天然抑菌剂在鲜食微藻中应用的可行性分析

摘要:微藻营养丰富,目前市售产品主要以高温干燥处理后的微藻粉制得,然而干燥过程会导致活性成分大量流失,营养价值大打折扣,因此,基于新鲜微藻开发的鲜食产品极具市场价值。在鲜食微藻保藏过程中,防腐且保留藻细胞活性是面临的首要问题。天然抑菌剂因为绿色安全已经被应用于水产品、肉制品、奶制品等食品中,具有良好的防腐保鲜效果。文章分析了几种天然抑菌剂的抑菌机制及其应用现状,并分析了将其应用于鲜食微藻的可行性。
关键词:鲜食微藻;天然抑菌剂;微藻防腐;保鲜
中图分类号:TS202.3""""" 文献标志码:A"""" 文章编号:1000-9973(2024)11-0195-05
Feasibility Analysis of Application of Natural Antimicrobial Agents in
Fresh-Eating Microalgae
LIU Ya-nan1, ZHOU Zhen-zhen2*, ZHANG Jun-jie1, CONG Wei2, DUAN Rui1
(1.School of Ocean Food and Biological Engineering, Jiangsu Ocean University, Lianyungang 222005,
China; 2.Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China)
Abstract: Microalgae is rich in nutrients. At present, commercially available products are mainly made from microalgae powder after high-temperature drying treatment. However, the drying process can lead to a significant loss of active components, greatly reducing its nutritional value. Therefore, fresh-eating products developed based on fresh microalgae are of great market value. During the preservation of fresh-eating microalgae, anti-corrosion and retaining algal cell activity is the primary issue. Natural antimicrobial agents have been applied in aquatic products, meat products, dairy products and other foods due to their green and safe nature, and have good anti-corrosion and preservation effects. In this paper, the antimicrobial mechanism and the application status of several natural antimicrobial agents are analyzed, and the feasibility of applying them to fresh-eating microalgae is analyzed.
Key words: fresh-eating microalgae; natural antimicrobial agent; anti-corrosion of microalgae; preservation
收稿日期:2024-05-28
基金项目:国家重点研发计划(2022YFD1300703)
作者简介:刘亚楠(1999—),女,硕士,研究方向:农(水)产品贮藏与保鲜。
*通信作者:周真真(1991—),女,助理研究员,博士,研究方向:微藻产品加工与应用。
微藻胞内营养丰富,作为可食用资源已有几百年的历史[1]。螺旋藻中蛋白质含量高达60%~70%,含有全部必需氨基酸,且与人体所需的比例极为接近[2],蛋白核小球藻中蛋白质含量高达60%,多糖含量约为25%,可用作优质的营养补充剂。
为了便于保存和运输,目前市场上的微藻多为干燥状态,主要通过喷雾干燥的方式将采收的微藻泥脱水。高温能快速达到干燥的效果且去除杂菌,但会导致胞内的热敏性成分损失高达70%[3-6]。为了较完整地保留微藻的营养成分,开发鲜食微藻产品逐渐引起人们的关注。
鲜食微藻是将采收后的湿藻泥不经干燥直接制成可食用形态。湿藻很难常温储存,一方面,藻细胞代谢会消耗胞内营养;另一方面,湿藻由于营养全面会不断富集环境中的细菌和霉菌,导致藻泥腐败。冷冻保存虽然可以解决上述问题,但解冻后细胞破碎,胞内物质溶出,难以制成鲜藻产品。因此,在适宜的存储温度下添加抑菌剂有望解决微藻的存储保鲜问题。
1 微藻制品中微生物污染的来源及种类
1.1 微生物污染的来源
微藻的微生物污染主要来源于培养过程和采收过程。微藻的规模化培养主要采用开放式和半开放式,无法避免环境中的浮游植物、细菌和原生动物等的污染,且微藻能通过光合作用产生胞外多糖和蛋白质等有机质,进而富集环境中的微生物,导致污染严重[7],微藻的采收主要采用自然沉降法、离心法和过滤法等。自然沉降法是浓缩藻液在自然环境中依靠重力将上清液与藻细胞分离[8],此过程中的微生物污染类似培养过程;离心法是将藻液高速旋转达到分离效果,配合过滤洗涤,去除一部分微生物,但该过程可能会受到空气中微生物的污染[9];过滤法是将藻液通过带孔的膜进行分离,培养液通过膜而藻细胞被截留[10],根据藻细胞和污染物的尺寸选择膜孔径,从而除去部分污染物,但无法保证完全无菌。此外,鲜藻产品加工过程中由于加工者的操作不当可能会发生交叉污染,如金黄色葡萄球菌(Staphylococcus aureus)等致病菌的污染[11],通过严格要求加工者规范操作,保障生产车间的卫生安全,可以降低此类污染发生的概率。
1.2 微生物的种类
不同藻种培养过程中污染的微生物种类不同。研究表明,螺旋藻培养液中的污染主要来源于细菌和原生动物,主要细菌为变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)等,原生动物种类主要是褶皱臂尾轮虫(Brachionus plicatilis)和异叶足变形虫(Euplaesiobystra hypersalinica)[12]。蛋白核小球藻培养液中污染的细菌主要有寄生细菌吸血弧菌(Vampirovibrio chlorellavorus)[13]、假单胞菌属(Pseudomonas)[14]、梭状芽孢杆菌(Clostridium)[15]、非硫紫色细菌(Rhodopseudomonas sp.)[16];且容易被其他蓝藻污染,如铜绿微囊藻(Microcystis aeruginosa)产的微囊藻毒素(microcystin)对人体有毒[17],不能食用;此外,还有原生动物如纤毛虫等的污染,但可过滤去除[7]。
湿藻存储时,除了自身携带的伴生菌外,还会受到环境中其他腐败菌的污染。在一项连续两年对新鲜和干燥的可食用海藻Alariaes culenta和Saccharina latissimi进行微生物存在评估的研究中,发现鲜藻储存时主要的腐败菌为假单胞菌属(Pseudomonas)、芽孢杆菌属(Bacillus)、葡萄球菌属(Staphylococcus)和微球菌属(Micrococcus),并且检测出了酵母菌属(Saccharomyces)[18]。因此,采用抑菌剂控制湿藻中的微生物对于延长其保藏期具有重要的意义。
2 天然抑菌剂的抑菌机制和在食品中的应用
在选择生鲜产品时,消费者对天然、绿色、健康、无污染、有“清洁标签”的食品越来越感兴趣[19]。从植物、动物或微生物中提取的天然抗菌剂具有绿色天然、安全无毒和广谱抗菌的特性,正在成为食品保鲜的首选[20]。
2.1 ε-聚赖氨酸的抑菌机制及应用
ε-聚赖氨酸(ε-PL)是L-赖氨酸的-α-羧基与-ε-氨基通过酰胺键连接而成的天然强效抑菌物质,由白色链霉菌(Streptomyces albus)发酵产生[21-22]。ε-PL带正电荷,可以与细胞膜上带负电荷的磷脂分子头部以静电作用相结合[23],破坏细胞膜的完整性,使菌体自溶死亡,还可能进入细胞质与遗传物质作用影响其正常功能[24]。ε-PL是一种广谱抑菌剂,对多种细菌、酵母菌和真菌有抑制作用[25-27]。
ε-PL作为符合GB 2760—2014的食品添加成分,目前已有广泛的应用。ε-PL能有效地抑制冷鲜鳙鱼片中假单胞菌(Pseudomonas)和希瓦氏菌(Shewanella)的繁殖[28]。喷洒1 000 mg/L的ε-PL可将赣南脐橙的保鲜期延长至180 d,坏果率仅为38.89%[29]。此外,还可与其他抗菌物质复配制成更高效的抑菌剂。Hu等[30]将ε-PL和大豆蛋白复配制成静电复合物并应用于柑橘的保鲜,仅需2.5 mg/L ε-PL即可抑制大肠杆菌(Escherichia coli)、金黄色葡萄球菌(S. aureus)的活性。尹卓凡[31]研究表明,与单独使用ε-PL相比,香芹酚/ε-PL纳米乳液的抗菌活性更高,对金黄色葡萄球菌(S. aureus)、肠出血性大肠杆菌(Enterohemorrhagic E.coli)和黑曲霉(Aspergillus niger)有明显的抑制作用,对芒果的保鲜效果良好。综上,单独或复配使用ε-PL可以有效提高生鲜食品的防腐保鲜效果。
2.2 溶菌酶的抑菌机制及应用
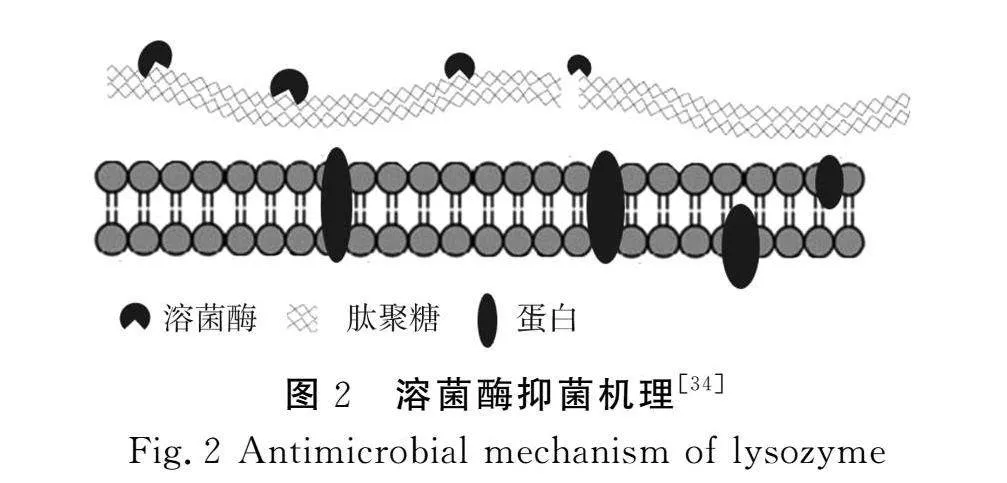
溶菌酶是一种能水解细菌黏多糖的碱性酶,主要从动物乳汁和禽类蛋白中提取。其肽链中Glu-35和Asp-52所构成的活性中心可以水解细菌细胞壁中N-乙酰氨基葡萄糖(NAG)和N-乙酰氨基胞壁酸(NAM)残基构成的β-(1,4)-糖苷键,使不溶性的黏多糖骨架结构断裂,进而导致细胞破裂[32-33]。
研究表明,浓度为0.4%的溶菌酶可抑制霉菌和酵母菌的生长,可用于高品质酸奶的生产[33]。溶菌酶在果蔬和水产品等保鲜中的应用研究也有许多报道。Xu等[35]将猕猴桃在0.08%溶菌酶中浸泡2 min储存20 d后,腐烂发生率降低60%。三文鱼保鲜存储时,在新鲜三文鱼的表面涂抹浓度为0.1%~0.7%的溶菌酶可抑制菌落总数的增长[36]。从溶菌酶与阴离子防御肽(GMAP2)的研究中发现,与抗革兰氏阳性菌活性水平相比,溶菌酶对革兰氏阴性菌的作用较弱,而与GMAP2联用可以协同对抗革兰氏阴性菌[37]。因此,溶菌酶需要与其他防腐剂联用以提高在食品中的抗菌效果。将溶菌酶与壳聚糖联用于草莓的保鲜中发现,活性比游离溶菌酶增强了256%,金黄色葡萄球菌(S. aureus)和单核细胞增生李斯特氏菌(Listeria monocytogenes)的抑菌圈增加1 mm左右[38]。溶菌酶和纳米纤维素联用可显著增强抗细菌和真菌的活性[39],与植酸联用时可将草鱼片的保质期延长2 d[40]。
2.3 纳他霉素的抑菌机制及应用
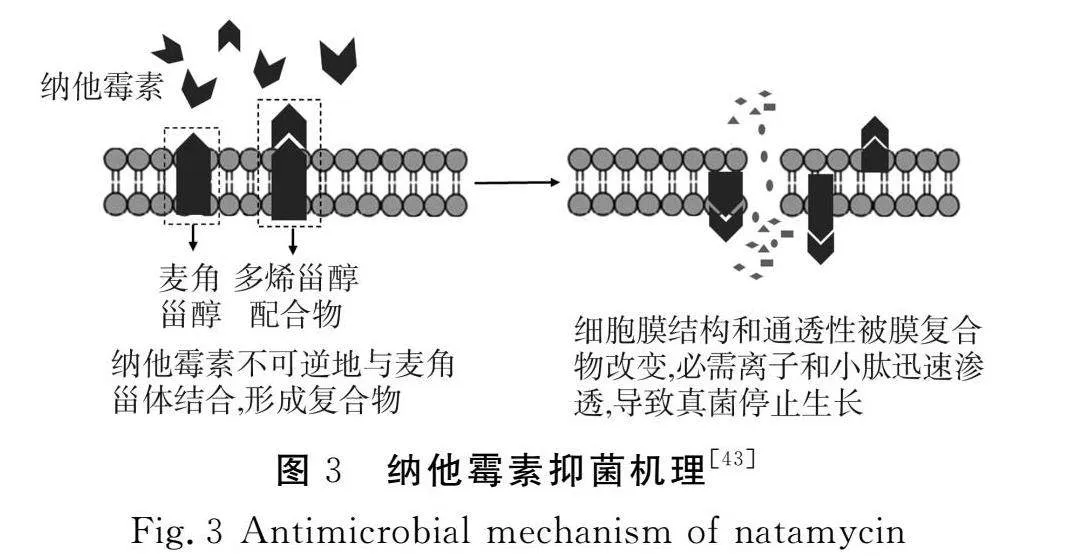
纳他霉素是由纳他链霉菌(Streptomyces natalensis)产生的一种天然多烯大环内酯类生物杀菌剂,对人体毒性较低,被美国食品药品监督管理局(FDA)认定为“普遍认为安全(GRAS)”的物质[41]。目前已在40多个国家被批准作为食品添加剂。纳他霉素通过与霉菌(酵母菌)等真菌细胞膜上的甾醇,尤其是麦角甾醇,结合形成复合物,改变细胞膜的通透性,从而杀死细胞[42],对细胞膜上无甾醇的细菌和病毒无效。
纳他霉素通常用于食品的表面处理,通过喷洒或浸泡即可有效地抑制霉菌和酵母菌。使用500 mg/L纳他霉素可将酸腐病发生率降低近30%[44]。含70%纳他霉素的薄膜可有效降低奶酪表面黑曲霉的生长速度[45]。纳他霉素浓度高于2.23 mg/L时可以显著抑制导致蓝莓腐败的链格孢霉菌(Alternaria)的分生孢子萌发和生长,从而延长蓝莓的保藏期。食品保鲜不仅要抑制真菌,而且需抑制细菌。因此,将纳他霉素与细菌抑菌剂如ε-PL和溶菌酶等联合使用,可以扩展抑菌范围,从而增强防腐保鲜效果。例如Wu等[46]将纳他霉素与ε-PL复配后应用于番茄炒蛋中可有效抑制假单胞菌(Pseudomonas)、不动杆菌(Acinetobacter)、镰刀菌(Fusarium)和曲霉(Aspergillus)等典型的腐败/病原微生物。
2.4 乳酸链球菌素的抑菌机制及应用
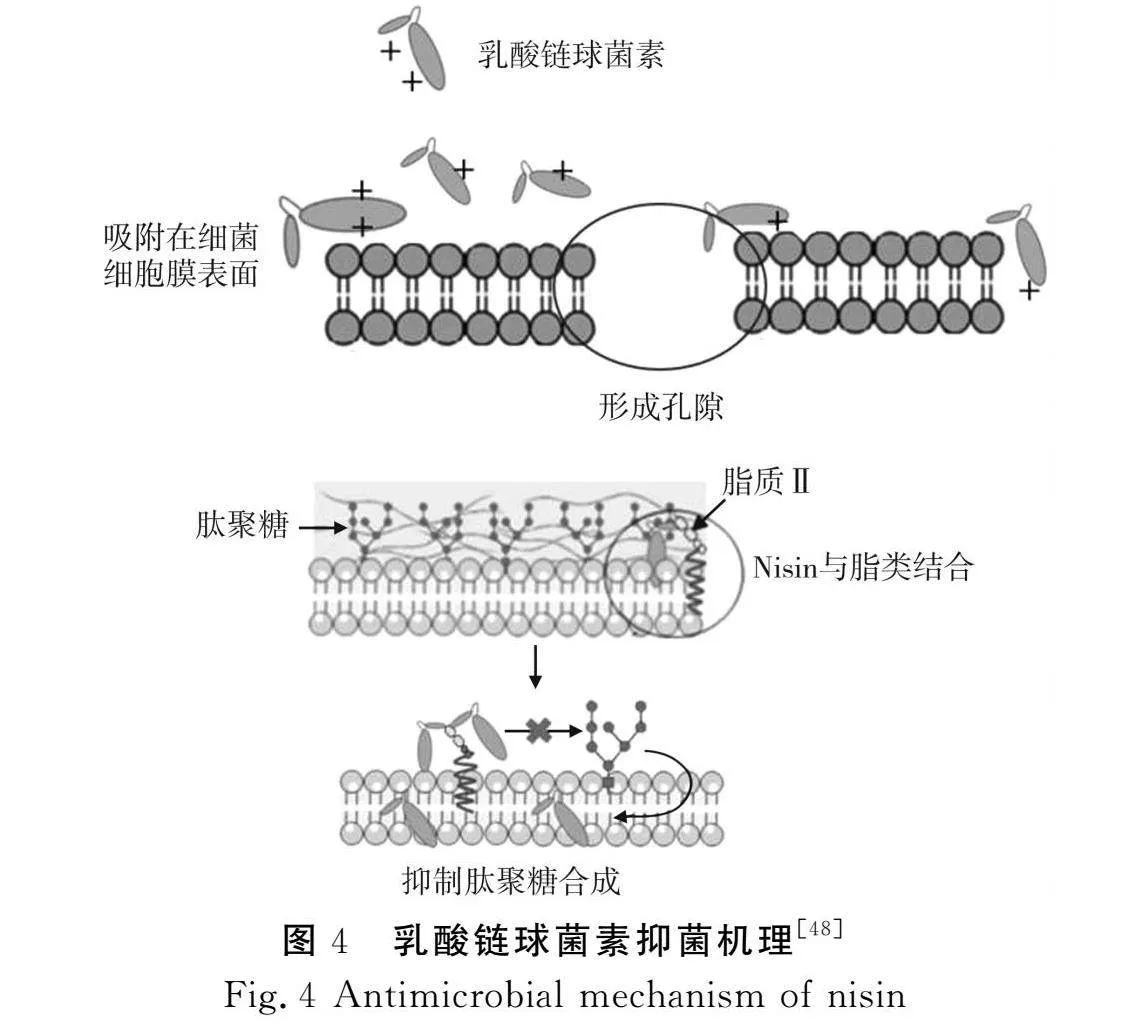
乳酸链球菌素由34个氨基酸残基组成,是乳酸菌产生的一种天然抗菌肽[47]。抑菌机理基于细菌膜水平产生双重作用机制:一方面,带正电的乳酸链球菌素与带负电的细菌细胞膜表面通过静电相互作用,形成一定孔径的通道,细胞质内小分子和离子通过孔道流失,导致细胞溶解死亡。另一方面,乳链菌肽的N末端与位于细菌细胞质膜上的肽聚糖前体脂质Ⅱ相互作用,使乳链菌肽的C末端穿过细菌细胞质膜,抑制肽聚糖合成[48]。
仅添加12 mg/L的乳酸链球菌素即可抑制干酪中的蜡状芽孢杆菌(Bacillus cereus)和金黄色葡萄球菌(S. aureus)[49]。应用于火腿表面时,乳酸链球菌素可使金黄色葡萄球菌(S. aureus)和单核细胞增生李斯特氏菌(Listeria monocytogenes)分别减少约5.53,5.62 log10 CFU/cm2[50]。乳酸链球菌素能有效地抑制革兰氏阳性菌和芽孢的生长和繁殖,而对革兰氏阴性菌、霉菌和酵母菌的抑制作用不明显。根据此特性可以与纳他霉素和溶菌酶等生物防腐剂配制成更广谱的抑菌剂制品。赵长青等[51]将0.50 g/kg乳酸链球菌素与0.50 g/kg溶菌酶复配后用于猪肉干的储存,在3个月的贮藏期内未检测出霉菌和金黄色葡萄球菌。也有研究表明,2.5%乳酸链球菌素和50%植酸具有协同作用,可有效减少冷藏牛肉中革兰氏阳性菌如大肠杆菌的数量[52]。
3 天然抑菌剂在鲜食微藻中应用的潜力
3.1 鲜食螺旋藻
螺旋藻是螺旋状多细胞蓝藻,为原核生物,没有细胞膜,细胞壁主要由粗蛋白和纤维素组成[53]。筛选鲜食螺旋藻抑菌剂时,要求抑菌剂不能破坏螺旋藻的细胞活性,根据上述生物防腐剂的抑菌机制,ε-PL能抑制鲜食螺旋藻中的杂菌,且不破坏螺旋藻细胞活性,因此有望成为鲜食螺旋藻的抑菌剂。
溶菌酶杀菌的作用位点与螺旋藻细胞壁的结构不一致,因此也不影响螺旋藻活性。螺旋藻泥存储过程中污染的酵母和霉菌使用适宜浓度的纳他霉素即可有效地清除。此外,溶菌酶和乳酸链球菌素在理论上也不会影响螺旋藻活性,可与ε-PL和纳他霉素联用,提高抑菌效率。
3.2 鲜食小球藻
蛋白核小球藻属于真核细胞,具有细胞膜结构,细胞膜的阴离子可能与ε-PL反应导致藻细胞裂解死亡,因此ε-PL不适用于小球藻。
纳他霉素属于内酯类抗生素,会迫使小球藻细胞脂质过氧化程度加剧,影响小球藻细胞中叶绿素合成、DNA复制与修复相关基因的表达[54]。因此,纳他霉素不适合作为蛋白核小球藻的抑菌剂。
溶菌酶主要作用于细胞壁的肽聚糖,而蛋白核小球藻的细胞壁成分主要为几丁质,因此不会抑制蛋白核小球藻的生长,可以应用于蛋白核小球藻的鲜食产品中。但由于其抑菌范围有限,必须配合使用其他广谱性的抑菌剂,例如脱氢乙酸钠等,才能达到食品抑菌效果。已有研究表明,脱氢乙酸钠可以降低培养过程中对无菌性的要求,且促进微藻生长[55]。
虽然目前天然抑菌剂在微藻产品中应用的研究还比较少,但随着研究的深入,天然抑菌剂将在鲜食微藻产品的开发中发挥重要的作用。
参考文献:
[1]VILLAR-COS S, GUZMN SNCHEZ J L, ACIN G, et al. Research trends and current requirements and challenges in the industrial production of Spirulina as a food source[J].Trends in Food Science amp; Technology,2024,143(2):104280.
[2]TAVAKOLI S, HONG H, WANG K, et al. Ultrasonic-assisted food-grade solvent extraction of high-value added compounds from microalgae Spirulina platensis and evaluation of their antioxidant and antibacterial properties[J].Algal Research,2021,60:102493.
[3]LARROSA A P Q, COMITRE A A, VAZ L B, et al. Influence of air temperature on physical characteristics and bioactive compounds in vacuum drying of Arthrospira spirulina[J].Journal of Food Process Engineering,2017,40(2):12359.
[4]COSTA B R, ROCHA S F, RODRIGUES M C K, et al. Physicochemical characteristics of the Spirulina sp. dried in heat pump and conventional tray dryers[J].International Journal of Food Science amp; Technology,2016,50(12):2614-2620.
[5]PAPADAKI S, KYRIAKOPOULOU K, STRAMARKOU M, et al. Environmental assessment of industrially applied drying technologies for the treatment of Spirulina platensis[J].Journal of Environmental Science, Toxicology and Food Technology,2017,11(1):41-46.
[6]FBIO F N, MARIANA D, GIUSTINO T. Drying and Quality of Microalgal Powders for Human Alimentation[M]//MILADA V. Microalgae—From Physiology to Application,Rijeka:Intech Open,2019:4.
[7]WANG H, ZHANG W, CHEN L, et al. The contamination and control of biological pollutants in mass cultivation of microalgae[J].Bioresource Technology,2013,128:745-750.
[8]YIN Z H, ZHU L D, LI S X, et al. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions[J].Bioresource Technology,2020,301(6):122804.
[9]LIU Z Y, HAO N H, HOU Y Y, et al. Technologies for harvesting the microalgae for industrial applications:current trends and perspectives[J].Bioresource Technology,2023,387(14):129631.
[10]UDAYAN A, SIROHI R, SREEKUMAR N, et al. Mass cultivation and harvesting of microalgal biomass: current trends and future perspectives[J].Bioresource Technology,2022,344(15):126406.
[11]AIYEGORO O A. Microbial Contamination of Processed meat[M]//DIKEMAN M. Encyclopedia of Meat Sciences (Third Edition), Oxford: Elsevier,2024:484-490.
[12]YUAN D N, YAO M M, WANG L, et al. Effect of recycling the culture medium on biodiversity and population dynamics of bio-contaminants in Spirulina platensis mass culture systems[J].Algal Research,2019,44:101718.
[13]HIRAYAMA A, SUEYOSHI M N, NAKANO T, et al. Development of large-scale microalgae production in the Middle East[J].Bioresource Technology,2022,343(4):126036.
[14]ZHOU W L, QIAO X T, SUN J F, et al. Ecological effect of Z-QS01 strain on Chlorella vulgaris and its response to UV-B radiation stress[J].Procedia Environmental Sciences,2011,11(6):741-748.
[15]MU R M, FAN Z Q, PEI H Y, et al. Isolation and algae-lysing characteristics of the algicidal bacterium B5[J].Journal of Environmental Sciences,2007,19(11):1336-1340.
[16]HAN D X, BI Y H, HU Z Y. Industrial Production of Microalgal Cell-Mass and Secondary Products-Species of High Potential: Nostoc[M]//Handbook of Microalgal Culture: Biotechnology and Applied Phycology,Oxford:Wiley-Blackwell,2004:304-311.
[17]LIN S Q, PAN J L, LI Z H, et al. Characterization of an algicidal bacterium Brevundimonas J4 and chemical defense of Synechococcus sp. BN60 against bacterium J4[J].Harmful Algae,2014,37:1-7.
[18]LYTOU A E, SCHOINA E, LIU Y, et al. Quality and safety assessment of edible seaweeds Alaria esculenta and Saccharina latissima cultivated in Scotland[J].Foods,2021,10(9):2210.
[19]GRANT A Q, PARVEEN S. All natural and clean-label preservatives and antimicrobial agents used during poultry processing and packaging[J].Journal of Food Protection,2017,80(4):540-544.
[20]CHEN Y, MIAO W H, LI X X, et al. The structure, properties, synthesis method and antimicrobial mechanism of ε-polylysine with the preservative effects for aquatic products[J].Trends in Food Science amp; Technology,2023,139:104131.
[21]ZHANG Y Z, ZHAO C Q, ZHAO X X, et al. Application of ε-polylysine in extending the storage period of pork jerky[J].Food Science amp; Nutrition,2021,9(6):3250-3257.
[22]庄孝东,白森萌,李博彦,等.ε-聚赖氨酸微生物生产及其应用研究进展[J].中国抗生素杂志,2023,48(10):1081-1095.
[23]常森林.ε-聚赖氨酸的发酵生产、提取纯化及修饰改性的研究[D].北京:中国科学院大学,2022.
[24]WEI M L, GE Y H, LI C Y, et al. Antifungal activity of epsilon-poly-L-lysine on Trichothecium roseum in vitro and its mechanisms[J].Physiological and Molecular Plant Pathology,2018,103:23-27.
[25]LIU K W, ZHOU X J, FU M R. Inhibiting effects of epsilon-polylysine (ε-PL) on Pencillium digitatum and its involved mechanism[J].Postharvest Biology amp; Technology,2017,123:94-101.
[26]ZHANG L M, LI R C, DONG F, et al. Physical, mechanical and antimicrobial properties of starch films incorporated with ε-poly-L-lysine[J].Food Chemistry,2015,166:107-114.
[27]CHANG S S, LU W Y, PARK S H, et al. Control of foodborne pathogens on ready-to-eat roast beef slurry by ε-polylysine[J].International Journal of Food Microbiology,2010,141(3):236-241.
[28]LIU X C, LI D P, LI K F, et al. Monitoring bacterial communities in ε-polylysine-treated bighead carp (Aristichthys nobilis) fillets using culture-dependent and culture-independent techniques[J].Food Microbiology,2018,76:257-266.
[29]陈秋映,杨美艳,高向阳,等.ε-聚赖氨酸对赣南脐橙保鲜效果的研究[J].食品工业,2021,42(12):143-147.
[30]HU Q Y, JIN Y Y, XIAO Y W, et al. ε-Polylysine and soybean protein isolate form nanoscale to microscale electrostatic complexes in solution: properties, interactions and as antimicrobial edible coatings on citrus[J].International Journal of Biological Macromolecules,2023,253(1):126616.
[31]尹卓凡.香芹酚/ε-聚赖氨酸抗菌纳米乳液的制备及其在芒果保鲜中的应用[D].长春:吉林大学,2023.
[32]曹涛,刘同军,王艳君.微生物溶菌酶的研究及应用[J].中国调味品,2011,36(3):23-26,32.
[33]AWAD D A B, EL-HADARY A, OSMAN A, et al. Yogurt fortified with enzyme-modified egg white lysozyme impact on sensory, physicochemical, and microbiological properties and potential for functional product development[J].Journal of Agriculture and Food Research,2023,14(4):100670.
[34]王武,王碧,白瑜,等.改造溶菌酶抑菌活性研究进展[J].中国畜牧兽医,2023,50(3):942-951.
[35]XU F X, LIU S Y, LIU Y F, et al. Effectiveness of lysozyme coatings and 1-MCP treatments on storage and preservation of kiwifruit[J].Food Chemistry,2019,288:201-207.
[36]WANG Z X, HU S F, GAO Y P, et al. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation[J].LWT-Food Science and Technology,2017,75:59-64.
[37]ZDYBICKA-BARABAS A, MAK P, KLYS A, et al. Synergistic action of Galleria mellonella anionic peptide 2 and lysozyme against Gram-negative bacteria[J].Biochimica et Biophysica Acta (BBA)-Biomembranes,2012,1818(11):2623-2635.
[38]NIU X D, ZHU L, XI L J, et al. An antimicrobial agent prepared by N-succinyl chitosan immobilized lysozyme and its application in strawberry preservation[J].Food Control,2020,108(6):106829.
[39]JEBALI A, HEKMATIMOGHADDAM S, BEHZADI A, et al. Antimicrobial activity of nanocellulose conjugated with allicin and lysozyme[J].Cellulose,2013,20:2897-2907.
[40]SUN X H, HONG H, JIA S L, et al. Effects of phytic acid and lysozyme on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets stored at 4 ℃[J].Food Microbiology,2020,86(9):103313.
[41]ZHANG C L, GONG H S, LIU Y L. Effects of postharvest coating using chitosan combined with natamycin on physicochemical and microbial properties of sweet cherry during cold storage[J].International Journal of Biological Macromolecules,2022,214:1-9.
[42]SAITO S, WANG F, XIAO C L. Natamycin as a postharvest treatment to control gray mold on stored blueberry fruit caused by multi-fungicide resistant Botrytis cinerea[J].Postharvest Biology and Technology,2022,187:111862.
[43]WANG X H, SONG X J, ZHANG D J, et al. Preparation and characterization of natamycin-incorporated agar film and its application on preservation of strawberries[J].Food Packaging and Shelf Life,2022,32:100863.
[44]FERNNDEZ G, SBRES M, LADO J, et al. Postharvest sour rot control in lemon fruit by natamycin and an Allium extract[J].International Journal of Food Microbiology,2022,368:109605.
[45]FANG M H, WANG J L, FANG S, et al. Fabrication of carboxymethyl chitosan films for cheese packaging containing gliadin-carboxymethyl chitosan nanoparticles co-encapsulating natamycin and theaflavins[J].International Journal of Biological Macromolecules,2023,246:125685.
[46]WU W F, LI Y R, ZHU X Y, et al. Antimicrobial activity enabled by chitosan-ε-polylysine-natamycin and its effect on microbial diversity of tomato scrambled egg paste[J].Food Chemistry:X,2023,19:100872.
[47]UCAR Y, OZOGUL Y, DURMU M, et al.The effects of nisin on the growth of foodborne pathogens and biogenic amine formation: in vivo and in vitro studies[J].Food Bioscience,2021(2):101266.
[48]WU M J, MA Y, DOU X, et al.A review of potential antibacterial activities of nisin against Listeria monocytogenes: the combined use of nisin shows more advantages than single use[J].Food Research International,2023,164(1):112363.
[49]SEN C, RAY P R, HOSSAIN S, et al. Influence of nisin on water activity, textural and other quality attributes of paneer (Indian cottage cheese) during storage[J].Food and Humanity,2023,1(3):1134-1144.
[50]PATTANAYAIYING R, H-KITTIKUN A, CUTTER C N. Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods[J].International Journal of Food Microbiology,2015,207:77-82.
[51]赵长青,张益卓,代锦苹,等.天然复配防腐剂对三种口味猪肉干贮藏品质的影响[J].中国调味品,2023,48(12):7-15.
[52]ZHAO G, KEMPEN P J, ZHENG T, et al.Synergistic bactericidal effect of nisin and phytic acid against Escherichia coli O157:H7[J].Food Control,2023,144(10):109324.
[53]张立彬,甄二英,李振永.螺旋藻的营养价值及培养[J].饲料研究,2006(1):31-32.
[54]李吉平.蛋白核小球藻对典型大环内酯类抗生素胁迫的响应及机制[D].南京:南京林业大学,2023.
[55]王兆伟,彭小伟.一种微藻培养基及应用:中国,CN114621874B[P].2022-06-14.

