超高效液相色谱-三重四级杆质谱联用法同时测定荔枝中4种细胞分裂素
黄雨莲 赵明磊 马兴帅 李建国 徐婧
摘 要:【目的】应用超高效液相色谱-三重四级杆质谱联用仪(UPLC-MS/MS),建立一种同时测定荔枝反式玉米素核苷(tZR)、异戊烯基腺嘌呤核苷(IPR)、二氢玉米素(DHZ)、异戊烯基腺嘌呤(IP)4种细胞分裂素含量的方法。【方法】0.25 g荔枝样品用乙腈-水(80∶20,体积比)溶液浸提8 h,经Bond Elut Plexa PCX固相萃取柱纯化,2.5%氨水甲醇洗脱后过0.22 ?m有机滤膜检测。选取XSelect HSS T3色谱柱,以甲醇和5 mmol·L-1甲酸铵水溶液为流动相进行7 min梯度洗脱,采用电喷雾正离子(ESI+)模式电离,选择反应监测(MRM)模式对细胞分裂素进行定量。【结果】4种细胞分裂素的检出限和定量限分别低于18.12和60.39 pg·g-1,在0.05~50 ng·mL-1质量浓度范围内呈良好的线性关系,线性相关系数(r2)大于0.999。在高、中、低浓度三种加标水平下,4种细胞分裂素的平均回收率为80.0%~108.2%,标准偏差为0.8%~15.5%。采用建立的方法可以同时检测出荔枝果皮、果肉、种子、幼果、叶片和果柄离区中4种细胞分裂素含量。【结论】该方法简便、快速、灵敏、准确,适用于荔枝不同组织中细胞分裂素含量的测定。
关键词:荔枝;液质联用;细胞分裂素;定量测定
中图分类号:S667.1 文献标志码:A 文章编号:1009-9980(2024)06-1228-11
Simultaneous determination of four cytokinins in litchi by ultra-high performance liquid chromatography triple quadrupole mass spectrometry
HUANG Yulian1, ZHAO Minglei1, 2, MA Xingshuai1, 2, LI Jianguo1, 2*, XU Jing1*
(1Key Laboratory of Biology and Genetic Improvement of Horticultural Crops (South China), Ministry of Agriculture and Rural Affairs/College of Horticulture, South China Agricultural University, Guangzhou 510642, Guangdong, China; 2Guangdong Litchi Engineering Research Center, Guangzhou 510642, Guangdong, China)
Abstract: 【Objective】 A method was developed to simultaneously measure four types of cytokinins (tZR, IPR, DHZ and IP) in litchi using ultra-high performance liquid chromatography-tandem triple quadrupole mass spectrometry (UPLC-MS/MS). 【Methods】 The experiment was conducted using litchi pericarp 50 days after flowering. The XSelect HSS T3 chromatographic column was selected. Methanol and 5 mmol·L-1 ammonium formate aqueous solution were used as the mobile phase with a flow rate of 0.3 mL·min-1. A gradient elution was conducted for 7 minutes. Positive electrospray ionization (ESI+) and the multiple reaction monitoring (MRM) mode were set for mass spectrometry detection. Under these conditions [ion source temperature 150 ℃, capillary voltage 0.35 kV, dissolvent gas temperature 500 ℃, atomization gas flow rate 1000 L·h-1, cone hole gas flow 50 L·h-1, and collision gas (argon gas) 0.17 L·h-1], quantification of cytokinins was achieved. The effects of different extraction solvents (80% methanol, 80% acetone, and 80% acetonitrile), different extraction times (4 h, 8 h, 12 h, and 16 h), different purification methods (PCX column, C18 column, HLB column, and no column), different concentrations of ammonium-methanol eluent (0.5%, 1.0%, 2.5%, and 5.0%), and different aqueous phases when methanol is the organic phase (0.05% formic acid solution, 0.1% formic acid solution, 1 mmol·L-1 ammonium formate solution, and 5 mmol·L-1 ammonium formate solution) were separately examined for their impact on cytokinin extraction, enrichment, and separation efficiency. Compared to other endogenous hormones, cytokinins are unique in that they have a purine ring and are alkaline. The PCX column is a strong cation exchange column filled with polymer cation exchange resin, which concentrates alkaline analytes and thereby enhances the detection sensitivity of alkaline compounds. Cytokinins exist as cations in the acidified extraction solution (pH≈2-3). They are tightly adsorbed by the filler during passage through the PCX column, and then rinsed with a 0.1% formic acid methanol solution (pH≈5-6). At this point, most acidic and neutral compounds are removed, leaving the target substances on the column to be eluted with a 2.5% ammonia-methanol solution (pH≈11.3). Target substances flow out with the eluent are collected ultimately. In addition, cytokinins are also polar substances which can be separated according to the principles of C18 column and HLB column separation, namely the principle of similar solubility. The stationary phase of C18 and HLB reverse phase columns is non-polar. When the polarity of the mobile phase is greater than that of the stationary phase, the target substance is eluted with the polar mobile phase. 【Results】 As for the extraction conditions, the extraction effect was better with extraction solvent of 80% acetonitrile than the other extraction solutions and the extraction time was 8 hours, resulting in smooth, sharp peaks and high response values. Regarding the purification conditions, solid phase extraction with PCX was chosen, and the concentration of the ammonia methanol eluent was 2.5%. Under this condition, chromatogram had less interference, offering the best enrichment separation effect. For the mobile phase conditions, when methanol was used as the organic phase, a 5 mmol·L-1 aqueous solution of ammonium formate was most effective, with which the response intensity of the target substance was high and the peak time was appropriate. Fresh litchi samples were taken and ground into fine powder in a mortar with liquid nitrogen. 0.25 g of the sample was put into a 15 mL plastic centrifuge tube, and added with 2.5 mL of 80% acetonitrile pre-cooled at 4 ℃. Cytokinins were extracted at 4 ℃ for 8 hours, during which the sample was shaken twice. After extraction, the extract was centrifuged at a speed of 10 000 r·min-1 for 10 minutes at 4 ℃, and the supernatant was collected. An equal volume of 80% acetonitrile was added to the residue, shaken for 10 minutes, and centrifuged at a speed of 10 000 r·min-1 for 10 minutes at 4 ℃. The supernatant was combined, added with formic acid to adjust the pH value to 2-3, and mixed well to obtain the crude extract. Consecutively, 2.5 mL methanol and an equal volume of 2% aqueous solution of formic acid were added for activation and balancing. The crude extract was passed through the column at a rate of 1-2 drops per second, rinsed with an equal volume of 0.1% formic acid methanol solution, and finally washed twice with an equal volume of 2.5% ammonia-water methanol solution. The eluent was collected in a test tube, blown to almost dryness with nitrogen, and added with 0.25 mL 15% methanol solution to re-dissolve. After vortex mixing, the eluent was filtered through a 0.22 ?m organic membrane for testing. The limits of detection and quantification for the four cytokinins were below 18.12 and 60.39 pg·g-1, respectively, showing a good linear relationship within the concentration range of 0.05-50 ng·mL-1, with a correlation coefficient (r2) greater than 0.999. At three spiking levels of high, medium, and low concentrations (0.4, 2, and 20 ng·mL-1), the average recovery rates of the four cytokinins were between 80.0%-108.2%, with a standard deviation ranging from 0.8%-15.5%. The established method was used to measure the endogenous cytokinins in litchi pericarp, pulp, seed, young fruit, leaves, and the abscission zone, and all four cytokinins could be detected. 【Conclusion】 This method has the advantages of easy preprocessing, short detection cycle, good reproducibility, high sensitivity, and low cost. The final results are reliable, making it suitable for rapid screening and quantitative detection of cytokinins in various parts of litchi. The method is of great practical value.
Key words: Litchi; Liquid chromatography-mass spectrometry; Cytokinin; Quantitative determination
细胞分裂素是天然存在的腺嘌呤衍生物,是一类较活跃的植物激素,具有广泛的生物学活性,参与植物从种子萌发到衰老的各个阶段[1]。在植物中,它们主要以游离碱的形式存在,各种细胞分裂素分子结构不同,但它们都具有芳香族环和侧链,其中,芳香族环上带有羧基和甲基或羟基等官能团,侧链则包括苯丙氨基、异戊二烯基等官能团,这些官能团赋予了细胞分裂素特定的生理功能。根据侧链的不同,天然细胞分裂素又可以分为两类,分别是异戊二烯类细胞分裂素和苯环类细胞分裂素,且前者更为普遍[2]。反式玉米素核苷(tZR)、异戊烯基腺嘌呤核苷(IPR)、二氢玉米素(DHZ)、异戊烯基腺嘌呤(IP)都属于前者(图1)。细胞分裂素的主要存在形式在不同的植物、相同植物的不同组织以及发育阶段有很大的差异。例如在猕猴桃中,IPR是细胞分裂素最为常见的存在形式[3];而在葡萄中,tZR是最常见的存在形式[4]。作为一种植物生长必不可少的激素,细胞分裂素在荔枝发育的整个过程中都发挥了重要的作用,该文涉及的4种细胞分裂素在细胞分裂素的合成代谢过程中较为关键。
荔枝(Litchi chinensis Sonn.)为无患子科荔枝属常绿植物,是华南的重要经济果树,在热带农业产业中占有重要的地位[5]。中国是荔枝的原产地,现有荔枝种植面积55万hm2,主要分布在广东、广西、福建、海南等省区[6]。荔枝在中国南方已有数千年的种植历史,且因色、香、味俱佳,深受广大消费者喜爱。李伟才等[7]发现无核荔枝果实中高含量的IAA和低含量的细胞分裂素可能是其产生无核的一个重要原因。在花芽分化过程中,细胞分裂素含量的升高会促进芽的分化和侧芽发育,消除顶端优势,促进花芽分化[8-9]。环剥是抑制落果、提高产量的有效措施,环剥后叶片的细胞分裂素含量明显提高[10]。采前落果期果柄离区内细胞分裂素含量的增加会抑制果柄离层的形成,减少果实的脱落[11]。在荔枝体胚发育过程中,添加外源玉米素可以促进体胚的进一步发育,促进球形胚的形成[12]。这些研究结果均说明细胞分裂素对荔枝的生长发育和产量形成至关重要。然而细胞分裂素在植物体内的含量极低,且在提取和测定过程中受其他代谢物的干扰严重[13],因此,选择一种合适的测定方法对获取细胞分裂素准确的测定结果至关重要。
目前,荔枝上多采用酶联免疫法(ELISA)[14-17]和高效液相色谱法(HPLC)[18]测定细胞分裂素含量,且前人多针对细胞分裂素中的一种进行测定,这两种方法精密度不高,获得的数据差异大。植物体本身除了基质组成复杂外,检测过程中还存在取样难、称量难和分析难等问题,这就对植物的定量分析提出了更高的要求[19]。因此,具有检测限低、带有质谱准确性、检出准确率高等特点的高效液相色谱-串联质谱法,已成为目前植物激素分析的主流检测方法。荔枝果实的样品酚类物质含量高,需要探讨细胞分裂素提取的最佳方案,高效液相色谱-质谱联用检测的色谱和质谱条件也需要进一步的优化。笔者在本研究中以仙进奉荔枝为材料,首次建立了用超高效液相色谱-三重四级杆质谱联用仪(UPLC-MS/MS)同时测定荔枝不同部位(果皮、果肉、种子、幼果、叶片、果柄离区)中tZR、IPR、DHZ、IP的检测方法,为相关研究提供参考。
1 材料和方法
1.1 供试材料
以广州市增城石滩中荔园的仙进奉荔枝为试验材料,选花后50 d的荔枝果实,将荔枝果实的果皮、果肉、种子和果柄离区分别作为试验材料,采集冬梢叶片,置于冰盒迅速带回实验室,做好标记后,置于液氮速冻后存放于-80 ℃冰箱中备用。
1.2 仪器与试剂
Waters超高效液相色谱-三重四极杆串联质谱联用仪(ACQUITY UPLC H-Class/ Xevo TQD)、Eppendorf台式冷冻离心机、Eppendorf Thermo Mixer混匀仪、氮吹仪。
乙腈(质谱纯)、甲醇(质谱纯)、甲酸(质谱纯)均购自赛默飞世尔科技公司,甲酸铵(色谱纯,上海麦克林生化科技股份有限公司),28%~30%氨水(色谱纯,上海阿拉丁生化科技股份有限公司),Bond Elut Plexa PCX柱(安捷伦科技有限公司),试验用水为屈臣氏蒸馏水。细胞分裂素标准品tZR、IPR、DHZ和IP均购自上海源叶生物科技有限公司,纯度均≥98%。
1.3 标准溶液的配制
准确称取tZR、IPR、DHZ、IP的标准品,用质谱甲醇制成单标准储备液密封储存于-80 ℃冰箱中,用15%的质谱甲醇配制成0.1 ?g·mL-1的细胞分裂素混合标准溶液,密封储存于-20 ℃冰箱备用,使用时再稀释成相应浓度。
1.4 样品前处理
过程参考曹赵云等[20]的方法并加以改进,以花后50 d荔枝果皮为材料,分别考察不同提取剂(80%甲醇[21-23]、80%丙酮、80%乙腈[24])、不同提取时间(4 h、8 h、12 h、16 h)、不同纯化方式(PCX柱[25]、C18柱[26]、HLB柱[27]、不过柱)、不同体积分数的氨水甲醇洗脱液(0.5%、1.0%、2.5%、5.0%)对细胞分裂素提取富集及分离效果的影响;同时以5 ng·mL-1标准品为材料,考察甲醇为有机相时不同水相(0.05%甲酸水溶液、0.1%甲酸水溶液、1 mmol·L-1甲酸铵水溶液、5 mmol·L-1甲酸铵水溶液)对细胞分裂素提取富集及分离效果的影响,筛选出适合荔枝样品的前处理流程。
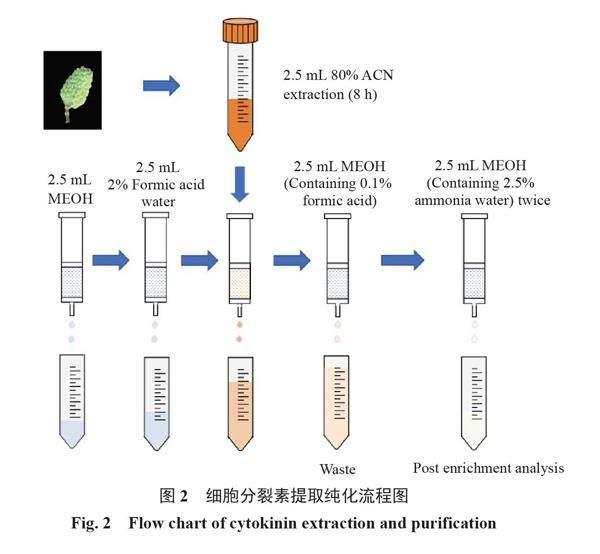
优化后的流程如图2所示。取新鲜的荔枝果皮样品,于研钵中加液氮磨细,用千分之一天平准确称取0.25 g样品于15 mL塑料离心管中,加入2.5 mL 4 ℃预冷的80%乙腈溶液,在4 ℃下浸提8 h,其间振摇2次。取出后于4 ℃在10 000 r·min-1的转速下离心10 min,收集上清液。在残渣中加入2.5 mL 80%乙腈,振荡10 min,于4 ℃在10 000 r·min-1的转速下离心10 min,将两次提取液合并,加甲酸调pH值为2~3,混匀,获得样品粗提液。
依次用2.5 mL甲醇和2.5 mL 2%甲酸水溶液进行活化和平衡固相萃取柱,约5 mL粗提液以每秒1~2滴的速度过柱,之后再用等体积甲醇溶液(含0.1%甲酸)淋洗,最后用2.5 mL甲醇溶液(含2.5%氨水)洗脱两次,洗脱液收集于试管,氮吹至10 ?L,加0.25 mL 15%甲醇水溶液复溶,漩涡混匀后过0.22 ?m有机滤膜后待测。
1.5 色谱和质谱条件
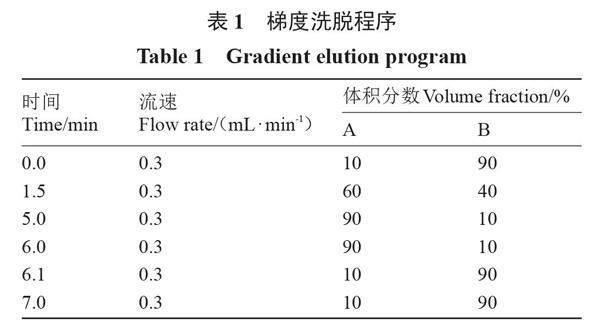
色谱条件:Waters公司X Select HSS T3柱(2.1 mm × 100 mm,2.5 ?m),流动相A为甲醇,流动B为5 mmol·L-1的甲酸铵水溶液,梯度洗脱程序见表1。
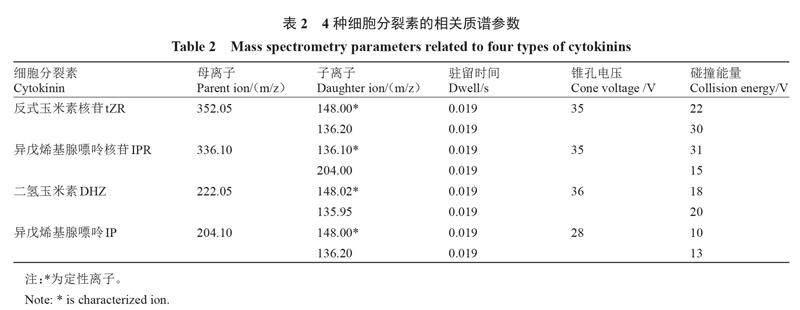
质谱条件为电喷雾离子源(ESI+),检测方式为多反应检测(MRM)模式。离子源温度为150 ℃,毛细管电压为0.35 kV,脱溶剂气温度500 ℃,雾化气氮气流速1000 L·h-1,锥孔气流速50 L·h-1;碰撞气(氩气)流速0.17 L·h-1。质谱参数见表2,4种细胞分裂素标准溶液的选择反应检测色谱图见图3。
2 结果与分析
2.1 提取条件优化
2.1.1 提取剂优化 在植物激素测定中,常用的提取剂有甲醇、乙腈和丙酮[28-30]。以仙进奉果皮为样品,在其他条件相同的情况下,考察了80%甲醇、80%丙酮、80%乙腈作为提取溶剂的提取效果(图4-A)。结果表明,对于tZR和IPR而言,80%乙腈的提取效果好;对于DHZ而言,80%甲醇的提取效果更好;对于IP而言,80%丙酮的提取效果更佳。经过综合比较,选择80%乙腈作为提取剂。
2.1.2 提取时间优化 选定80%乙腈作为提取剂,考察了提取时间分别为4 h、8 h、12 h、16 h时对细胞分裂素提取效果的影响。结果(图4-B)表明,提取时间从4 h延长到8 h时,tZR和DHZ的提取效果得到了显著提升;提取时间从8 h延长到12 h时,除IPR和IP提取效果略有下降外,其他两种细胞分裂素的提取效果没有发生显著变化;提取时间从12 h延长到16 h时,IP的提取效果略有下降,tZR的提取效果显著下降。综合比较,提取时间最终选择8 h。
2.2 纯化条件优化
2.2.1 固相萃取柱优化 纯化条件主要考虑两个重要参数:固相萃取柱和洗脱溶剂。目前比较常见的固相萃取柱有以十八烷基键合相硅胶为填料的C18柱,以亲水-亲油平衡聚合物材料为填料的HLB柱以及以混合阳离子交换材料为填料的PCX柱等。以仙进奉果皮为样品,选择C18柱、HLB柱、PCX柱,以及不纯化这4种处理方式进行比较(图5-A)。C18柱可有效去除果皮中酯类及色素类化合物;HLB柱无需活化,纯化步骤少,可有效除去磷脂、蛋白质和色素等大分子物质;PCX柱能够除去大量酸性和中性化合物;空白对照不进行除杂。选择PCX柱进行纯化时,4种细胞分裂素检测响应值高且分离度好。其他3种纯化方式,4种细胞分裂素检测响应值都较PCX柱低,DHZ和IP在色谱图中分离度差。综上所述,选择PCX柱对样品进行纯化。
2.2.2 洗脱液优化 根据选定PCX柱的特点,再结合细胞分裂素的理化性质,在空白样品中添加相同质量浓度(5 ng·mL-1)4种细胞分裂素,选择不同体积分数的氨水甲醇溶液进行洗脱,考察回收率差异(图5-B)。试验发现,不同体积分数氨水甲醇洗脱液均可影响细胞分裂素回收率。当氨水甲醇的体积分数为0.1%和1%时,碱性较弱,PCX柱上固定的细胞分裂素不能被充分洗脱;氨水甲醇体积分数增加到2.5%时,碱性增强,被洗脱的IPR和DHZ含量显著增加;氨水甲醇体积分数增加到5%时,碱性进一步增强,但IPR和DHZ洗脱效果不佳,其他几种细胞分裂素无明显变化。因此,综合比较之后,选择体积分数为2.5%的氨水甲醇溶液进行洗脱。
2.3 色谱条件优化
细胞分裂素是一类碱性物质,流动相的pH值和组成会对色谱分离和响应强度有较大的影响。试验采用甲醇分别与0.05%甲酸水溶液、0.10%甲酸水溶液、1 mmol·L-1甲酸铵水溶液和5 mmol·L-1甲酸铵水溶液组合,作为流动相进行考察,发现5 mmol·L-1甲酸铵水溶液(pH≈4.5)-甲醇为流动相时,4种细胞分裂素峰形较好,目标物响应值高(图6)。
2.4 方法准确度及精密度考察
2.4.1 线性关系和检出限 取质量浓度为50 ng·mL-1的混合标准溶液,依次稀释配制成系列质量浓度为0.05、0.10、0.50、1.00、5.00、10.00、50.00 ng·mL-1的混合标准溶液进行检测,绘制标准曲线,并进行线性回归,得到回归方程和相关系数(r2)。再分别以3倍信噪比(S/N)和10倍信噪比所对应的质量浓度确定检出限(LOD)和定量限(LOQ)。结果表明,tZR、IPR、DHZ、IP在0.05~50.00 ng·mL-1范围内线性良好,r2>0.999,LOD和LOQ分别为3.11~18.12和10.37~60.39 pg·g-1,说明该研究方法对细胞分裂素有较高的检测灵敏度(表3)。
2.4.2 回收率 为了评价该方法的准确性和可重复性,以荔枝果皮为样品,向其中添加低、中、高三种不同质量浓度的标品(0.4、2.0、20.0 ng·mL-1)进行回收率试验,做3次平行试验,计算平均加标回收率和相对标准偏差。结果(表4)显示,4种细胞分裂素的相对回收率为80.0%~108.2%,RSD为0.8%~15.5%。
2.4.3 实际样品分析 为了证明该方法对荔枝不同部位的普适性,利用所建立的方法对荔枝果皮、果肉、种子、叶片、果柄离区和幼果中的内源细胞分裂素进行测定(图7)。测定结果见表5,tZR含量(w,后同)以种子中最高(3.92 ng·g-1),果肉中最低(0.30 ng·g-1);IPR含量以叶片中最高(11.83 ng·g-1),其次为离区(7.86 ng·g-1),其他组织的含量均低于1.00 ng·g-1;DHZ在所有测定的组织中,只有叶片和种子中含量达到0.10 ng·g-1,其他组织均低于此值;IP含量以离区中最高(1.29 ng·g-1),种子种最低。上述部位中的4种细胞分裂素都能被检出,说明该法可用于荔枝不同部位的测定。
3 讨 论
对荔枝内源激素的测定多采用ELISA法以及HPLC法(目前难以胜任)。ELISA法利用抗原与抗体的特异反应将待测物与酶建立关联,当同一类结构相似激素存在时,抗体专一结合性差,导致检测值偏高;HPLC法根据各组分在固定相及流动相中吸附能力、分配系数、离子交换作用或分子尺寸大小的差异,在色谱柱中进行分离纯化,因此结构和极性相似的两种化合物可分离度较差。李伟才等[7]用液相色谱法测得荔枝谢花后80 d内果实中细胞分裂素的含量为0~800 ng·g-1,而周贤军等[31]用酶联免疫测量结果为0~760 pg·g-1。周昌敏等[10]用酶联免疫测的白点期前后叶片中细胞分裂素含量为300~600 ng·g-1,花静静[32]用普通液质联用(LC-MS)测出来的结果约2.74 ng·g-1。胡桂兵等[33]用酶联免疫测定结果约为335.36 ng·g-1,笔者在本研究中用超高效液相色谱-三重四级杆质谱联用法测定荔枝果皮花后50 d的IPR含量约为0.62 ng·g-1,金峰等[34]用液质联用测定冬梢叶片中IP和IPR含量分别为0.66和9.88 ng·g-1,与本文测定结果接近。以上结果说明ELISA法以及HPLC法的准确性和灵敏度较差。而质谱分析法(MS),是通过高能电子轰击,检测带电分子离子或碎片离子的质量数,最终确定带电离子质量的方法,具有高选择性和灵敏性。另外与传统的HPLC相比,超高效液相色谱(UPLC)在保留HPLC优点的同时,还提升了分析速度。UPLC-MS/MS法更是结合了UPLC的高分离性、高速度的特点以及MS/MS高选择性、高灵敏度的特点,成为了目前植物激素检测的主要手段之一,在石榴、文冠果、苹果等果树中也有相关报道[35-37]。
植物内源激素属于微量物质,难以分析,且样品基质效应复杂,所以合适的前处理过程以及分析方法显得尤为重要。当以80%丙酮和80%甲醇为提取剂时,对tZR和IP的提取效果不佳,且80%丙酮作为提取剂时,提取剂绿色很深,说明其中溶解了很多叶绿素,易对后续检测造成干扰;以80%乙腈为提取剂时,DHZ和IPR提取效果不如另两种,但tZR和IP的响应值显著高于另外两种,且色谱图分离度好。综合考虑,选用80%乙腈作为提取剂。在提取过程中,提取8 h可达最佳效果,无需过夜,可实现当天提取,当天测定,提高了检测结果的准确度。与其他植物内源激素相比,细胞分裂素较为特别的地方在于其具有嘌呤环,呈碱性。因此利用其特性,在纯化时选择PCX强阳离子交换柱,该柱以聚合阳离子交换树脂为填料,对弱碱性化合物吸附性较强,进而提高了碱性化合物的纯化效果。细胞分裂素在酸化后的提取液(pH≈2~3)中呈阳离子状态,过柱时被填料吸附,再用含0.1%甲酸的甲醇溶液(pH≈5~6)淋洗,此时大多数酸性和中性化合物被清除,留在柱上的目标物再用2.5%的氨水甲醇溶液洗脱(pH≈11.3),目标物随着洗脱液流出,最终实现目标物的收集。XSelect HSS T3色谱柱以高强度硅胶为填料,可延长极性目标物的保留时间,有利于样品组分更好的分离。同时方法优化了流动相条件,最终实现了荔枝果实中多种细胞分裂素有效分离和准确定量。
4 结 论
建立了高效液相色谱-三重四极杆质谱法同时测定荔枝中tZR、IPR、DHZ和IP这4种细胞分裂素的检测方法。样品通过80%乙腈低温提取,PCX纯化,X Select HSS T3分离,在ESI+、MRM模式下分析定量,实现了荔枝不同样品中4种关键细胞分裂素的高效提取和测定。样品平均加标回收率为80.0%~108.2%,RSD范围为0.8%~15.5%。经方法学验证,该方法具备前处理检测周期短、重现性好、灵敏度高等优点,适用于荔枝各部位细胞分裂素的快速筛查和定量测定。
参考文献References:
[1] BRIZZOLARI A,FOTI M C,SASO L,CIUFFREDA P,LAZAREVI? J,SANTANIELLO E. Evaluation of the radical scavenging activity of some representative isoprenoid and aromatic cytokinin ribosides (N6-substituted adenosines) by in vitro chemical assays[J]. Natural Product Research,2022,36(24):6443-6447.
[2] 马新梅. 拟南芥细胞分裂素O-糖基转移酶基因功能分析[D]. 济南:山东大学,2011.
MA Xinmei. Function analysis of a cytokinin O-glycosyltransferase gene in Arabidopsis thaliana[D]. Jinan:Shandong University,2011.
[3] 鲁敏,黄亚欣,王国立,安华明.‘贵长猕猴桃果实内源激素的动态分布及含量变化与果实形状发育的关系[J].植物生理学报,2020,56(10):2159-2167.
LU Min,HUANG Yaxin,WANG Guoli,AN Huaming. The correlation between the dynamic distribution and content of endogenous hormones and the kiwi fruit shape during ‘Guichang fruits development[J]. Plant Physiology Journal,2020,56(10):2159-2167.
[4] 王宁,张艳霞,李栋梅,王振平. 宁夏不同子产区霞多丽葡萄内源激素及果实品质差异研究[J]. 果树学报,2023,40(7):1374-1385.
WANG Ning,ZHANG Yanxia,LI Dongmei,WANG Zhenping. Effect of different terroir conditions on endogenous hormones and berry quality of Chardonnay at the East Helan Mountains[J]. Journal of Fruit Science,2023,40(7):1374-1385.
[5] 李建国,王惠聪,周碧燕,赵明磊,李彩琴,夏瑞,黄旭明. 荔枝花果发育生理和分子生物学研究进展[J]. 华南农业大学学报,2019,40(5):119-127.
LI Jianguo,WANG Huicong,ZHOU Biyan,ZHAO Minglei,LI Caiqin,XIA Rui,HUANG Xuming. Research advances in physiology and molecular biology of flower and fruit development in litchi[J]. Journal of South China Agricultural University,2019,40(5):119-127.
[6] 郭栋梁,黄石连,向旭. 2022年广东荔枝生产形势分析[J]. 广东农业科学,2022,49(6):130-137.
GUO Dongliang,HUANG Shilian,XIANG Xu. Analysis of Guangdong litchi production situation in 2022[J]. Guangdong Agricultural Sciences,2022,49(6):130-137.
[7] 李伟才,魏永赞,胡会刚,石胜友,王一承,谢江辉. 3种无核荔枝果实发育过程中内源激素含量变化动态[J]. 热带作物学报,2011,32(6):1042-1045.
LI Weicai,WEI Yongzan,HU Huigang,SHI Shengyou,WANG Yicheng,XIE Jianghui. Dynamic changes of endogenous hormone contents in the pericarp of seedless litchi during fruit growth and development[J]. Chinese Journal of Tropical Crops,2011,32(6):1042-1045.
[8] 肖华山,吕柳新,陈志彤. 荔枝花芽分化过程中内源激素含量的动态变化[J]. 宁德师专学报(自然科学版),2007,19(2):113-115.
XIAO Huashan,L? Liuxin,CHEN Zhitong. Dynamic changes of endogenous hormone inlitchi (Litchi chinensis Sonn.) during flower bud differentiation[J]. Journal of Ningde Teachers College (Natural Science),2007,19(2):113-115.
[9] 吴志祥,周兆德,陶忠良,王令霞. 妃子笑与鹅蛋荔枝花芽分化期间内源激素的变化[J]. 热带作物学报,2005,26(4):42-45.
WU Zhixiang,ZHOU Zhaode,TAO Zhongliang,WANG Ling-xia. Changes of endogenous hormones in Feizixiao and Edan litchi during flower bud differentiation[J]. Chinese Journal of Tropical Crops,2005,26(4):42-45.
[10] 周昌敏,何兆桓,杨苞梅,李国良,姚丽贤. 环剥对荔枝叶片营养及花果生长发育的影响[J]. 广东农业科学,2018,45(4):34-42.
ZHOU Changmin,HE Zhaohuan,YANG Baomei,LI Guoliang,YAO Lixian. Effect of girdling on litchi foliar nutrient and development of flower and fruit[J]. Guangdong Agricultural Sciences,2018,45(4):34-42.
[11] 付亚男. A4无核荔枝落果与裂果生理基础及其调控研究[D]. 海口:海南大学,2017.
FU Yanan. Studies on the physiological basis of the fruit drop and cracking and its regulation of A4 seedless litchi[D]. Haikou:Hainan University,2017.
[12] 车建美,赖钟雄,赖呈纯,郭志雄,刘鸿洲,黄志宏. 荔枝体细胞胚胎发生早期的3种内源激素含量变化[J]. 热带作物学报,2005,26(2):55-61.
CHE Jianmei,LAI Zhongxiong,LAI Chengchun,GUO Zhixiong,LIU Hongzhou,HUANG Zhihong. Changes of endogenous phytohormones during the early somatic embryogenesis in litchi (Litchi chinensis Sonn.)[J]. Chinese Journal of Tropical Crops,2005,26(2):55-61.
[13] 蔡保东,刘昭,丁俊,冯钰锜. 混合模式色谱-串联质谱联用快速和高灵敏检测植物组织内源性细胞分裂素[J]. 分析科学学报,2014,30(5):613-618.
CAI Baodong,LIU Zhao,DING Jun,FENG Yuqi. Rapid and highly sensitive determination of endogenous cytokinins in plant samples by mixed-mode liquid chromatography-tandem mass spectrometry[J]. Journal of Analytical Science,2014,30(5):613-618.
[14] 魏永赞,董晨,王弋,郑雪文,李伟才. 烯效唑对荔枝花期发育过程内源激素的影响[J]. 热带作物学报,2017,38(7):1188-1192.
WEI Yongzan,DONG Chen,WANG Yi,ZHENG Xuewen,LI Weicai. Effects of uniconazole on the endogenous phytohormone contents in the florescence period of litchi[J]. Chinese Journal of Tropical Crops,2017,38(7):1188-1192.
[15] CHEN X,TAO Z L,WU Z X,WANG L X,FU H Z,ZHOU Z D,FAN W B. Effect of paclobutrazol plus ethephon treatment on endogenous hormones and carbon and nitrogen nutrients in litchi variety ‘Feizixiao[J]. Agricultural Science & Technology,2013,14(8):1125-1131.
[16] 李建国,黄旭明,黄辉白,周碧燕. 大果型和小果型荔枝品种果实发育细胞学和生理学比较[J]. 果树学报,2002,19(3):158-162.
LI Jianguo,HUANG Xuming,HUANG Huibai,ZHOU Biyan. A cytological and physiological study of large-and small-sized litchi cultivars fruit[J]. Journal of Fruit Science,2002,19(3):158-162.
[17] 李建国,周碧燕. 大核和焦核“桂味” 荔枝果实发育及其发育期间果皮中内源激素含量的变化比较[J]. 植物生理学通讯,2005,41(5):587-590.
LI Jianguo,ZHOU Biyan. Comparison on fruit development and changes in endogenous hormone contents in pericarp between large- and aborted-seeded litchi (Litchi chinensis Sonn. cv. Guiwei)[J]. Plant Physiology Communications,2005,41(5):587-590.
[18] 曾庆钱,陈厚彬,鲁才浩,李建国. HPLC测定荔枝不同器官中内源激素流程的优化[J]. 果树学报,2006,23(1):145-148.
ZENG Qingqian,CHEN Houbin,LU Caihao,LI Jianguo. An optimized HPLC procedure for analyzing endogenous hormones in different organs of litchi[J]. Journal of Fruit Science,2006,23(1):145-148.
[19] 李玉璇,段春凤,关亚风. 植物样品中内源性植物激素时空分布的研究进展[J]. 色谱,2019,37(8):806-814.
LI Yuxuan,DUAN Chunfeng,GUAN Yafeng. Recent advances in spatio-temporal distribution of endogenous phytohormones[J]. Chinese Journal of Chromatography,2019,37(8):806-814.
[20] 曹赵云,马有宁,牟仁祥,于莎莎,陈铭学. 固相萃取-液相色谱-串联质谱法测定水稻中17种细胞分裂素[J]. 色谱,2015,33(7):715-721.
CAO Zhaoyun,MA Youning,MOU Renxiang,YU Shasha,CHEN Mingxue. Analysis of 17 cytokinins in rice by solid phase extraction purification and liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Chromatography,2015,33(7):715-721.
[21] ZHANG M,CHEN X J,ZHAO Y J,ZHANG J Q,HE Q Q,QIAN J Q,TAN G Y,LIU W,YANG X L,WANG B M. Quantification of six types of cytokinins:Integration of an ultra-performance liquid chromatographic-electrospray tandem mass spectrometric method with antibody based immunoaffinity columns equally recognizing cytokinins in free base and nucleoside forms[J]. Journal of Chromatography A,2022,1682:463497.
[22] 童建华,赵文魁,张雪芹,梁艳萍. 拟南芥中4种细胞分裂素的高效液相色谱法测定[J]. 亚热带植物科学,2008,37(4):18-21.
TONG Jianhua,ZHAO Wenkui,ZHANG Xueqin,LIANG Yanping. Simultaneous determination of four kinds of cytokinin components in Arabidopsis thaliana by high performance liquid chromatography[J]. Subtropical Plant Science,2008,37(4):18-21.
[23] ZHOU X,LI R Q,WANG C,MA X X,SUN Y,SONG W X,WEI X B,LI D H,MA X,WANG R Q. Simultaneous quantitation of cytokinin bases and their glycoconjugates with stable isotope labelling ultrahigh performance liquid chromatography mass spectrometry[J]. Journal of Chromatography A,2021,1636:461782.
[24] CAI B D,ZHU J X,SHI Z G,YUAN B F,FENG Y Q. A simple sample preparation approach based on hydrophilic solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry for determination of endogenous cytokinins[J]. Journal of Chromatography B,2013,942/943:31-36.
[25] CAO Z Y,SUN L H,MOU R X,ZHANG L P,LIN X Y,ZHU Z W,CHEN M X. Profiling of phytohormones and their major metabolites in rice using binary solid-phase extraction and liquid chromatography-triple quadrupole mass spectrometry[J]. Journal of Chromatography A,2016,1451:67-74.
[26] LI Y H,WEI F,DONG X Y,PENG J H,LIU S Y,CHEN H. Simultaneous analysis of multiple endogenous plant hormones in leaf tissue of oilseed rape by solid-phase extraction coupled with high-performance liquid chromatography-electrospray ionisation tandem mass spectrometry[J]. Phytochemical Analysis,2011,22(5):442-449.
[27] HAN Z,LIU G,RAO Q X,BAI B,ZHAO Z H,LIU H,WU A B. A liquid chromatography tandem mass spectrometry method for simultaneous determination of acid/alkaline phytohormones in grapes[J]. Journal of Chromatography B,2012,881/882:83-89.
[28] 张军,杜平. 高效液相色谱-串联质谱法测定葡萄中的吡效隆和赤霉素[J]. 色谱,2011,29(11):1133-1136.
ZHANG Jun,DU Ping. Determination of forchlorfenuron and gibberellin acid in the grapes using high performance liquid chromatography tandem mass spectrometry[J]. Chinese Journal of Chromatography,2011,29(11):1133-1136.
[29] 徐婧,王丹,陈庆欣,张艳青,王俊,赵明磊,李建国. 超高效液相色谱—三重四级杆质谱联用法同时定量荔枝果实组织中IAA和ABA[J]. 中国南方果树,2022,51(1):59-66.
XU Jing,WANG Dan,CHEN Qingxin,ZHANG Yanqing,WANG Jun,ZHAO Minglei,LI Jianguo. Simultaneous quantification of indole-3-aceticacid and abscisic acid in litchi fruit tissues by ultra-high performance liquid chromatography-tandem mass spectrometry[J]. South China Fruits,2022,51(1):59-66.
[30] IZUMI Y,OKAZAWA A,BAMBA T,KOBAYASHI A,FUKUSAKI E. Development of a method for comprehensive and quantitative analysis of plant hormones by highly sensitive nanoflow liquid chromatography-electrospray ionization-ion trap mass spectrometry[J]. Analytica Chimica Acta,2009,648(2):215-225.
[31] 周贤军,黄德炎,黄辉白,吴定尧. 螺旋环剥对‘糯米糍荔枝坐果与碳水化合物及激素的影响[J]. 园艺学报,1999,26(2):77-80.
ZHOU Xianjun, HUANG Deyan, HUANG Huibai, WU Dingyao. Carbohydrate and endohormone status in relation to fruit set as influenced by trunk spiral girdling of young litchi trees[J]. Acta Horticulturae Sinica,1999,26(2):77-80.
[32] 花静静. 无核荔枝促花技术及其生理研究[D]. 海口:海南大学,2021.
HUA Jingjing. Study on flower-promoting technology and physiology of seedless litchi[D]. Haikou:Hainan University,2021.
[33] 胡桂兵,陈大成,李平,欧阳若,高飞飞,王卫华. 荔枝果皮花青苷与内源激素含量的变化规律[J]. 福建果树,2000(1):1-3.
HU Guibing,CHEN Dacheng,LI Ping,OUYANG Ruo,GAO Feifei,WANG Weihua. Changes in anthocyanin and endogenous hormone content in litchi peel[J]. Fujian Fruits,2000(1):1-3.
[34] 金峰,向旭,邱燕萍,袁沛元,凡超. 乙氧氟草醚对桂味荔枝冬梢控杀效果及内源激素影响的研究[J]. 核农学报,2023,37(9):1894-1903.
JIN Feng,XIANG Xu,QIU Yanping,YUAN Peiyuan,FAN Chao. Study on oxyfluorfen control effect of oxyfluorfen on Guiwei litchi winter shoots (Litchi chinensis Sonn.) and its influence on endogenous hormones[J]. Journal of Nuclear Agricultural Sciences,2023,37(9):1894-1903.
[35] YILMAZ C,OZGUVEN A I. Hormone physiology of preharvest fruit cracking in pomegranate (Punica granatum L.)[J]. Acta Horticulturae,2006,727:545-550.
[36] 王鹏侠,阮成江,杜维,赵振. 文冠果种子不同发育期内源激素的动态变化[J]. 大连民族大学学报,2022,24(1):1-4.
WANG Pengxia,RUAN Chengjiang,DU Wei,ZHAO Zhen. Dynamic changes of endogenous hormones in Xanthoceras sorbifolium seeds during different developmental stages[J]. Journal of Dalian Minzu University,2022,24(1):1-4.
[37] 王庆杰,金仲鑫,周李杰,郝玉金,姚玉新. 苹果MdcyMDH过量表达对光合、激素和生长的影响[J]. 中国农业科学,2015,48(14):2868-2875.
WANG Qingjie,JIN Zhongxin,ZHOU Lijie,HAO Yujin,YAO Yuxin. Impacts of MdcyMDH overexpression on photosynthesis,hormone and growth in apple[J]. Scientia Agricultura Sinica,2015,48(14):2868-2875.
收稿日期:2024-02-06 接受日期:2024-03-24
基金项目:国家自然科学基金重点项目(32330092);国家现代农业产业技术体系建设项目(CARS-32-07)
作者简介:黄雨莲,女,在读硕士研究生,研究方向为植物激素测定。E-mail:1401175682@qq.com
*通信作者 Author for correspondence. E-mail:jxu@scau.edu.cn;E-mail:jianli@scau.edu.cn

