纳米短纤维复合水凝胶的制备及其生物医学应用






Preparation of short nanofiber composite hydrogels and their biomedical applications
摘要:
水凝胶以高水含量、卓越的生物相容性和药物包封性能而备受瞩目,由于其在力学性能与模拟细胞外基质(ECM)等方面存在不足,从而限制了其生物医学应用。引入具备高比表面积、高长径比和良好单分散性的纳米短纤维,是弥补这些局限并扩展其应用潜力的有效方法之一。为促进纳米短纤维复合水凝胶的开发和应用,本文综述了纳米短纤维的功能化方法及其与水凝胶的复合方式,并分类介绍了复合水凝胶在组织工程、药物递送及生物传感与检测这3个方面的生物医学应用。最后,结合短纤维复合水凝胶当前发展分析了其在生物医学领域应用中所面临的挑战和未来的发展前景。
关键词:
纳米纤维;功能化短纤维;水凝胶;组织工程;药物递送;传感与检测
中图分类号:
TS102.65
文献标志码:
A
文章编号: 1001-7003(2024)11-0056-12
DOI: 10.3969/j.issn.1001-7003.2024.11.007
收稿日期:
20240302;
修回日期:
20241009
基金项目:
中华人民共和国商务部茧丝绸发展专项一般项目(23070148-C)
作者简介:
于杨(1997),男,硕士研究生,研究方向为纤维增强水凝胶复合材料的制备及其抗肿瘤应用。通信作者:赵毅丽,讲师,yzhao@zstu.edu.cn。
高分子材料科学和纳米成形技术的迅速发展推动了水凝胶和纳米纤维在生物医学领域的广泛应用。水凝胶由亲水性聚合物交联形成,具备高含水量和孔隙率、良好的生物相容性及药物包封能力。通过设计控制其组成结构,可以创造出不同机械性能、降解速率和刺激—响应能力的智能水凝胶。然而,传统水凝胶在模拟细胞外基质(ECM)方面存在局限,且单一的物理化学交联常使其力学性能不足。因此,研究者借鉴宏观复合材料的纤维增强策略,将直径在数十至数百纳米内的纳米纤维整合入水凝胶中。这些纳米纤维具有较大的长径比和比表面积,可模拟细胞外基质的微结构,形成的纳米纤维复合水凝胶在药物递送和组织工程等生物医学应用中表现出卓越性能。如Jang等[1]通过将聚己内酯(PCL)与乙醇进行同轴静电纺丝,实现了纳米纤维在接收器表面的海藻酸钠/CaCO3凝胶薄层上逐层堆叠。这一方法有效地分散了纳米纤维,并将其整合入水凝胶之中,从而显著提升了最终复合材料的抗压强度和刚度。Zhang等[2]采用模具对聚乳酸—羟基乙酸共聚(PLCL)纳米纤维进行三维塑形,并利用负压技术实现角蛋白凝胶与纤维的紧密结合,创造了具有优良界面黏附力的双层结构。
然而,纳米纤维增强水凝胶仍面临几个关键挑战。首先,以静电纺丝为主的连续纳米纤维毡/膜,孔隙小,密度大,限制了气液物质交换[3]。其次,二维纳米纤维膜既难以模拟ECM复杂的微/纳结构,也无法应用于对材料三维形态有要求的领域。此外,将纤维稳定而均匀地分散于凝胶网络中,是构建稳定的纳米纤维复合水凝胶的一项技术挑战[4]。最后,纳米纤维长丝于水凝胶内部形成的聚集体会限制材料的可注射性,影响微创治疗应用[5]。
近年来,制备纳米短纤维并用于增强水凝胶的研究备受关注。通过施加高剪切应力来切割并制备均质化的纳米短纤维,是获取短纤维的基本方法之一[6-9]。为了提高长度一致性,研究者们进一步探索了冷冻切割法[10-12]和超声破碎法[13-15]。研究表明,均匀分散于水凝胶中的纳米短纤维,不仅改善了其分布不均和流动性受限的问题,也使三维构型纤维复合水凝胶具备可3D打印与可注射的性能[16,17]。然而,目前对纳米短纤维复合水凝胶材料的理解尚不全面。本文综述了纳米短纤维的功能化方法及其与水凝胶的复合方式对材料性能的影响,并概括了其在生物医学领域的应用局限性和未来发展前景。
1" 短纤维功能化
功能化的短纤维在水凝胶材料中扮演着多重角色,从药物或纳米颗粒物理掺杂、纤维表面改性、成纤聚合物改性,到特殊工艺制备的本征功能化短纤维,这些方法在提高水凝胶性能、模拟特定组织微结构、促进医学应用等方面发挥关键作用。
1.1" 物理掺杂
利用静电纺丝技术的高可拓展性,将纳米药物负载于纳米短纤维中作为水凝胶的次级负载平台,赋予水凝胶特定的功能。Ghaderinejad等[18]采用离心纺丝/冷冻切割技术,将含有超顺磁氧化铁磁性纳米颗粒(SPIONs)的PCL纳米短纤维混入海藻酸盐可注射水凝胶中,制得一种在外部磁场下可实现取向排列的复合材料,磁场取向后的磁性纳米纤维显著提高了水凝胶的模量,并加速了嗅黏膜间充质干细胞(OE-MSCs)的定向神经分化。
此外,功能化的纳米短纤维能更有效地模拟特定生理组织的胞外微结构。Cheng等[19]设计了一种旨在治疗骨缺损的酶催化交联丝素蛋白(SF)水凝胶,其中包含了负载硅纳米颗粒的丝素蛋白纳米短纤维(SiNPs@NFs)以增强其性能与模仿天然骨细胞ECM的结构,如图1所示,SiNPs@NFs(5%)的添加将水凝胶的压缩模量从30.9 kPa显著增加至234.6 kPa,同时还促进了细胞的黏附、增殖和成骨分化,为治疗大型颅骨缺损提供了一种新方法。
同时,静电纺丝技术可实现在短纤维内负载多种药物,在同一凝胶中发挥组合功效。Wei等[20]经静电纺丝/冷冻切割技术制备了含有软骨分化诱导剂KGN和抗炎药Celecoxib的聚乙二醇聚乳酸(PELA)短纤维(Fk,Fc),随后与骨髓间充质干细胞(MSC)共同培养组装形成细胞球体(CS),加入到透明质酸(HA)水凝胶中,形成Fk-CS@HA-FC纤维复合水凝胶。加入短纤维后,水凝胶的力储能模量从860 Pa增加至2 150 Pa,并为MSC分化、ECM沉积和软骨再生提供了必要的生化和机械条件,展现了治疗骨软骨缺损和骨关节炎的潜力。
纳米颗粒、生物及化学等药物被直接掺入纳米纤维,这种方法直观、简便,制备过程易于操作且具有高度的可扩展性,该技术对于纤维材料的选择具有较大的灵活性。然而,其局限性包括药物释放缺乏可控性和短纤维处理后药物负载量减少,这些问题需要在未来的研究中得到深入研究和改进。
1.2" 纤维表面改性
高比表面积是纳米纤维材料的显著优势之一,其在转化为短纤维时仍然保留,并且由于摆脱了连续结构的限制,短纤维可均匀分散并与水凝胶自由结合。Li等[21]通过等离子体
活化/冷冻切割制备了表面羧基密度和长度分布可控的PCL纳米短纤维,纤维表面引入马来酰亚胺(MAL)后与巯基化透明质酸(HA-SH)水凝胶结合形成MAL-PCL@HA纤维复合凝胶,显著改善其结构均一性与机械性能。此外,Wang等[22]通过静电纺丝/均质化技术得到的聚乳酸—甘醇酸(PLGA)短纤维在氨基化处理后(APLGA),亲水性提高、长度更短、直径略小,并且其表面的氨基与明胶甲基丙烯酸酯/氧化右旋糖酐(GM/ODex)水凝胶基质的醛基反应,使其分散性得以改善。
此外,将细胞因子药物偶联于纳米纤维表面是提高其稳定性、留存性并延缓失效的一种方法。Bruggeman等[23]将脑源性神经生长因子(brain-derived neuotrophyic factor,BDNF)分别以物理掺杂和胺—巯基共价固定两种方式负载于聚乳酸(PLA)短纤维上,掺入自组装肽(SAP)中制成短纤维复合凝胶支架,浸泡6 d后,共价固定的BDNF释放量仅有物理共混的1/12,凸显了该方法的长期稳定性。类似地,Wang等[24]同样利用胺—巯基共价固定将胶质细胞源性神经营养因子(glialcellline-derived neurotrophic factor,GDNF)固定在聚左旋乳酸(PLLA)纳米短纤维表面,加入到木聚糖水凝胶中形成复合支架,显著改善了多巴胺祖细胞(dopaminergic progenitors cells)的移植效果,提高了2倍存活率,促进轴突生长,展现出在神经退行性疾病治疗领域的巨大潜力。
表面功能化的短纤维,不仅可以实现细胞因子等药物的稳定负载,还能改善纤维—水凝胶的界面相互作用,提高了水凝胶的机械性能。然而,短纤维的功能化表面被水凝胶包裹,不与外界环境直接接触,在一定程度上限制了其功能的发挥。
1.3" 成纤聚合物改性
由于制造技术的限制,对纤维进行后续的复杂化学转化或加工可能面临一些实际困难。为应对这一问题,利用静电纺丝工艺的简便性和易成型性,通过对纳米纤维的基体材料进行选择、设计和预处理,有望创造出具有特定本征性能的功能化纤维。Zhang等[25]将环氧开环聚合(ROP)和原子转移自由基聚合(ATRP)合成的聚(2-二甲氨基)甲基丙烯酸乙酯(PDMAEMA)嵌段共聚物进行季铵盐化,得到PDMAEMA-Q,然后通过静电纺丝/均质化方法制备了PLA/PDMAEMA-Q纳米短纤维。季铵盐改性后的短纤维在羧甲基纤维素(CMC)水凝胶中表现出更强的亲水性和分散性,增强了相互作用,使复合水凝胶的储能模量G′从390 Pa提高至960 Pa。
1.4" 本征功能纳米短纤维
静电纺丝并不是制造纳米纤维的唯一方法,相比“由大变小”地将纤维长丝短切,利用生物合成[26-28]、大分子组装[29-31]或无机材料[32-34]生长等自然界中“由小变大”形成纳米纤维结构的反应与现象,开发具有本征功能性的纳米短纤维是一种新的思路。如Zhi等[35]通过大肠杆菌培养获得了大量长约900 nm的丝状噬菌体M13病毒纤维,在其表面偶联苯硼酸衍生物(DOCPBA),构建了对多元醇敏感的纳米纤维状生物偶合物PBA-M13,加入PVA溶液中通过硼酸酯动态共价键交联,制备了一种葡萄糖触发胰岛素释放的可注射水凝胶。Lee等[36]通过PCR扩增与重组蛋白技术制造了基于酵母Sup35和SSB/La蛋白的Sup35-SSB/La融合蛋白,经超声破碎、自组装后形成100~400 nm的管状SuPNPs蛋白纤维探针,通过生物素化或乙烯基化分散于链霉亲和素/丙烯酰胺水凝胶中复合,构建了可以检测SSB/La抗体标记的SuPNPs纤维复合水凝胶免疫检测平台。此外,无机纳米线也是常见的一种功能短纤维,Xue等[37]采用甲基丙烯酸化明胶(GelMA)作为水凝胶基质,以还原法制备的银纳米线(AgNW)作为导电掺杂剂,通过定向冷冻技术制备了一种用于肌肉缺损修复的各向异性导电水凝胶支架。
特殊工艺制备的纳米短纤维是静电纺丝技术的一个重要补充,通过将所得短纤维应用于水凝胶中,成功提高了整体性能,为实现具有特定性能的纳米复合材料提供了一种可行途径。
2" 复合方法
水凝胶与纳米短纤维的复合丰富了生物医学材料的设计与制备。水凝胶以三维结构、生物相容性和药物包封能力而著称,而纳米短纤维则具备微纳结构、高比表面积和各向异性,能够模拟细胞基质微环境。通过物理混合、共价交联等手段,巧妙地组合两者,充分发挥各自优势,为材料性能和应用提供新可能。
2.1" 物理共混
纳米短纤维复合水凝胶常使用简单物理共混实现纳米短纤维在水凝胶中的均匀分散。Poveda等[38]通过静电纺丝和湍流凝固域法分别制备了PLLA纳米纤维(PLLA-ES和PLLA-HT),均质短纤维化后超声分散于氨化明胶溶液中,经戊二醛交联得到短纤维复合凝胶。对比分析显示,PLLA-ES纤维比PLLA-HT结构更细致,比表面积更大,表现出更佳的分散性和增强效应,能够更好地嵌入凝胶基质以支持细胞生长。Regev等[39]在载玻片上均匀涂布明胶溶液,静电纺丝沉积牛血清白蛋白(BSA)纳米纤维,多次重复形成纤维明胶分层复合结构,冷冻切割成小方块后熔化,葡聚糖交联形成凝胶,制备了短纤维长度、浓度可调的复合水凝胶,提高了其弹性模量和凝胶化速度,同时保留了可注射性,适用于心肌、软骨等柔软组织再生。光刻技术在形成微观精细结构上有一定的优势,Matera等[40]将掺杂光引发剂I2959的乙烯基磺化改性葡聚糖(DexVS)静电纺纳米纤维沉积在玻璃板上,利用栅式光掩膜紫外交联得到定制长度的DexVS短纤维,分散在甲基丙烯酸酯—明胶(GelMA)溶液中,紫外辐照下形成DexVS/GelMA复合凝胶,丰富了凝胶支持微结构,提升了细胞的有序性和传播速率,如图2所示。
物理共混作为一种简单的结合方法,操作方便、易于实施,快速获得复合材料。然而,纤维与水凝胶之间的结合相对较弱,容易发生纤维团聚的现象,不仅降低了共混材料的均匀性,还可能破坏凝胶的结构,从而影响整体性能。
2.2" 共价交联
共价交联可在短纤维与凝胶基质间形成稳定的化学键,从而提高整体稳定性和耐久性。Wang等[22]通过在明胶甲基丙烯酸酯/氧化右旋糖酐(GM/ODex)水凝胶中引入氨基化PLGA短纤维(APLGA),与富含醛基的ODex发生席夫碱反应,制得了GM/ODex-APLGA原位水凝胶,展现出良好的纤维分散性和更均匀的孔隙,大幅提升了压缩模量。类似地,Juan等[41]以2,2,6,6-四甲基哌啶氧化物和碘酸盐对纤维素纳米纤维(CNFs)氧化改性,得到含醛基的纤维素纳米纤维(MCNFs),以该纤维作为交联剂与乙二醇壳聚糖(GC)之间发生席夫碱反应交联形成高自愈合效率、良好注射性及良好生物相容与降解性的GC-MCNF水凝胶。
共价作用在将短纤维与水凝胶结合方面提供了可靠的手段,形成更紧密的整体结构,使得复合材料在一些特定应用中表现出色。然而,这一方法的选材受到限制,需要选择能够有效形成共价键的材料,增加了研究和生产的复杂性。
2.3" 非共价作用
除了共价交联,非共价作用也广泛应用于纳米短纤维复合水凝胶的制备。利用氢键、范德华力等非共价相互作用手段,使纳米纤维与水凝胶基质发生相互作用,从而调控水凝胶的结构与性能。Li等[42]通过均质化处理将天然桑蚕丝短纤维化(SNF)并分散在壳聚糖(CS)溶液中,冷冻干燥制成SNF/CS复合支架。在添加SNF后,CS水凝胶的羟基红外吸收峰发生了位移,证明了氢键的形成。相较于纯CS支架,添加短纤维的多孔SNF/CS支架在干态和湿态下均表现出压缩模量和强度的显著提高。
仿生学为研究工作提供了丰富的启示。一些生物分子相互作用,由于其强烈的亲和力和特异性,被应用于新材料的开发。Lee等[36]通过基因技术构建编码Sup35-SSB/La融合蛋白的质粒表达载体,转化大肠杆菌以合成生物素化的bt-SuPNPs融合蛋白,自组装为短纤维后加入链霉亲和素/丙烯酰胺水凝胶中,利用链霉亲和素与生物素的特异结合使蛋白短纤维非共价固定在凝胶内,提高了SSB/La抗体检测的灵敏度与稳定性。
虽然在材料和功能化的选择上有所限制,但生物体内常见的生物基材料,如生物素、多糖和肽类物质,天然地以非共价作用结合,展现出高度特异性和生物亲和,为纤维复合水凝胶在生物医学领域的应用提供了有利特性。
2.4" 外界作用辅助混合
同样是一维纳米材料,短纤维相比长丝纳米纤维有着更大的可活动空间,在外界作用辅助下纳米短纤维聚合体能在水凝胶内部形成特殊的微环境。Islam等[43]开发了一种利用磁性纳米纤维水凝胶实现对干细胞分化动态调控的方法,如图3所示。首先通过静电纺丝/超声破碎制备了负载Fe3O4磁性颗粒的明胶纳米短纤维,将其与干细胞(ADSCs)混合注入丙烯酸改性明胶—甲基丙烯酸酐水凝胶基质中,光交联形成了短纤维/干细胞—水凝胶复合材料,可在磁场作用下,增强纤维间相互作用,形成取向结构,提升水凝胶的刚度并增强细胞多核化和肌管形成,从而促进成骨分化。
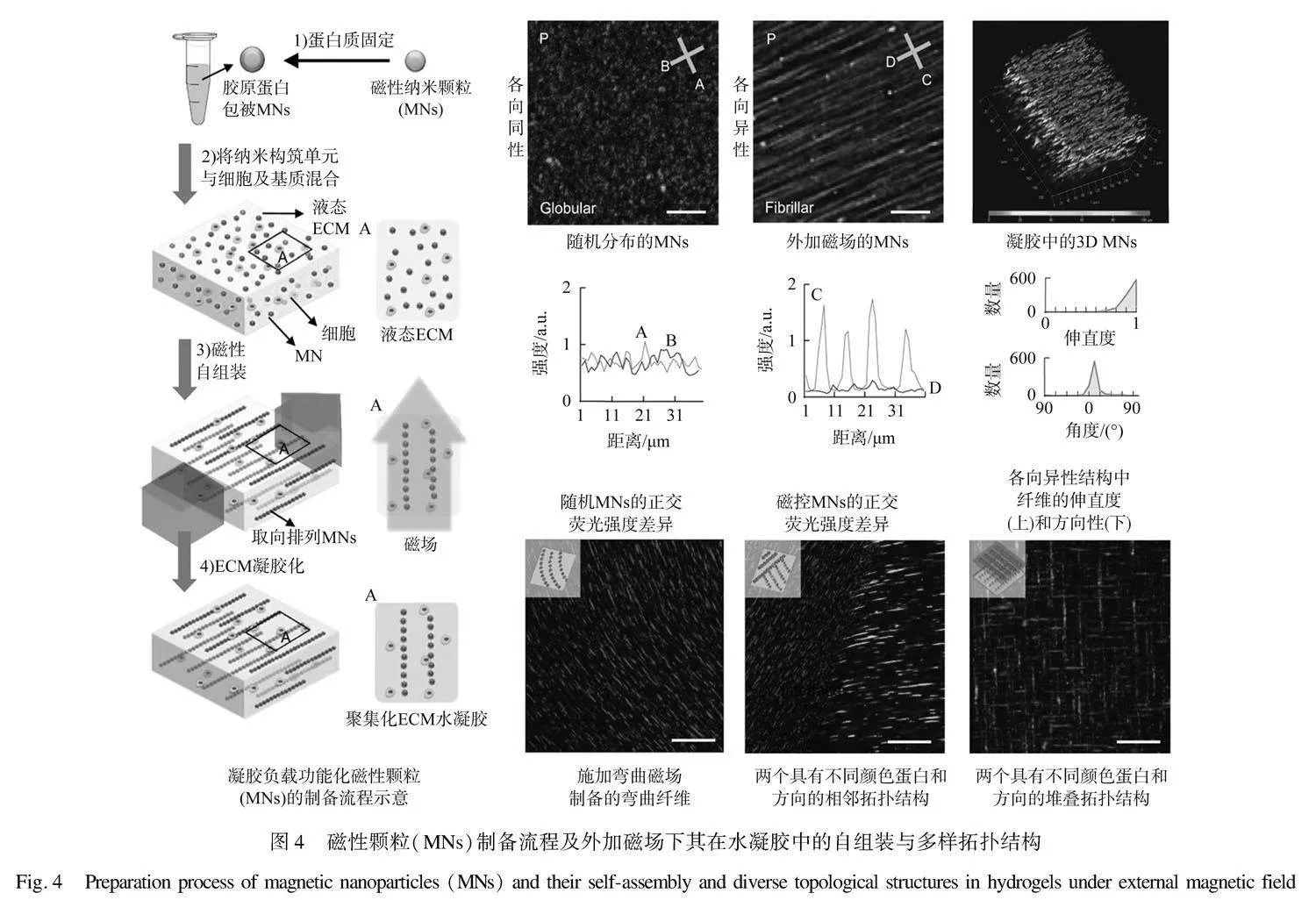
此外,磁性材料在外加磁场中的有序排列使得纳米颗粒聚集并在纳米尺度上展现出连续线性结构,在物理性质和功能上表现出一维材料,即纳米纤维的典型特征。Kim等[44]采用共沉淀法合成了Fe3O4磁性纳米颗粒,通过配位作用和酰胺化与胶原蛋白纤维偶联,投入胶原蛋白水凝胶中,在外加磁场下,磁性颗粒取向排列形成类似纳米纤维的一维线性结构,带动蛋白纤维在水凝胶中组装为高度取向的微结构,如图4所示。相较于未取向的水凝胶,成纤维细胞在这种取向水凝胶中展现出更显著的延伸性,生长方向往往平行于颗粒链方向,这表明纳米颗粒在三维基质中形成的取向结构为细胞生长提供了引导。
纳米短纤维不仅可以增强水凝胶,其本身也可作为水凝胶的主体材料。生物大分子,如肽和蛋白质,借助其自组装能力及盐析效应,往往可以直接与水结合形成各种结构的水凝胶,为在纳米尺度上设计制备具有特定功能的水凝胶提供了一种高效途径。Zhang等[45]利用固相肽合成技术成功合成了一种温度敏感的羧端基两亲多肽,其在特定离子环境下能够自组装成纳米短纤维。通过升温—冷却处理,多肽溶液的黏度显著增加,再经机械拉伸注入含盐溶液中,可形成均匀双折射性的面条状凝胶。在室温下,溶液中的多肽分子呈薄片结构,直接盐析会形成随机纠缠的凝胶结构,而加热诱导的脱水和自组装过程会使其形成有序排列的纳米纤维束,可促进干细胞在三维环境中的取向和迁移。类似地,Mredha等[46]将鱼鳔胶原蛋白溶液注入磷酸氢二钠溶液,借助注射流剪切力与盐扩散的协同作用,构建出兼具纵向排列和同心定向纳米纤维结构,通过在体系中引入聚(N,N′-二甲基丙烯酰胺)(PDMAAm)作为第二交联网络,显著增强了胶原凝胶的韧性,最终得到一种基于胶原纤维的新型双网络(DN)水凝胶,具备作为体内承重材料的应用潜力。本文对纳米短纤维功能化方法及其与水凝胶复合方式进行了总结,如表1所示。
3" 生物医学应用
3.1" 组织工程
将纳米短纤维引入水凝胶,在组织工程中发挥协同作用,显著提升了水凝胶的力学性能和稳定性,同时模拟细胞外基质微环境,为细胞的黏附、增殖和分化提供了理想支持。Kosik等[47]通过XanoShear工艺,在黏性分散介质(甘油/水)
中,利用Poiseuille剪切流,拉伸PLA溶液滴,沉淀收集亚微米短纤维,添加到海藻酸盐溶液中,形成纤维增强的3D打印墨水,应用于软骨组织工程,显著提高了3D支架的强度,并在为期14 d的体外培养中实现了约80%的细胞存活。此外,Shen等[48]将静电纺丝/均质化得到的PLGA短纤维(Fib)分散于甲壳素/柠檬酸(CC)溶液中,形成Fib/CC混合液(图5),随
后将软骨脱细胞(CDM)分散到Fib/CC中,通过冻融循环形成凝胶,再冷冻干燥制备成仿生壳聚糖支架。短纤维和CDM的引入不仅提升了其力学性能,还促进了软骨细胞的黏附和增殖,支持成熟软骨组织的形成。
此外,对于神经、心肌等天然取向组织,在设计组织工程材料时对其有序性提出要求,水凝胶中的短纤维能够在一系列外部引导下有序排列,创造了一种引导神经细胞定向生长的有效方式。Omidinia等[49]通过静电纺丝/冷冻切片制备了负载SPIONs的PLGA短纤维,经荧光聚乙二醇涂层标记后,加入纤维蛋白凝胶中,可在注射后外加磁场形成原位取向的短纤维复合水凝胶(Anisogel),荧光标记便于追踪纤维位置,如图6所示。与随机纤维水凝胶相比,Anisogel中神经细胞在外加磁场下呈现更协调同步的钙信号和更有序的生长活动。
类似地,Karimi等[50]通过湿法静电纺丝/超声破碎制备含有SPIONs的明胶磁性纳米短纤维(M.SNF),与OE-MSCs一同加入海藻酸钠溶液中,CaCl2交联形成磁性短纤维/海藻酸盐水凝胶(M.SNF/Alg),可在外加磁场下使OE-MSCs表现出更高的神经样分化和细胞增殖率,有助于促进神经细胞
的存活、分化和各向异性生长。同样,心肌组织也对定向的细胞黏附有着一定的需求。Zhang等[45]研发了一种温度敏感的两亲性多肽,经加热—冷却处理后可自组装成纳米短纤维,将心肌细胞(HL-1)加入其中并牵拉取向形成干细胞—凝胶线,HL-1沿凝胶轴向延伸迁移,细胞体和伪足与纳米纤维束取向相同,并展现了自发的电活动,有望恢复组织电通信与治疗心律失常。
3.2" 药物递送
在药物递送领域,纳米短纤维复合水凝胶以其可注射的形态灵活性在微创领域得到了广泛应用。Wang等[51]利用静电纺丝/冷冻切割制备了载有阿霉素(DOX)的PELA纳米短纤维(FDOX),均匀分散在负载血管抑制剂(CA4P)的PLGA-PEG-PLGA水凝胶中,构建了一种可注射的双重载药短纤维复合水凝胶(GCA4P/FDOX)。CA4P水溶性好,其释放过程主要受到扩散控制,在5 d内以爆发式迅速的释放完成,而DOX的释放不仅取决于扩散,还受到复合基质降解的影响,最终在30 d内释放了87%的药物。类似地,李慧娟等[52]通过静电纺丝/表面涂覆制备了多巴胺(PDA)涂层的PLCL纳米纤维,均质化制成短纤维,与淫羊藿苷(ICA)混合于CC溶液中,冷冻循环得到ICA-PDA@PLCL/CC水凝胶,短纤维表面的多巴胺和凝胶基质中的壳聚糖发生氢键作用,增强了支架的力学性能,同时缓慢释放的ICA展现出显著的抗纤维化和抗炎症效果。
尽管水凝胶是三维结构,但通常不能提供细胞附着底物,导致干细胞移植后存活率较低,而纳米短纤维构建的微环境则可以实现更高的存活率。Hsieh等[53]利用静电纺丝/超声破碎制备了聚(ε-己内酯-co-D,L-乳酸)纳米短纤维(P(CL:DLLA)),将其与神经干细胞/祖细胞(neural stem/progenitor cell,NSPC)混合加入透明质酸/甲基纤维素(HAMC)温敏凝胶前体液中,可在37 ℃下迅速凝胶化,形成适用于受损脊髓干细胞传递的可注射纤维复合水凝胶。短纤维的引入有效减少了细胞凝聚和坏死,促进了NSPC向神经元和少突胶质细胞表型(oligodendrocytic phenotypes)分化。
总的来说,短纤维复合凝胶在药物递送领域表现出色,保留了可注射性,增强了稳定性,能够避免药物突释,还具备细胞活性封装的功能。然而,有时纳米短纤维的释放动力学不够可控,导致药物释放不稳定,影响治疗效果的精细调控。
3.3" 生物传感与检测
纳米短纤维复合水凝胶材料通过其大比表面积和优越的生物相容性,可用于构建高灵敏的生物传感器,实现对生物信号快速、精准地传感与检测。银纳米线(AgNWs)是十分常见的导电填料[54-56],Ding等[57]研发了一种复合水凝胶,利用疏水改性聚丙烯酰胺(HMPAM)构建非共价键网络,同时通过N,N-双丙烯酰胱胺(BACA)修饰AgNWs形成可逆Ag-S配位键,并借助PAM与葡聚糖的氢键作用形成三重网络结构,有效改善了刚性纳米线与聚合物基质间的力学匹配问题,在人体运动和肌电信号检测方面表现突出。Jeong等[58]将与病毒标靶对应的引物DNA以硫—金键修饰到金纳米线表面,一旦接触到目标靶点就能通过DNA聚合酶驱动的高效滚环扩增(RCA)产生大量与引物DNA互补的长链DNA,形成密集的DNA网络水凝胶,实现对特定病毒的灵敏检测。此外,Wu等[59]利用多巴胺诱导效应并控制反应温度,成功地将多巴胺包覆在双金属AuPt材料表面,形成了具有三维多孔纳米线网络结构的水凝胶,可用于固定乙酰胆碱酯酶(AChE)并构建用于高灵敏有机磷检测的酶基生物传感器。凭借独特的各向异性构造,纳米短纤维在应对诸如运动、应变等涉及空间异质性的精密传感任务中展现出显著优势,而其出众的高比表面积特性则进一步赋能于生物信号的高效捕获与精确探测。
4" 结" 论
将纳米短纤维整合入水凝胶网络,其一维结构与高自由度既增强了材料的机械性能又不损失可注射性,纳米材料的高比表面积拓展了检测灵敏度,纤维的各向异性在模拟细胞外基质的同时引导细胞定向生长。然而,当前的复合凝胶仍面对一些挑战:1) 纳米短纤维制备流程复杂、长度均一性差,目前形成纳米短纤维的方法多为“由大变小”的破碎法或剥离法,需要开发更加高效的纳米短纤维制备方法,同时提高所得短纤维的长度均一性;2) 短纤维在凝胶中是随机分布的,即使能通过各种外加磁场或热处理等方法使短纤维在水凝胶中取向,但其控制精度不足,这限制了复合凝胶性能的提高,需要进一步研究两者的结合机制,更精细地操控短纤维;3) 虽然水凝胶基质与纳米短纤维的结合在一定程度上提高了复合材料的力学强度,但仍不足以满足临床应用的高要求,在保证短纤维生物相容性的前提下寻找更高强的材料是一种选择。相信随着短纤维制造和水凝胶复合技术的不断发展,纳米短纤维复合水凝胶有望大规模应用于药物递送、组织工程等医学领域中,助力于人类的健康与幸福生活。
《丝绸》官网下载
中国知网下载
参考文献:
[1]JANG J, LEE J, SEOL Y-J, et al. Improving mechanical properties of alginate hydrogel by reinforcement with ethanol treated polycaprolactone nanofibers[J]. Composites Part B: Engineering, 2013, 45(1): 1216-1221.
[2]ZHANG M, XU S, DU C, et al. Novel PLCL nanofibrous/keratin hydrogel bilayer wound dressing for skin wound repair[J]. Colloids and Surfaces B: Biointerfaces, 2023, 222: 113119.
[3]张蓓蕾, 沈明武, 史向阳. 静电纺短纤维的制备及其生物医学应用[J]. 纺织学报, 2021, 42(5): 1-8.
ZHANG B L, SHEN M W, SHI X Y. Preparation and biomedical applications of electrospun short fibers[J]. Journal of Textile Research, 2021, 42(5): 1-8.
[4]CHOI C, YUN E, CHA C. Emerging technology of nanofiber-composite hydrogels for biomedical applications[J]. Macromolecular Bioscience, 2023, 23: 2300222.
[5]ZHANG M, XU S, WANG R, et al. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications[J]. Journal of Materials Science amp; Technology, 2023, 162: 157-178.
[6]RAJA C, SAWAWI M, SAHARI S, et al. A review on electrospun short fiber production[J]. International Journal of Integrated Engineering, 2023, 15(5): 28-34.
[7]CHEN W, MA J, ZHU L, et al. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering[J]. Colloids and Surfaces B: Biointerfaces, 2016, 142: 165-172.
[8]YE K, LIU D, KUANG H, et al. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration[J]. Journal of Colloid and Interface Science, 2019, 534: 625-636.
[9]YUAN Z, REN Y, SHAFIQ M, et al. Converging 3D printing and electrospinning: Effect of poly(l-lactide)/gelatin based short nanofibers aerogels on tracheal regeneration[J]. Macromolecular Bioscience, 2022, 22(1): 2100342.
[10]BODA S, CHEN S, CHU K, et al. Electrospraying electrospun nanofiber segments into injectable microspheres for potential cell delivery[J]. Acs Applied Materials amp; Interfaces, 2018, 10(30): 25069-25079.
[11]LIAO X, HU P, AGARWAL S, et al. Impact of the fiber length distribution on porous sponges originating from short electrospun fibers made from polymer yarn[J]. Macromolecular Materials and Engineering, 2020, 305(2): 1900629.
[12]WEI J, LEI D, CHEN M, et al. Engineering HepG2 spheroids with injectable fiber fragments as predictable models for drug metabolism and tumor infiltration[J]. Journal of Biomedical Materials Research Part B-Applied Biomaterials, 2020, 108(8): 3331-3344.
[13]NIEMCZYK-SOCZYNSKA B, DULNIK J, JEZNACH O, et al. Shortening of electrospun PLLA fibers by ultrasonication[J]. Micron, 2021, 145: 103066.
[14]SAWAWI M, RAJA C, TANJUNG S, et al. Effects of uv irradation on electrospun PLLA and PAN in the production of short electropun fibres using ultrasonication method[J]. Pertanika Journal of Science and Technology, 2023, 31(5): 2441-2451.
[15]SAWAWI M, WANG T, NISBET D, et al. Scission of electrospun polymer fibres by ultrasonication[J]. Polymer, 2013, 54(16): 4237-4252.
[16]TULADHAR S, CLARK S, AHASAN H. Tuning shear thinning factors of 3D bio-printable hydrogels using short fiber[J]. Materials, 2023, 16(2): 572.
[17]TULADHAR S, CLARK S, HABIB M. Controlling rheological properties of hybrid hydrogel using short fiber for extrusion-based 3D bioprinting process[C]//Proceedings of the ASME 2023 International Manufacturing Science and Engineering Conference. New Brunswick, New Jersey: American Society of Mechanical Engineers, 2023: MSEC2023-104233, V001T03A006.
[18]GHADERINEJAD P, NAJMODDIN N, BAGHER Z, et al. An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering[J]. Chemical Engineering Journal, 2021, 420: 130465.
[19]CHENG Y, CHENG G, XIE C, et al. Biomimetic silk fibroin hydrogels strengthened by silica nanoparticles distributed nanofibers facilitate bone repair[J]. Advanced Healthcare Materials, 2021, 10(9): 2001646.
[20]WEI J, RAN P, LI Q, et al. Hierarchically structured injectable hydrogels with loaded cell spheroids for cartilage repairing and osteoarthritis treatment[J]. Chemical Engineering Journal, 2022, 430: 132211.
[21]LI X, CHO B, MARTIN R, et al. Nanofiber-hydrogel composite-mediated angiogenesis for soft tissue reconstruction[J]. Science Translational Medicine, 2019, 11(490): aau6210.
[22]WANG M, DU J, LI M, et al. In situ forming double-crosslinked hydrogels with highly dispersed short fibers for the treatment of irregular wounds[J]. Biomaterials Science, 2023, 11(7): 2383-2394.
[23]BRUGGEMAN K, WANG Y, MACLEAN F, et al. Temporally controlled growth factor delivery from a self-assembling peptide hydrogel and electrospun nanofibre composite scaffold[J]. Nanoscale, 2017, 9(36): 13661-13669.
[24]WANG T, BRUGGEMAN K, KAUHAUSEN J, et al. Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of parkinson’s disease[J]. Biomaterials, 2016, 74: 89-98.
[25]ZHANG X, MEGONE W, PEIJS T, et al. Functionalization of electrospun PLA fibers using amphiphilic block copolymers for use in carboxy-methyl-cellulose hydrogel composites[J]. Nanocomposites, 2020, 6(3): 85-98.
[26]LIU M, ZHENG H, CHEN J, et al. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering[J]. Carbohydrate Polymers, 2016, 152: 832-840.
[27]陈楚楚, 吴启静, 王怡仁, 等. 仿生高强度甲壳素纳米纤维/明胶水凝胶的制备与性能[J]. 高分子材料科学与工程, 2020, 36(8): 152-157.
CHEN C C, WU Q J, WANG Y R, et al. Synthesis and characterization of bioinspired chitin nanofiber/gelatin hydrogels with high mechanical properties[J]. Polymer Materials Science amp; Engineering, 2020, 36(8): 152-157.
[28]YIN K, DIVAKAR P, WEGST U. Plant-derived nanocellulose as structural and mechanical reinforcement of freeze-cast chitosan scaffolds for biomedical applications[J]. Biomacromolecules, 2019, 20(10): 3733-3745.
[29]KAUR H, SHARMA P, PATEL N, et al. Accessing highly tunable nanostructured hydrogels in a short ionic complementary peptide sequence via pH trigger[J]. Langmuir, 2020, 36(41): 12107-12120.
[30]LIGORIO C, ZHOU M, WYCHOWANIEC J, et al. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications[J]. Acta Biomaterialia, 2019, 92: 92-103.
[31]POPESCU M-T, LIONTOS G, AVGEROPOULOS A, et al. Injectable hydrogel: Amplifying the pH sensitivity of a triblock copolypeptide by conjugating the N-termini via dynamic covalent bonding[J]. ACS Applied Materials amp; Interfaces, 2016, 8(27): 17539-17548.
[32]YU S, ZHANG W, AN J, et al. Flexible, multifunctional aerogel films based on PBO nanofibers and their application in wearable electronic devices[J]. Electrochimica Acta, 2023, 441: 141802.
[33]郭秋艳, 刘牛, 张凡, 等. 3D打印可拉伸、自愈合型水凝胶的制备及应用研究[J]. 现代化工, 2023, 43(3): 177-182.
GUO Q Y, LIU N, ZHANG F, et al. Preparation and application of stretchable self-healing hydrogel based on 3D printing[J]. Modern Chemical Industry, 2023, 43(3): 177-182.
[34]史杰中, 贾昊旸, 刘冬生. 单根DNA短链构筑pH响应超分子水凝胶[J]. 高分子学报, 2017(1): 135-142.
SHI J Z, JIA H Y, LIU D S. pH-responsive supramolecular hydrogel based on one short strand DNA[J]. Acta Polymerica Sinica, 2017(1): 135-142.
[35]ZHI X, ZHENG C, XIONG J, et al. Nanofilamentous virus-based dynamic hydrogels with tunable internal structures, injectability, self-healing, and sugar responsiveness at physiological pH[J]. Langmuir, 2018, 34(43): 12914-12923.
[36]LEE D, PARK J, LEE E, et al. A protein nanofiber hydrogel for sensitive immunoassays[J]. Analyst, 2013, 138(17): 4786-4794.
[37]XUE Y, LI J, JIANG T, et al. Biomimetic conductive hydrogel scaffolds with anisotropy and electrical stimulation for in vivo skeletal muscle reconstruction[J]. Advanced Healthcare Materials, 2024, 13(4): 2302180.
[38]POVEDA-REYES S, MELLERA-OGLIALORO L, MARTNEZ-HAYA R, et al. Reinforcing an injectable gelatin hydrogel with PLLA microfibers: Two routes for short fiber production[J]. Macromolecular Materials and Engineering, 2015, 300(10): 977-988.
[39]REGEV O, REDDY C, NSEIR N, et al. Hydrogel reinforced by short albumin fibers: Mechanical characterization and assessment of biocompatibility[J]. Macromolecular Materials and Engineering, 2013, 298(3): 283-291.
[40]MATERA D, WANG W, SMITH M, et al. Fiber density modulates cell spreading in 3D interstitial matrix mimetics[J]. ACS Biomaterials Science amp; Engineering, 2019, 5(6): 2965-2975.
[41]JUAN L-T, LIN S-H, WONG C-W, et al. Functionalized cellulose nanofibers as crosslinkers to produce chitosan self-healing hydrogel and shape memory cryogel[J]. ACS Applied Materials amp; Interfaces, 2022, 14(32): 36353-36365.
[42]LI L, YANG H, LI X, et al. Natural silk nanofibrils as reinforcements for the preparation of chitosan-based bionanocomposites[J]. Carbohydrate Polymers, 2021, 253: 117214.
[43]ISLAM M, MOLLEY T, HUNG T, et al. Magnetic nanofibrous hydrogels for dynamic control of stem cell differentiation[J]. ACS Applied Materials amp; Interfaces, 2023, 15(44): 50663-50678.
[44]KIM J, STAUNTON J, TANNER K. Independent control of topography for 3D patterning of the ECM microenvironment[J]. Advanced Materials, 2016, 28(1): 132-137.
[45]ZHANG S, GREENFIELD M, MATA A, et al. A self-assembly pathway to aligned monodomain gels[J]. Nature Materials, 2010, 9(7): 594-601.
[46]MREDHA M, KITAMURA N, NONOYAMA T, et al. Anisotropic tough double network hydrogel from fish collagen and its spontaneous in vivo bonding to bone[J]. Biomaterials, 2017, 132: 85-95.
[47]KOSIK-KOZIOL A, COSTANTINI M, BOLEK T, et al. PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration[J]. Biofabrication, 2017, 9(4): 044105.
[48]SHEN Y, XU Y, YI B, et al. Engineering a highly biomimetic chitosan-based cartilage scaffold by using short fibers and a cartilage-decellularized matrix[J]. Biomacromolecules, 2021, 22(5): 2284-2297.
[49]OMIDINIA-ANARKOLI A, BOESVELD S, TUVSHINDORJ U, et al. An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells[J]. Small, 2017, 13(36): 1702207.
[50]KARIMI S, BAGHER Z, NAJMODDIN N, et al. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactorymucosa stem cells bioactivity for potential nerve regeneration application[J]. International Journal of Biological Macromolecules, 2021, 167: 796-806.
[51]WANG T, YANG L, XIE Y, et al. An injectable hydrogel/staple fiber composite for sustained release of CA4P and doxorubicin for combined chemotherapy of xenografted breast tumor in mice[J]. Journal of Southern Medical University, 2022, 42(5): 625-632.
[52]李慧娟, 王先流, 沈炎冰, 等. 负载淫羊藿苷的壳聚糖基仿生支架的促软骨形成和炎症缓解作用[J]. 生物工程学报, 2022, 38(6): 2308-2321.
LI H J, WANG X L, SHEN Y B, et al. Chondrogenic and ameliorated inflammatory effects of chitosan-based biomimetic scaffold loaded with icariin[J]. Chinese Journal of Biotechnology, 2022, 38(6): 2308-2321.
[53]HSIEH A, ZAHIR T, LAPITSKY Y, et al. Hydrogel/electrospun fiber composites influence neural stem/progenitor cell fate[J]. Soft Matter, 2010, 6(10): 2227-2237.
[54]HAN Q, CHEN Y, SONG W, et al. Fabrication of agarose hydrogel with patterned silver nanowires for motion sensor[J]. Bio-Design and Manufacturing, 2019, 2(4): 269-277.
[55]ZHANG X, LI Z, LIU C, et al. Silver nanowire/silver/poly(dimethylsiloxane) as strain sensors for motion monitoring[J]. ACS Applied Nano Materials, 2022, 5(10): 15797-15807.
[56]ZOU L, CHANG B, LIU H, et al. Multiple physical bonds cross-linked strong and tough hydrogel with antibacterial ability for wearable strain sensor[J]. ACS Applied Polymer Materials, 2022, 4(12): 9194-9205.
[57]DING J, QIAO Z, ZHANG Y, et al. NIR-responsive multi-healing HMPAM/dextran/AgNWs hydrogel sensor with recoverable mechanics and conductivity for human-machine interaction[J]. Carbohydrate Polymers, 2020, 247: 116686.
[58]JEONG J, KIM H, LEE J. Enzymatic polymerization on DNA modified gold nanowire for label-free detection of pathogen DNA[J]. International Journal of Molecular Sciences, 2015, 16(6): 13653-13660.
[59]WU Y, JIAO L, XU W, et al. Polydopamine-capped bimetallic AuPt hydrogels enable robust biosensor for organophosphorus pesticide detection[J]. Small, 2019, 15(17): 1900632.
Preparation of short nanofiber composite hydrogels and their biomedical applications
ZHANG Chi, WANG Xiangrong
YU Yang, ZOU Yujiao, ZHAO Yili, LI Ni
(College of Textile Science and Engineering (International Institute of Silk), Zhejiang Sci-Tech University, Hangzhou 310018, China)
Abstract:
Nanofiber hydrogel composites integrate the high water content and biocompatibility of hydrogels with the high surface area-to-volume ratio, nano-scale dimensions, and ability to mimic the extracellular matrix (ECM). This convergence presents promising applications in biomedicine. Advances in biomedical engineering have spurred high expectations for the performance and functionality of composite hydrogels, notably in biomimetic materials, drug delivery, and tissue engineering. Nonetheless, traditional nanofiber membranes struggle to replicate complex three-dimensional micro-nano environments. Their small pore sizes and high densities restrict nutrient transport. Furthermore, the inadequate dispersion of continuous nanofibers hampers the materials’ minimally invasive use. To address these limitations, researchers have progressively introduced controllable-length short nanofibers into hydrogel networks. These nanofibers offer superior dispersibility, enhancing their integration with hydrogels and the emulation of complex physiological conditions.
To comprehensively understand the research status and performance advantages of short nanofiber composite hydrogels, and to promote their development and application, this paper reviews recent progress. It focuses on the functionalization and composite methods of short nanofibers, summarizing their applications in the biomedical field, and proposing current challenges and future directions. Functionalized short nanofibers serve crucial roles in composite hydrogels. Firstly, by encapsulating cytokines or nanoparticles, these fibers create a microenvironment within the hydrogel, enhancing cell growth. Secondly, surface modifications that immobilize therapeutic or monitoring agents on the fiber surface can prevent drug loss, thereby enhancing therapeutic efficacy. Additionally, these modifications increase the contact area between the drug and its environment, improving detection sensitivity. Furthermore, by modifying precursor materials prior to electrospinning, short fibers can be functionally customized, imparting specific properties and improving compatibility with the hydrogel matrix. The application of biotechnology, such as the self-assembly of large molecules like DNA and peptides into short nanofibers, not only endows them with specific biological functionality but also enables interaction with hydrogels, enhancing their effects and further expanding the potential applications of short fibers in hydrogels. The binding mechanisms between short fibers and hydrogels are crucial. Besides simple physical blending, the interfacial interaction between the two can be strengthened through a series of interactions including covalent bonding, hydrogen bonding, biotin interaction, etc. Furthermore, methods such as heat treatment, mechanical stretching, solvent diffusion, and magnetic field control can orderly arrange short fibers in gels, forming oriented structures, which aid in mimicking the microstructure of natural oriented tissues such as muscles and nerves, promoting tissue regeneration.
The enhancement and functionalization of hydrogels become a significant research focus in biomedical materials science, with the innovative incorporation of short fibers offering new pathways for improving hydrogel performance. This approach not only enhances the mechanical properties of hydrogels but also introduces new functionalities in a simple and effective manner. Short fiber-reinforced hydrogels demonstrate extensive potential applications in regulating cellular behavior, optimizing drug delivery mechanisms, and enhancing biosensing capabilities. These advancements provide a new material foundation and therapeutic strategy for regenerative medicine and precision medicine.
However, current research on composite hydrogels faces several significant challenges. Firstly, the primary fabrication techniques for short nanofibers suffer from scalability issues and inconsistent fiber morphology. Innovative fabrication methods that enhance production efficiency and achieve uniform fiber dimensions are urgently needed. Secondly, the precise control of the three-dimensional distribution and orientation of short fibers within the gel matrix is critical for optimizing the performance of composite hydrogels. This necessitates a deeper understanding of interfacial interactions and the development of advanced fiber assembly strategies. Lastly, while the incorporation of short nanofibers improves the mechanical properties of composite materials, they still fall short of the high-strength requirements for applications such as bone and joint repair. Thus, advanced composite strategies that can match or exceed the mechanical properties of natural tissues are essential to ensure long-term stability and functionality in complex biomechanical environments.
Key words:
nanofibers; functionalized short nanofibers; hydrogel; tissue engineering; drug delivery; biosensing and diagnostics

