-58 (2008).
10 Yamada, O., Yin, Y., Tanaka, K., Kita, H., Okamoto, K., “Polymer electrolyte fuel cells based on main-chain-type sulfonated polyimides”,., 50, 2655-2659 (2005).
11 Song, J.M., Miyatake, K., Uchida, H., Watanabe, M., “Investigation of direct methanol fuel cell performance of sulfonated polyimide membrane”,., 51, 4497-4504 (2005).
12 Othman, M.H.D., Ismail, A.F., Mustafa, A., “Proton conducting composite membrane from sulfonated poly(ether ether ketone) and boron orthophosphate for direct methanol fuel cell application”,..., 299, 156-165 (2007).
13 Lin, Y.F., Hsiao, Y.H., Yen, C.Y., Chiang, C.L., Lee, C.H., Huang, C.C., Ma, C.C.M., “Sulfonated poly(propylene oxide) oligomers/Nafion®acid-base blend membranes for DMFC”,., 172, 570-577 (2007).
14 Ramya, K., Dhathathreyan, K.S., “Methanol crossover studies on heat-treated Nafion®membranes”,..., 311, 121-127 (2008).
15 Park, K.T., Jung, U.H., Choi, D.W., Chun, K., Lee, H.M., Kim, S.H., “ZrO2-SiO2/Nafion®composite membrane for polymer electrolyte membrane fuel cells operation at high temperature and low humidity”,., 177, 247-253 (2008).
16 Zhao, C., Li, X., Na, H., “Synthesis of sulfonated poly(ether ether ketone) (S-PEEKs) material for proton exchange membrane”,..., 280, 643-650 (2006).
17 Mikhailenko, S.U.D., Wang K.P., Kaliaguine, S., Xing, P.X., Robertson, G.P., Guiver, M.D., “Proton conducting membranes based on cross-linked sulfonated poly(ether ether ketone) (SPEEK)”,..., 233, 93-99 (2004).
18 Silva, V.S., Ruffmann, B., Silva, H., Gallego, Y.A., Mends, A., Madeira, L.M., Nunes, S.P., “Proton electrolyte membrane properties and direct methanol fuel cell performance (I) Characterization of hybrid sulfonated poly (ether ether ketone)/zirconium oxide membranes”,., 140, 34-40 (2005).
19 Mikhailenko, S.U.D., Zaidi, S.M.J., Kaliaguine, S., “Electrical properties of sulfonated polyether ether ketone/polyetherimide blend membranes doped with inorganic acids”,...,:.., 33, 1386-1395 (2000).
20 Kerres, J., Ullrich, A., Meier, F., Haring, T., “Synthesis and characterization of novel acid-base polymer blends for application in membrane fuel cells”,., 125, 243-249 (1999).
21 Manea, C., Mulder, M., “Characterization of polymer blends of polyether sulfone/sulfonated polysulfone and polyether sulfone/sulfonated polyetherether ketone for direct methanol fuel cell applications”,..., 206, 443-453 (2002).
22 Nagarale, R.K., Gohil, G.S., Shahi, V.K., “Sulfonated poly(ether ether ketone)/polyaniline composite proton-exchange membrane”,..., 280, 389-396 (2006).
23 Zaidi, S.M.J., “Preparation and characterization of composite membranes using blends of SPEEK/PBI with boron phosphate”,., 50, 4771-4777 (2005).
24 Jung, H.Y., Park, J.K., “Blend membranes based on sulfonated poly(ether ether ketone) and poly(vinylidene fluoride) for high performance direct methanol fuel cell”,., 52, 7464-7468 (2007).
25 Zaidi, S.M.J., Mikhailenko, S.D., Robertson, G.P., Guiver, M.D., Kaliaguine, S., “Proton conducting composite membranes from polyetherether ketone and heteropolyacids for fuel cell applications”,..., 173, 17-34 (2000).
26 Jung, B., Kim, B., Yang, J.M., “Transport of methanol and protons through partially sulfonated polymer blend membranes for direct methanol fuel cell”,..., 245, 61-69 (2004).
27 Swier, S., Ramani, V., Fenton, J.M., Kunz, H.R., Shaw, M.T., Weiss, R.A., “Polymer blends based on sulfonated poly(ether ketone ketone) and poly(ether sulfone) as proton exchange membranes for fuel cells”,..., 256, 122-133 (2005).
28 Jia, L.D., Xu, X.F., Zhang, H.J., Xu, J.P., “Permeation of nitrogen and water vapor through sulfonated polyetherethersulfone membrane”,...,:.., 35, 2133-2140 (1997).
29 Li, L., Wang, Y.X., “Sulfonated polyethersulfone Cardo membranes for direct methanol fuel cell”,..., 246, 167-172 (2005).
30 Wu, P.X., Zhang, L.C., Polymer Blending Modification, China Light Industry Press, Beijing, 29-31 (1996).
31 Tricoli, V., Carretta, N., Bartolozzi, M., “A comparative investgation of proton and methanol transport in fluorinated ionomeric membranes”,..., 147, 1286-1290 (2000).
32 Huang, M.Y., Wang, Y.X., Cai, Y.Q., Xu, L., “Sulfonated poly(ether ether ketone)/zirconium tricarboxybutylphosphonate composite proton-exchange membranes for direct methanol fuel cells”,, 4, 337-342 (2007).
33 Li, Q.F., Huang, R.H., “Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100ºC”,.., 15, 4896-4915 (2003).
2008-11-09,
2009-08-18.
the State Key Development Program for Basic Research of China (2008CB617502), the National Natural Science Foundation of China (20606025), and Program for Changjiang Scholars and Innovative Research Team in University of China (IRT0641).
** To whom correspondence should be addressed. E-mail: xuli620@eyou.com
 Chinese Journal of Chemical Engineering2009年6期
Chinese Journal of Chemical Engineering2009年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Working Temperature on the Resistance Characteristic of aPleated Stainless Steel Woven Filter*
- The Numerical Simulation of Collapse Pressure and Boundary of the Cavity Cloud in Venturi*
- Performance of Inner-core Supersonic Gas Separation Device with Droplet Enlargement Method*
- Void Fraction Distributions in Cold-gassed and Hot-sparged Three Phase Stirred Tanks with Multi-impeller*
- Kinetics of COD Removal in a Biological Aerated Filter in thePresence of 2,4,6-Trinitrophenol (Picric Acid)*
- Representation of Phase Behavior of Ionic Liquids Using the Equation of State for Square-well Chain Fluids with Variable Range*
