机器人辅助下乳腺癌根治术患者行超声引导下Ⅱ型胸神经阻滞术后镇痛效果及影响因素

摘要 目的:分析机器人辅助下乳腺癌根治术患者行超声引导下Ⅱ型胸神经阻滞(PECS Ⅱ)术后镇痛效果及影响因素。方法:采取前瞻性研究,选取2023年2月—2024年8月于西北妇女儿童医院收治的80例行机器人辅助下乳腺癌根治术患者作为研究对象,按随机数字表法分为对照组(40例,采取胸椎旁神经阻滞)和观察组(40例,采取超声引导下PECS Ⅱ)。比较两组患者术后恢复情况及舒芬太尼用量,不同时点(术后1 h、5 h、8 h、12 h)
采用视觉模拟评分法(VAS)评分;术前、术后12 h炎症指标TNF-α、IL-6;分析影响机器人辅助下乳腺癌根治术患者术后镇痛效果的因素。结果:观察组术后镇痛时间长于对照组,自主呼吸恢复、拔除喉罩、完全清醒时间短于对照组,舒芬太尼用量少于对照组(Plt;0.05);术后12 h,两组患者TNF-α、IL-6水平均高于术前,且观察组低于对照组(Plt;0.05);术后5 h、8 h、12 h,两组患者VAS评分均高于术后1 h,并呈上升趋势,观察组各时点VAS评分均低于对照组(Plt;0.05);术后12 h,采用VAS评分评估患者镇痛效果,其中镇痛效果可或差27例,镇痛效果优或良53例,镇痛效果可差组肿瘤部位为上象限、术前焦虑或抑郁患者占比高于镇痛效果优良组,血清TNF-α、IL-6水平高于镇痛效果优良组(Plt;0.05);回归分析显示,术前焦虑或抑郁、血清TNF-α、IL-6高表达为影响机器人辅助下乳腺癌根治术患者镇痛效果的因素(ORgt;1,Plt;0.05)。结论:超声引导下PECS Ⅱ能够有效延长机器人辅助下乳腺癌根治术患者术后镇痛时间,减少麻醉药物用量,减轻患者术后炎症反应,且术前焦虑或抑郁、血清TNF-α、IL-6水平与患者术后疼痛密切相关。
关键词 乳腺癌根治术;机器人辅助手术;镇痛效果;超声引导;胸神经阻滞;Ⅱ型胸神经
中图分类号 R737.9 文献标识码 A 文章编号 2096-7721(2025)01-0045-08
Postoperative analgesic effect and influencing factors of ultrasound-guided pectoral nerve block type Ⅱ in patients undergoing robot-assisted radical mastectomy
LI Zhen1 , XU Jialei1, DING Liang2
(1.Anesthesia Operating Room, Northwest Women and Children’s Hospital, Xi’an 710061, China;
2.Department of Pharmacy, Chang’an Hospital, Xi’an 710018, China)
Abstract Objective: To analyze the postoperative analgesic effect and influencing factors of ultrasound-guided pectoral nerve block type Ⅱ (PECS Ⅱ) in patients undergoing robot-assisted radical mastectomy. Methods: A prospective study was conducted. 80 patients who underwent robot-assisted radical mastectomy in the Northwest Women and Children’s Hospital from Feb. 2023 to Aug. 2024 were selected and divided into the control group [thoracic paravertebral nerve block (TPVB), n=40] and the observation group (ultrasound-guided PECS Ⅱ block, n=40) using a random number table. The postoperative recovery and
sufentanil dosage, visual analogue scale (VAS) scores at different timepoints (1, 5, 8, and 12 h after surgery), and inflammatory indicators [tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6)] before and 12 h after surgery were compared between the two groups. The factors affecting analgesic effect after robot-assisted radical mastectomy were analyzed. Results: Compared with the control group, the time of postoperative analgesia was longer, the time of spontaneous breathing recovery, removal of the laryngeal mask and full awakening were shorter, and the dosage of sufentanil was less in the observation group than those in the control group (Plt;0.05). At 12 h after surgery, the levels of TNF-α and IL-6 in the two groups were higher than those before surgery, and they were relatively lower in the observation group (Plt;0.05). VAS scores in both groups at 5, 8, and 12 h after operation were higher than those at 1 h after operation and exhibited an upward trend. The VAS scores of the observation group at each timepoint were lower than those of the control group (Plt;0.05). At 12 h after surgery, VAS scores showed that 27 cases had poor analgesic effect and 53 cases had good analgesic effect. In the poor analgesic effect group, the proportion of patients with tumors located in the upper quadrant, preoperative anxiety or depression, and levels of serum TNF-α and IL-6 were higher than the good analgesic effect group (Plt;0.05). Regression analysis showed that preoperative anxiety or depression and high expression of serum TNF-α and IL-6 were factors affecting the analgesic effect after robot-assisted radical mastectomy (ORgt;1, Plt;0.05). Conclusion: Ultrasound-guided PECS II block can effectively prolong the postoperative analgesia in patients undergoing robot-assisted radical mastectomy, reduce the dosage of anesthetic drugs, and lower the postoperative inflammatory response. Preoperative anxiety or depression and serum TNF-α and IL-6 levels are closely related to postoperative pain.
Key words Ridical Mastectomy; Robot-assisted Surgery; Analgesic Effect; Ultrasound-guiding; Pectoral Nerve Block; Type Ⅱ Pectoral Nerves
随着机器人技术的发展,机器人辅助下乳腺癌根治术也逐步被临床所应用,机器人手术系统可为术者提供高分辨率3D视觉及机械臂7个方向的自由度,可有效提升手术操作的精准度[1]。但乳腺癌根治术对患者机体创伤较大,机体应激反应强烈,患者术后疼痛发生率较高。临床实践发现,合理的麻醉方案,能够有效减轻患者围手术期疼痛,提升镇痛效果。Ⅱ型胸神经阻滞(Pectoral Nerve Block Type Ⅱ,PECS Ⅱ)
被临床主要应用于胸部或肋骨骨折麻醉中。PECSⅡ不仅能够支配T2~T6节段乳腺皮区的肋间神经外侧皮支,还可阻滞胸长神经、胸背神经等[2]。据相关研究显示,超声引导下PECS Ⅱ可有效提升机器人辅助下冠状动脉旁路移植术患者围手术期镇痛效果[3]。鉴于此,本研究着重分析机器人辅助下乳腺癌根治术行PECS Ⅱ术后镇痛效果及影响因素。现报道如下。
1 资料与方法
1.1一般资料 采取前瞻性研究,选取2023年2月—2024年8月于西北妇女儿童医院收治的80例行机器人辅助下乳腺癌根治术患者作为研究对象,按随机数字表法分为对照组和观察组,各40例。观察组年龄24~53岁,平均年龄(36.80±5.36)岁;发病侧:左侧26例(65.00%),右侧14例(23.33%);ASA[4]分级:Ⅰ级21例(52.50%),Ⅱ级19例(47.50%);TNM分期:Ⅰ期15例(37.50%),Ⅱ期25例(62.50%)。对照组年龄25~51岁,平均年龄(36.90±4.81)岁;发病侧:左侧22例(55.00%),右侧18例(45.00%);ASA分级:Ⅰ级18例(45.00%),Ⅱ级22例(55.00%);TNM分期:Ⅰ期14例(35.00%),Ⅱ期26例(65.00%)。两组患者一般资料比较,差异有统计学意义(Pgt;0.05),具有可比性。患者已签署知情同意书。
1.2纳入与排除标准 纳入标准:①乳腺癌符合相关指南[5]的诊断标准;②年龄≥18岁;③均为单侧发病;④均为女性;⑤卡式[6]评分gt;70分;⑥均行淋巴结清扫。排除标准:①合并感染者;②术前接受放化疗者;③合并肝肾功能障碍者;④基础疾病控制不佳者;⑤有局部麻醉药物过敏史者。
1.3方法
1.3.1对照组 采取胸椎旁神经阻滞。辅助患者取坐位,通过初诊寻找第3胸椎椎间隙,使用超声(Siemens Medical Solutions USA,Inc.,国
械注进20173061080)获得胸膜、横突及肋间突韧带图像。沿探头外侧缘缓慢进针,通过进针点注入2%利多卡因(北京益民药业,规格2 mL∶40 mg,国药准字H11020322)行局部麻醉,将针尖(SPIDENT Co.,Ltd.,国械注进20173142471)推入至肋横突韧带,注射2 mL生理盐水,注入25 mL 0.5%罗哌卡因(AstraZeneca AB,规格10 mL∶20 mg,批准文号H20140766),回抽无血及脑脊液。
1.3.2观察组 采取PECS Ⅱ。辅助患者取平卧位,手术侧手臂外展,于锁骨中线部位使用超声探头寻找腋静脉及腋动脉,沿腋前线向外侧移动至T3节段皮区水平,获取胸小肌及深部前锯肌图像。局麻及进针方式与对照组一致,针尖由内向外,通过超声图像确认针尖位于前锯肌与胸小肌之间,注入15 mL 0.5%罗哌卡因,随后将针尖推进胸大肌、胸小肌间隙,再次注入10 mL 0.5%罗哌卡因。
1.3.3麻醉诱导及维持麻醉 两组患者阻滞成功后,采取喉罩(Teleflex Medical,国械注进20182080458)麻醉,采用丙泊酚(Corden Pharma S.P.A.,规格20 mL∶200 mg,批准文号H20171277)1.5μg/mL,舒芬太尼[江苏恩华药业,规格2 mL∶100μg(按C22H30N2O2S计),国药准字H20203651] 0.1μg/kg,苯磺顺阿曲库铵(GLAXOSMITHKLINE MANUFACTURING SPA,规格10 mL∶20 mg,国药准字HJ20181159)进行麻醉诱导。术中根据患者调节丙泊酚输注效应室浓度,间断性推注舒芬太尼维持麻醉效果,使脑电波双频指数维持在40~60。术后采取静脉自控镇痛,曲马多(Grunenthal GmbH,规格1 mL∶50 mg,批准文号H20140812)1 mg/kg
作为负荷剂量,使用静脉自控镇痛泵(B. Braun Melsungen AG,国械注进20223140086),曲马多400 mg,芬太尼[江苏恩华药业,规格10 mL∶
0.5 mg(以芬太尼计),国药准字H20113509]
0.2 mg与生理盐水混合,总量为120 mL,设定剂量为2 mL/h,每次自控镇痛剂量为2 mL,锁定间隔时间15 min。
1.3.4机器人辅助下乳腺癌根治术 患者取平卧位,患侧肩下垫枕。麻醉后,取3 cm切口行前哨淋巴结活检,随后经切口置入2号臂观察孔Trocar。距Trocar 6 cm腋顶处做小切口,置入
1号臂操作孔Trocar。于乳房外侧缘近下皱襞处置入3号臂操作孔Trocar,沿下皱襞距3号臂操作孔Trocar 6 cm处行小切口,置入4号臂操作孔Trocar。游离胸大小肌间隙,充分松弛肌肉后,充气使腔内气压稳定在8~10 mmHg,1号臂抬起胸大肌,3号臂使用永久电钩沿胸小肌浅面及肋骨表面游离,离断胸大肌内侧部分及下方起点,向下游离直至术前划定的下皱襞标志线。以相同方法行乳房后间隙游离。最后进行皮下组织游离,皮瓣厚度约5 mm,由1号臂置入有孔双极镊牵拉腺体,4号臂将皮瓣抬起,由3号臂行游离操作。1号臂和4号臂可根据部位不同进行牵拉或上抬,直至将整个腺体切除。皮下腺体切除完毕后,撤出手术机器人,腺体由主切口取出,冲洗术腔完成手术。
1.3.5镇痛效果评估及分组方法 于患者术后12 h
使用视觉模拟评分法(Visual Analogue Scale,VAS)[7]评估患者镇痛效果,总分0~10分,0~2分为优,3~5分为良,6~8分为可,9~10为差,将评分为优或良患者纳入镇痛效果优良组,将评分为可或差患者纳入镇痛效果可差组。
1.3.6基线资料 统计两组患者基线资料,包括年龄、BMI、肿瘤部位、术前焦虑或抑郁[于患者术前,使用焦虑自评量表(Self-rating Anxiety Scale,
SAS)[8]、抑郁自评量表(Self-rating Depression Scale,SDS)[9]评估患者焦虑、抑郁水平,SDS、SAS均20个条目,每个条目1~4分,总分=各条目之和×1.25,分界值分别为50、53分,SDS评分≥50分为焦虑,SAS评分≥53分为抑郁]、临床分期、文化程度、婚姻状况、合并高脂血症、合并高血压、合并糖尿病、吸烟、饮酒。血清指标:采集患者术前、术后12 h空腹静脉血
3 mL,使用离心机(Fresenius Kabi AG,国械注进20183100418),离心参数:转速3200 r/min,
离心半径10 cm,离心10 min,离心取血清,使用全自动干式生化分析仪FUJI DRI-CHEM NX700i(FUJIFILM Corporation,国械注进20202220005)检测TNF-α、IL-6水平。
1.4观察指标 ①术后恢复情况:记录患者术后镇痛、自主呼吸恢复、拔除喉罩完全清醒时间及芬太尼用量。②疼痛水平:记录患者术后不同时点(术后1 h、5 h、8 h、12 h)VAS评分评估患者疼痛水平,总分0~10分,分数越高疼痛越明显。③炎症水平:记录患者术前、术后12 h
TNF-α、IL-6水平。
1.5统计学方法 所有数据均采用SPSS 25.0软件进行统计学分析,计量资料以均数±标准差(x±s)表示,计数资料以例数(百分比)[n(%)]表示,计量资料两组间比较采用独立样本t检验,组内比较采用配对t检验,两组多时点数据分析采用重复测量检验,计数资料采用 χ2检验;采用二元Logistic回归分析影响机器人辅助下乳腺癌根治术患者术后镇痛效果的因素。检验水平
α=0.05。以Plt;0.05为差异有统计学意义。
2 结果
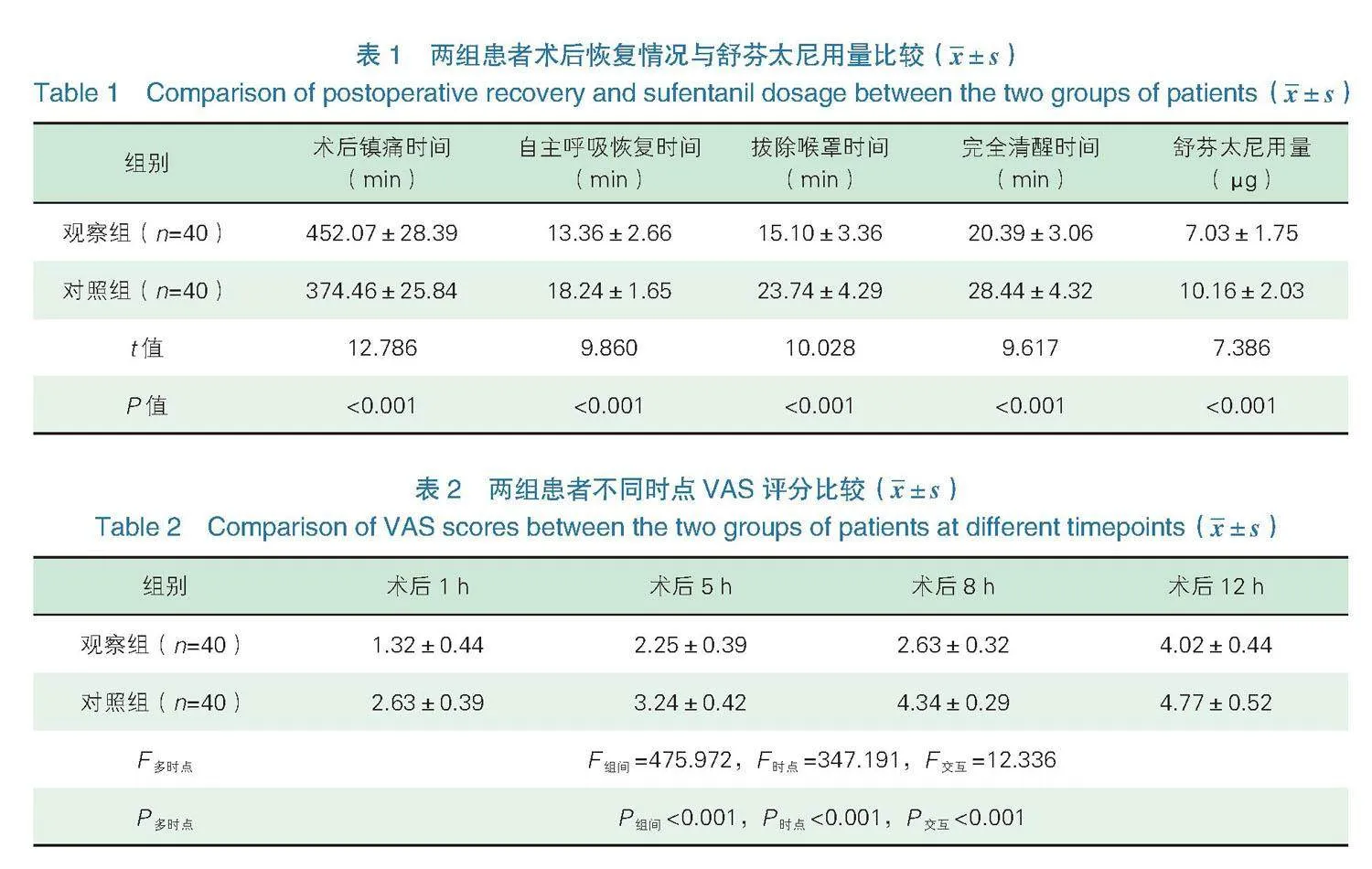
2.1术后恢复情况与舒芬太尼用量 观察组术后镇痛时间长于对照组,自主呼吸恢复、拔除喉罩、完全清醒时间短于对照组,舒芬太尼用量少于对照组,差异有统计学意义(Plt;0.05),见表1。
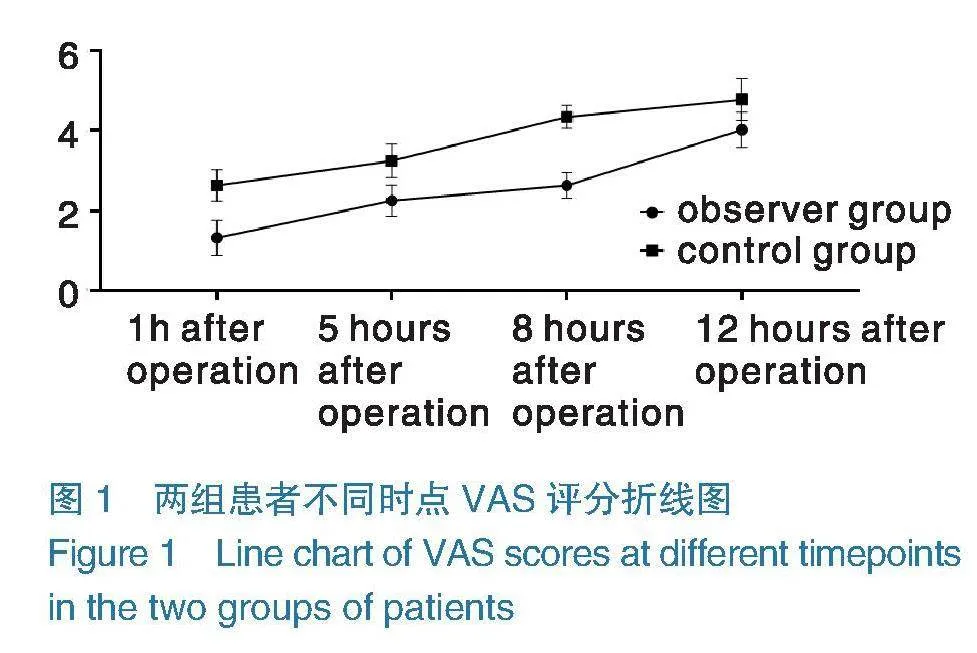
2.2不同时点VAS评分 两组患者术后5 h、8 h、12 h VAS评分均高于术后1 h,并呈上升趋势,且观察组评分低于对照组,两组组间、时点及交互比较,差异有统计学意义(Plt;0.05),见表2,
如图1。
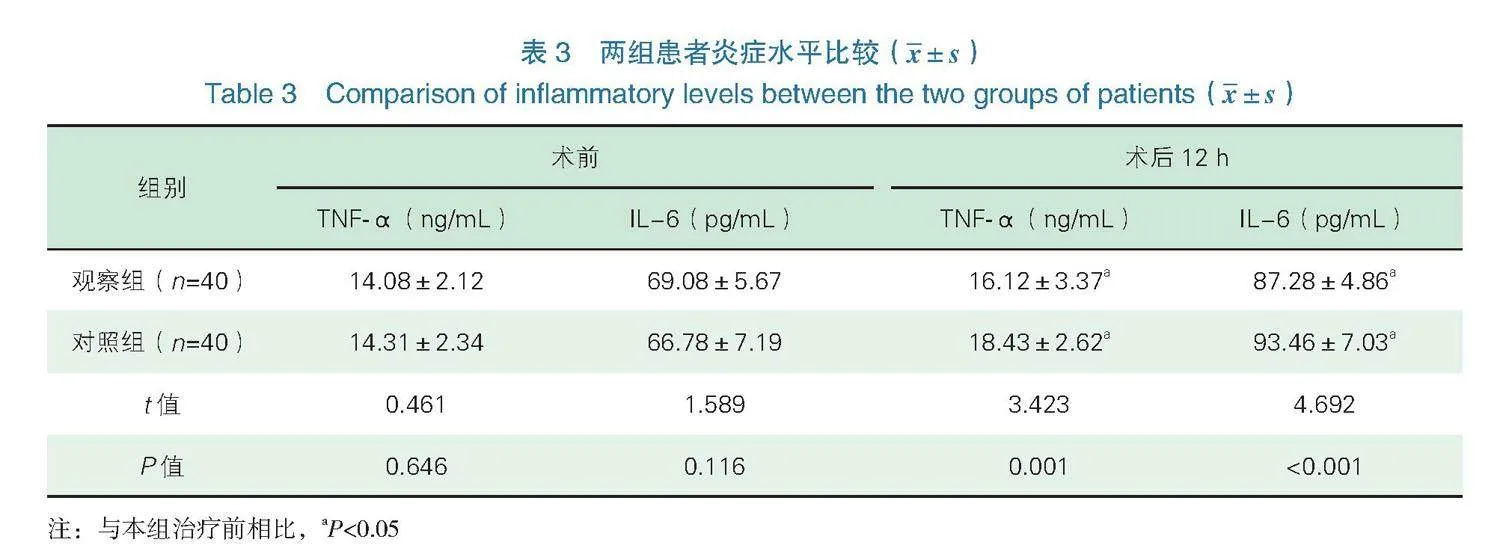
2.3炎症水平 术后12 h,两组患者TNF-α、IL-6水平均高于术前,且观察组低于对照组,差异有统计学意义(Plt;0.05),见表3。
2.4基线资料 术后12 h,采用VAS评估患者镇痛效果,其中镇痛效果可或差27例,镇痛效果优或良53例,镇痛效果可差组肿瘤部位为上象限、术前焦虑或抑郁患者占比高于镇痛效果优良组,血清TNF-α、IL-6高于镇痛效果优良组,差异有统计学意义(Plt;0.05),见表4。
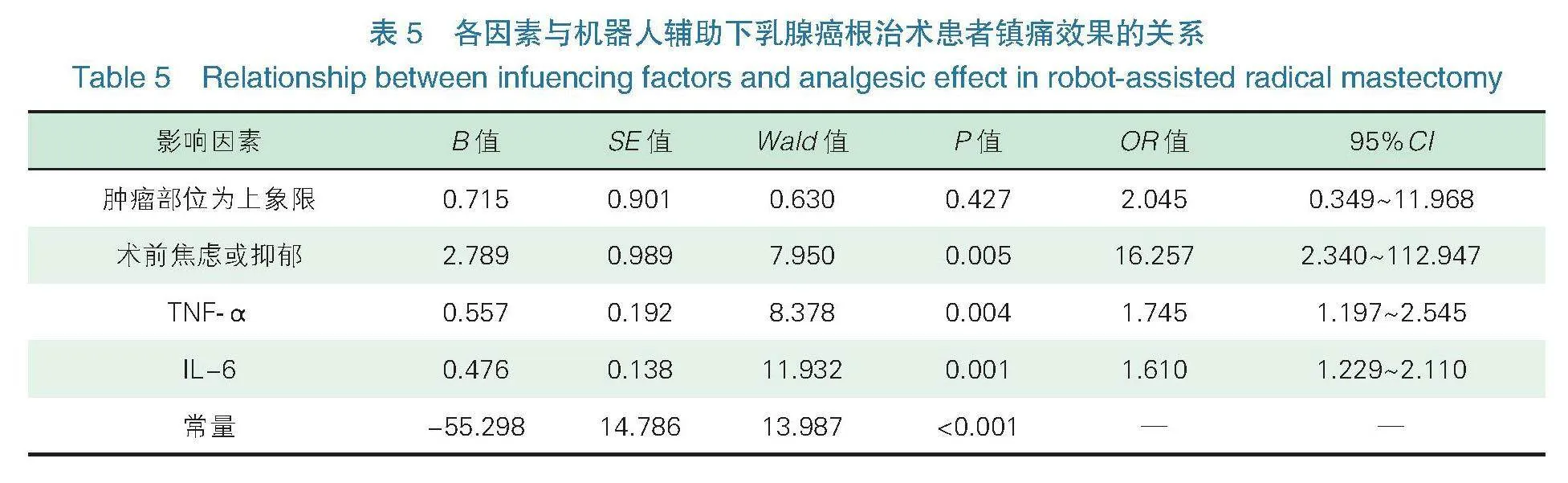
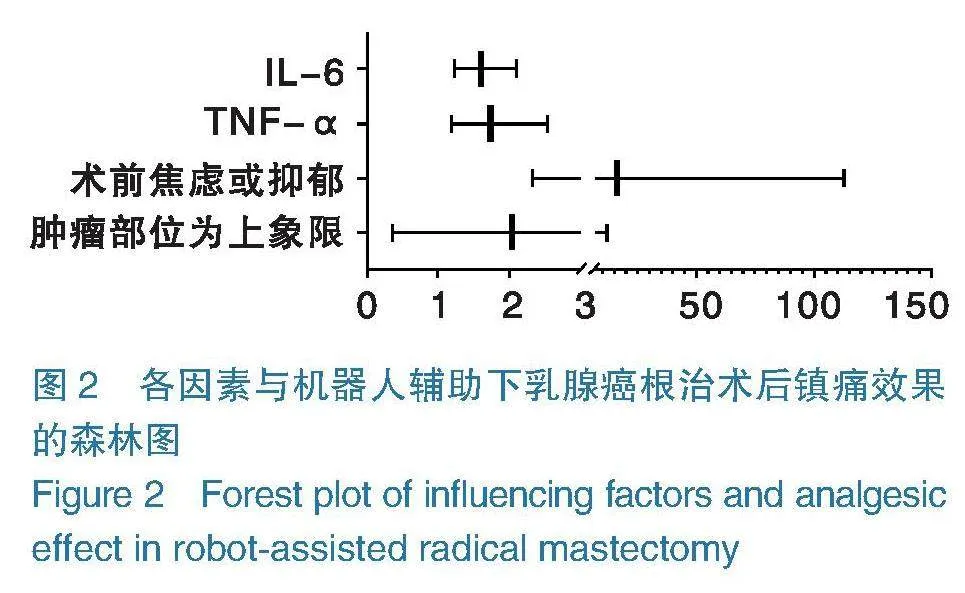
2.5各因素与机器人辅助下乳腺癌根治术患者镇痛效果的关系 将机器人辅助下乳腺癌根治术患者镇痛效果作为因变量(“0”=镇痛效果优良组,“1”=镇痛效果中差组),将表4中差异有统计学意义的变量(肿瘤部位、术前焦虑或抑郁、TNF-α、IL-6)作为协变量,二元logistic回归分析术前焦虑或抑郁、血清TNF-α、IL-6高表达是影响机器人辅助下乳腺癌根治术患者镇痛效果的危险因素(ORgt;1,Plt;0.05),见表5,如图2。
3 讨论
有研究显示,乳腺癌约占女性恶性肿瘤的15%,并以每年3%的速度增长,严重威胁女性生命安全及生活质量[10]。机器人辅助下乳腺癌根治术是治疗乳腺癌的重要方式。但乳腺癌根治术患者往往需配合淋巴结清扫,对患者机体损伤较大,部分患者术后疼痛较为明显。研究显示,行腋窝淋巴结清扫患者术后疼痛发生率约50%,中重度疼痛可高达25%,如镇痛不充分,则严重影响患者术后康复及生活质量[11]。因此临床需积极探寻更有效的镇痛方案,并探寻影响镇痛效果的影响因素,进行针对性干预。
本次研究结果显示,两组患者术后5 h、8 h、12 h VAS评分均高于术后1 h,并呈上升趋势,且观察组低于对照组,表明超声引导下PECS Ⅱ能够有效缓解患者术后疼痛,提高临床镇痛效果。分析其原因在于,PECS Ⅱ是由PECS Ⅰ发展而来,于前锯肌与胸小肌、胸小肌与胸大肌之间的间隙注入局部麻醉药物,从而阻滞胸内、外侧神经及胸长神经等神经,能够更好地阻滞肋间臂神经,阻滞范围更广泛,进而发挥更好的镇痛效果[12-13]。同时PECS Ⅱ麻醉药物会向两端扩散,除可阻滞T2~T4节段皮区外,甚至可达到T6节段皮区感觉阻滞,从而减轻患者手术切口疼痛,缓解患者术侧胸壁的肌筋膜
疼痛[14]。
TNF-α属于肿瘤坏死因子受体超家族蛋白之一,TNF-α对部分肿瘤细胞具有类似生长因子作用,可促进肿瘤细胞增殖[15]。IL-6是临床较为重要的炎性因子,主要由单核巨噬细胞、血管内皮细胞产生,具有调节机体免疫功能的作用[16]。本研究结果显示,观察组术后镇痛时间长于对照组,术后恢复时间短于对照组,舒芬太尼用量少于对照组,术后12 h,两组患者TNF-α、IL-6水平均高于术前,且观察组低于对照组,表明超声引导下PECS Ⅱ能够有效延长镇痛时间,促进患者术后恢复,减轻机体炎症反应。创伤及手术疼痛可激活机体应激反应,从而影响机体代谢平衡及免疫状态,刺激机体释放大量炎症因子,进而引起机体炎症反应[17-18]。但超声引导下PECS Ⅱ范围更广,包括T2~T6节段皮区,可有效避免因中枢敏化,降低疼痛阈值,从而缓解机体刺激,控制机体炎症
反应[19-20]。
二元Logistic回归分析术前焦虑或抑郁、血清TNF-α、IL-6高表达是影响机器人辅助下乳腺癌根治术患者镇痛效果的危险因素。分析原因在于:①术前焦虑或抑郁。据相关研究显示,交感神经是疼痛与焦虑之间的一个潜在联络环节,研究显示,刺激交感神经系统,可降低患者疼痛阈值,增加伤害性感受器的自发活动[21-22]。同时焦虑、抑郁患者可激活前扣带回、眶额叶皮层、脑岛及海马区,而焦虑、抑郁及疼痛三者激活的脑回路有所重叠,因此焦虑、抑郁患者所出现的心理应激,可下调额叶皮层苯二氮受体,激活下丘脑-垂体-肾上腺素轴,从而影响患者术后疼痛水平[23-24]。②血清TNF-α、IL-6高表达。研究显示TNF-α可通过激活p38-丝裂原活化蛋白激酶,促进钠离子内流,降低兴奋性阈值[25]。同时TNF-α可提高谷氨酸诱导的兴奋性电流,从而造成神经病理性疼痛,增加患者术后疼痛[26]。据动物实验研究显示,IL-6是向高级中枢提供伤害信息的神经递质[27]。IL-6除了可以与TNF-α发挥相似兴奋电流作用外,还可增加环磷腺苷反应元件结合蛋白磷酸化产生热敏痛,影响患者术后疼痛[28-29]。但本研究未能发现肿瘤部位与术后疼痛的关系。有相关研究显示,肿瘤部位可能与疼痛程度相关,其认为肿瘤位于上象限的患者肿瘤部位与腋窝及上肢较近,患者因病灶切除及腋窝淋巴结清扫,术后需进行加压包扎,放置引流管,从而限制患者手术活动范围,稍做运动便会引起疼痛[30-31]。
可能与本研究纳入患者较少有关,未来可联合多中心、延长研究时间、增加患者数量,进而提高临床研究价值。
综上所述,超声引导下PECS Ⅱ能够有效延长机器人辅助下乳腺癌根治术患者术后镇痛时间,减少麻醉药物用量,可减轻患者术后炎症反应,且术前焦虑或抑郁、血清TNF-α、IL-6水平与患者术后疼痛密切相关。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:李珍负责设计论文框架,起草论文,论文修改,实验操作及研究过程的实施;徐嘉蕾负责数据收集,统计学分析,绘制图表;丁亮负责拟定写作思
路,指导撰写文章并最后定稿。
参考文献
[1] 张聚良, 杨柳, 张明坤, 等. 达芬奇机器人在乳腺癌根治切除并一期假体重建中的临床应用[J]. 中国普外基础与临床杂志, 2022, 29(11): 1415-1420.
[2] Tavares Mendonça F, de Assis Feitosa Junior A, Nogueira H, et al. Efficacy of type-I and type-II pectoral nerve blocks (PECS I and II) in patients undergoing mastectomy: a prospective randomised clinical trial[J]. Anaesthesiol Intensive Ther, 2022, 54(4): 302-309.
[3] YU L L, ZHOU Q, LI W, et al. Effects of esketamine combined with ultrasound-guided pectoral nerve block type II on the quality of early postoperative recovery in patients undergoing a modified radical mastectomy for breast cancer: a randomized controlled trial[J]. J Pain Res, 2022, 11(15): 3157-3169.
[4] Wagener G, Bezinover D, Wang C, et al. Fluid management during kidney transplantation: a consensus statement of the committee on transplant anesthesia of the american society of anesthesiologists[J]. Transplantation, 2021, 105(8): 1677-1684.
[5] 中国抗癌协会乳腺癌专业委员会. 中国抗癌协会乳腺癌诊治指南与规范(2021年版)[J].中国癌症杂志, 2021, 31(10): 954-1040.
[6] Rades D, Staackmann C, Schild S E. Karnofsky performance score-an independent prognostic factor of survival after palliative irradiation for sino-nasal cancer[J]. Anticancer Res, 2021, 41(5): 2495-2499.
[7] Shafshak T S, Elnemr R. Likert scale vs visual analog scale for assessing facial pleasantness[J]. J Clin Rheumatol, 2021, 27(7):
282-285.
[8] Campo-Arias A, Pinto-Vásquez H L, Pedrozo-Pupo J C. Confirmatory factor analysis of the brief spanish zung self-rating depression scale in patients with chronic pulmonary obstructive disease[J].Perspect Psychiatr Care, 2022, 58(3): 889-893.
[9] Campo-Arias A, Blanco-Ortega J D, Pedrozo-Pupo J C. Brief spanish zung self-rating anxiety scale: dimensionality, internal consistency, nomological validity, and differential item functioning among chronic obstructive pulmonary disease patients in colombia[J]. J Nurs Meas, 2022, 30(3): 407-418.
[10] Xu Z, Sandler D P, Taylor J A. Blood DNA methylation and breast cancer: a prospective case-cohort analysis in the sister study[J]. J Natl Cancer Inst, 2020, 112(1): 87-94.
[11] LU Z H, WANG Q, SUN X D, et al. Transcutaneous electrical acupoint stimulation before surgery reduces chronic pain after mastectomy: A randomized clinical trial[J]. J Clin Anesth, 2021, 11(74): 110453.
[12] Sethuraman R M. Pectoral nerve blocks for breast augmentation surgery: comment[J]. Anesthesiology, 2023, 138(2): 220-221.
[13] ZHANG Z Y, LI Z Y, ZHANG Z X, et al. Analgesic efficacy of pectoral nerve blocks in implant-based mammoplasty: a systematic review and meta-analysis[J]. Aesthetic Plast Surg, 2023, 47(1):
106-115.
[14] Campos J H, Seering M. A novel technique for postoperative analgesia in video-assisted thoracoscopic surgery: “A modified pectoral nerve block”[J]. J Cardiothorac Vasc Anesth, 2022, 36(2): 497-499.
[15] HUANG Z, CAI Y, YANG Y F, et al. Effects of ultrasound-guided lumbar-sciatic nerve block and epidural anesthesia on the levels of IL-6, IL-8, TNF-alpha and coagulation factors in peripheral blood of elderly patients after hip arthroplasty[J]. J Med Biochem, 2022, 41(4): 433-440.
[16] Tantri A R, Rahmi R, Marsaban A H M, et al. Comparison of postoperative IL-6 and IL-10 levels following Erector Spinae Plane Block (ESPB) and classical Thoracolumbar Interfascial Plane (TLIP) block in a posterior lumbar decompression and stabilization procedure: a randomized controlled trial[J]. BMC Anesthesiol, 2023, 23(1): 13.
[17] Kuş A, Yörükoğlu U H, Aksu C, et al. The effect of thoracic paravertebral block on seroma reduction in breast surgery-a randomized controlled trial[J]. Braz J Anesthesiol, 2020, 70(3): 215-219.
[18] 王辉, 廖永锋, 程飞. 全身麻醉联合胸椎旁神经阻滞对胸腔镜肺叶切除患者术后炎症因子及疼痛介质水平的影响[J]. 临床和实验医学杂志, 2023, 22(12): 1334-1338.
[19] 胡建, 邢凡, 丰陈, 等. 胸神经阻滞对乳腺癌根治性切除术患者镇痛效果及免疫功能影响[J].重庆医学, 2023, 52(11):
1671-1676.
[20] LIU Y H, LI G, LIU Z Y, et al. Efficacy of ultrasound-guided intercostal nerve block on pain management and physiological outcomes in breast cancer mastectomy: A retrospective study[J]. Medicine (Baltimore), 2024, 103(31): e38738.
[21] Reynolds C A, Minic Z. Chronic pain-associated cardiovascular disease: the role of sympathetic nerve activity[J]. Int J Mol Sci, 2023, 24(6): 5378.
[22] WANG C, WEI M, WU Y, et al. Epigenetic up-regulation of ADAMTS4 in sympathetic ganglia is involved in the maintenance of neuropathic pain following nerve injury[J]. Neurochem Res, 2023, 48(8): 2350-2359.
[23] Boakye P A, Tang S J, Smith P A. Mediators of neuropathic pain; focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β[J]. Front Pain Res (Lausanne), 2021, 25(2): 698157.
[24] Kayacan Y, Derebasi D G, Ucar C, et al. The hypothalamic-pituitary-adrenal axis activity in archers: cortisol release, stress, anxiety and success[J]. J Sports Med Phys Fitness, 2022, 62(1):
139-148.
[25] Akhter N, Wilson A, Arefanian H, et al. Endoplasmic reticulum stress promotes the expression of TNF-alpha in THP-1 cells by mechanisms involving ROS/CHOP/HIF-1alpha and MAPK/NF-kappaB pathways[J]. Int J Mol Sci, 2023, 24(20): 15186.
[26] 张悦, 周冬梅, 李伟, 等. 肿瘤坏死因子-α在糖尿病痛性神经病变患者中的表达及其意义[J]. 中国实验诊断学, 2020, 24(1): 36-40.
[27] YE G Y, LIN C Y, ZHANG Y, et al. Quercetin alleviates neuropathic pain in the Rat CCI model by mediating AMPK/MAPK pathway[J].
J Pain Res, 2021, 19(14): 1289-1301.
[28] SUN J, ZHOU Y Q, XU B Y, et al. STING/NF-kappaB/IL-6-Mediated inflammation in microglia contributes to spared nerve Injury (SNI)-induced pain initiation[J]. J Neuroimmune Pharmacol, 2022, 17(3-4): 453-469.
[29] 韩庆波, 李永敏, 刘洋, 等. 基于术后疼痛及应激介质分泌研究帕瑞昔布钠超前镇痛用于腹腔镜手术的价值[J]. 海南医学院学报, 2020, 26(8): 598-601.
[30] Ranieri G, Laface C, Fazio V, et al. Local treatment with deep percutaneous electrochemotherapy of different tumor lesions: pain relief and objective response results from an observational study[J]. Eur Rev Med Pharmacol Sci, 2020, 24(14): 7764-7775.
[31] Jacobs A, Lemoine A, Joshi G P, et al. PROSPECT guideline for oncological breast surgery: a systematic review and procedure-specific postoperative pain management recommendations[J]. Anaesthesia, 2020, 75(5): 664-673.
收稿日期:2024-10-30
编辑:赵敏
基金项目:陕西省自然科学基金项目(2019JM-sf-089)
Foundation Item: Natural Science Foundation of Shaanxi Province (2019JM-sf-089)
通讯作者:丁亮,Email:285459392@qq.com
Corresponding Author: DING Liang, Email: 285459392@qq.com
引用格式:李珍,徐嘉蕾,丁亮. 机器人辅助下乳腺癌根治术患者行超声引导下Ⅱ型胸神经阻滞术后镇痛效果及影响因素[J].机器人外科学杂志(中英文),2025,6(1):45-52.
Citation: LI Z, XU J L, DING L. Postoperative analgesic effect and influencing factors of ultrasound-guided pectoral nerve block type Ⅱ in patients undergoing robot-assisted radical mastectomy[J]. Chinese Journal of Robotic Surgery, 2025, 6(1): 45-52.

