Leveraging metformin to combat hepatocellular carcinoma: its therapeutic promise against hepatitis viral infections
Ali Shojaeian, Mohsen Nakhaie, Zahra Sobhi Amjad, Armin Khaghani Boroujeni, Somayeh Shokri,5,Shahab Mahmoudvand,5
1Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan 6517838736, Iran.
2Gastroenterology and Hepatology, Research Center, Institute of Basic and Clinical Physiology Science, Kerman University of Medical Science, Kerman 7616913555, Iran.
3Department of Microbiology, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah 6715847141, Iran.
4Skin Disease and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran.
5Department of Virology, School of Medicine, Hamadan University of Medical Sciences, Hamadan 6517838736, Iran.
Abstract Hepatocellular carcinoma (HCC) is categorized among the most common primary malignant liver cancer and a primary global cause of death from cancer.HCC tends to affect males 2-4 times more than females in many nations.The main factors that raise the incidence of HCC are chronic liver diseases, hepatotropic viruses like hepatitis B (HBV) and C (HCV), non-alcoholic fatty liver disease, exposure to toxins like aflatoxin, and nonalcoholic steatohepatitis (NASH).Among these, hepatitis B and C are the most prevalent causes of chronic hepatitis globally.Metformin, which is made from a naturally occurring compound called galegine, derived from the plant Galega officinalis (G.officinalis), has been found to exhibit antitumor effects in a wide range of malignancies,including HCC.In fact, compared to patients on sulphonylureas or insulin, studies have demonstrated that metformin treatment significantly lowers the risk of HCC in patients with chronic liver disease.This article will first describe the molecular mechanism of hepatitis B and C viruses in the development of HCC.Then, we will provide detailed explanations about metformin, followed by a discussion of the association between metformin and hepatocellular carcinoma caused by the viruses mentioned above.
Keywords: Hepatocellular carcinoma, HCC, hepatitis B virus, hepatitis C virus, metformin
INTRODUCTION
Over 700,000 cases of hepatocellular carcinoma (HCC), a form of liver cancer, are reported each year,making it one of the main causes of cancer-related deaths globally.More than 80% of this global health problem occurs in developing countries[1].HCC is more commonly diagnosed in males, with 2-4 times higher incidence rates than in females in many countries[2].The primary factors that increase the incidence of HCC include chronic liver disorders, hepatotropic viruses such as hepatitis B (HBV) and C (HCV)viruses, non-alcoholic fatty liver disease, the use of/toxins such as aflatoxin, and non-alcoholic steatohepatitis (NASH) [Figure 1][3,4].Hepatitis B and C are the primary causes of chronic hepatitis globally,among these factors.Chronic viral hepatitis can cause cirrhosis or HCC if treatment is not received[5].

Figure 1.Risk factors associated with HCC development.
The eight different genotypes of the circular, double-stranded DNA virus known as HBV (A to H)[6].HCV,on the other hand, is a single-stranded RNA virus that is divided into six different genotypes and shows significant genetic variability (I to VI)[7].HBV-HCV co-infection can raise the chance of developing HCC in cirrhosis patients[8].The incidence of HCC can increase by 2.9 times when combined with alcohol[9].Due to its strong ability to increase insulin sensitivity and safety, the oral drug metformin has been extensively utilized for nearly 60 years to treat metabolic syndrome and type 2 diabetes[10].The medication functions by stimulating AMP-activated protein kinase (AMPK), which decreases hepatic gluconeogenesis and raises skeletal muscle uptake of glucose.A key indicator of the state of cellular energy, AMPK is activated in human fibroblasts and several forms of cancer[11].Intriguingly, recent reports have shown that metformin exhibits antitumor effects in many different cancers, such as HCC[12].In actuality, patients with chronic liver disease who take metformin instead of sulphonylureas or insulin have a lower chance of developing HCC[13].Metformin may have a direct effect on tumor-initiating HCC cells.In a study, rats were injected with celastrol, celastrol plus metformin for the treatment of HCC, and the potential of apoptosis was enhanced.The levels of caspase-9 and caspase-3 and Bax/BCL-2 were also observed.Metformin and celastrol suppressed NFκB, p65, TNFR, and TLR4 gene expression, preventing the activation caused by the phosphorylation of IκBκB and NFκBp65 and reducing the degradation of IκBα.Furthermore, the combined treatment of metformin plus celastrol suppressed angiogenesis, metastasis, and tumor proliferation, which was revealed by the decrease in the liver levels of VEGF, MMP-2.9, and cyclin D1 mRNA, respectively.
In summary, compared to using celastrol alone, the combination of metformin and celastrol appears to be more effective in treating HCC by blocking the NLRP3 inflammasome and NFκB signaling[14].Surgery is the primary treatment option for patients in the early stages of malignancy.However, if surgical resection or radiofrequency therapy is not feasible, patients can be treated with arterial chemoembolization[15], anticancer drug and lipiodol emulsion[16], or drug-eluting beads[17].Liver transplantation is a radical curative surgery that is commonly used as a standard treatment for HCC (hepatocellular carcinoma) using the Milan criteria[18].However, patients who require liver transplantation often face a shortage of donors and the progression of their cancer stage while waiting for a donor[19].In cases where other treatments fail, sorafenib(Nexavar), a kinase inhibitor drug, might be prescribed for patients.Sorafenib is approved to treat renal cell carcinoma (RCC), unresectable HCC, FLT3-ITD positive AML, and radioactively iodine-resistant advanced thyroid carcinoma.It exhibits activity against numerous protein kinases, including VEGFR, PDGFR, and RAF kinases.Preventing HCC and conducting thorough screening for high-risk populations are therefore essential[19,20].HCC is a primary liver cancer that primarily affects people with cirrhosis and chronic liver disease.However, Approximately 25% of patients have no history of cirrhosis or risk factors[18].Vaccination and antiviral therapy can have a positive effect in preventing the development of cirrhosis.Nevertheless, if antiviral therapy is delayed until cirrhosis develops, its preventive effect will be reduced[19].Interferon therapy is also ineffective in reducing the risk of HCC.There are other agents, such as metformin, retinoids,and propranolol, that may be effective in reducing HCC but require further prospective trials[10,20].Thus, the current study set out to find out whether metformin could aid in reducing HCC caused by the hepatitis B and C viruses.
MOLECULAR MECHANISMS OF HBV IN THE DEVELOPMENT OF HCC
Hepatitis B virus
TheHepadnaviridaefamily of enveloped DNA viruses includes HBV, which has partially double-stranded relaxed circular DNA (rcDNA).Globally, about 300 million people have HBV infection, which is a leading cause of cirrhosis development and its complications[21,22].HBV genome comprises four genes (C, S, X, and P).In addition, the Hepatitis B X protein (HBx) plays a critical role in the development of HCC at the molecular and cellular levels[23].One important component of the HBV-related HCC mechanism is HBV integration into the host genome.HBV is among the most important risk factors for liver cancer development.Numerous aspects of the host’s liver cells may be impacted by the infection, resulting in inflammation of the liver that may develop into chronic hepatitis, cirrhosis, and possibly HCC.Genomic stability, HBV gene expression, and host gene expression profiling can all be altered by HBV DNA integration into the host genome.Early in the infection process, this integration may take place.The host genes close to the integration sites may be impacted by the integrated fragment, which may ultimately contribute to carcinogenesis.The integrated fragment may express full-length or shortened HBV proteins.Interestingly, a significant proportion of HBV-related HCC can be seen in non-cirrhotic liver.This suggests that HBV integration into the host genome can lead to HCC even in the absence of cirrhosis[24].Furthermore, persistent HBV infection of the liver cells can lead to HCC, cirrhosis, fibrosis, or chronic hepatitis B.There are several HBV-related risk factors for HCC, including the level of HBV DNA load,genotypes of HBV, and full-long or truncated proteins of HBV.Overall, the integration of HBV into the host genome plays a crucial role in the development of HCC.It can occur early in the infection and can lead to HCC even in non-cirrhotic livers.This highlights the importance of early detection and treatment of HBV infection to prevent the development of HCC[25].Several known mechanisms play a crucial role in HBV-induced HCC, which are discussed below.
HBV-induced HCC mechanisms
Signaling pathways (including Hippo, Ras-Raf-MAPK, c-Src, PI3K-Akt, NF-κB, JAK-STAT, Wnt/β-catenin,TGF-βand p53)
The Hippo signaling pathway is essential for controlling stem cell self-renewal, apoptosis, and cell division[26].Furthermore, Yes-associated protein (YAP) is a downstream effector of this pathway and is considered to be a critical human oncogene.The Hippo pathway regulates YAP function, and the module phosphorylates YAP on serine residues, restraining YAP activity[27].Research on mice has demonstrated that overexpression of YAP stimulates the growth of liver cancer by controlling the transcription of specific target genes like c-myc, Ki-67, SRY-Box 4 (SOX4), H19, and α-fetoprotein (AFP)[28].Additionally,overexpression of YAP in transgenic mice increases liver size and eventually leads to cancer[29].Zhanget al.discovered that YAP was overexpressed in hepatoma HepG2.2.15 cell line, HCC samples, and liver cancer tissues from HBx-transgenic mice[30].Furthermore, a recent study found that the expressions of the HBx and YAP proteins are linked to metastasis and a poor prognosis[31].
By phosphorylating apoptosis-regulating factors such as Bad, Bim, Mcl-1, caspase 9, and the contentious Bcl-2, the Ras/Raf/MAPK cascade contributes to cell-cycle regulation, cell differentiation, and apoptosis[32].This pathway contributes to HCC development in a number of ways.For example, overexpression of Raf kinase is seen in the majority of HCC cases, and the Ras gene is mutated in approximately 30% of HCC cases.Furthermore, overexpression of several upstream growth factors, including transforming growth factor-α (TGF-α), platelet-derived growth factor-β (PDGF-β), vascular endothelial growth factor (VEGF),and epidermal growth factor (EGF) has been observed in HCC[33-35].Studies have shown that HBx promotes liver cancer metastasis by activating the ERK and p38 MAPK signaling pathways[36,37].Another study by Liaoet al.indicated that HBx upregulates the ERK and AKT pathways through the activation of the Notch1 pathway[38].Numerous biological processes, such as cell invasion, apoptosis, differentiation, proliferation,and survival, depend on the Notch pathway[39].
c-Src is a cytoplasmic non-receptor tyrosine kinase that mediates intracellular signal transmission by binding to various proteins in the Src family of kinases[40].By controlling angiogenesis, migration, invasion,apoptosis, proliferation, and angiogenesis, Src contributes significantly to the development of tumors and increases the progression of cancer[41].According to a Yanget al.study, c-Src plays a critical role in the development of HCC, and a poor prognosis is associated with its elevated expression[42].During developmental morphogenesis, a cellular process known as the epithelial-mesenchymal transition (EMT)gives stationary epithelial cells the capacity to migrate and invade[43].In HCC cells, HBx protein may induce EMT by activating c-Src[44].The PI3K/AKT pathway is crucial for controlling the motility, growth,proliferation, and metabolism of cells[45].Once activated, the PI3K/AKT pathway increases the expression of matrix metalloproteinases (MMPs) and snail transcriptional for EMT induction[46].Wanget al.reported a significant up-regulation of PI3K/AKT signaling in HCC Huh7 cells[47].Multiple studies have also demonstrated that HBx can activate the PI3K/AKT pathway, promoting cell proliferationin vitro[48-51].
The transcription factor nuclear factor kappa B (NF-κB) is essential for both innate and adaptive immunity as well as inflammation.It acts as a central link between hepatic injury, fibrosis, and HCC[52].Research has indicated that HBx can trigger NF-κB via multiple unique signaling pathways[53].Interleukin 6 (IL-6) is among a group of cytokines and growth factors that are produced when NF-κB signaling is activated in Kupffer cells.The most significant cytokine in determining whether HCC will survive is IL-6[54].In this regard, Quétieret al.indicated that HBx can promote the expression of IL-6 in hepatoma cells[55].Another study revealed that HBeAg can cause HBV replication and sustain persistent viral infection by suppressing NF-κB activation[56].It is true that NF-κB has two sides.HBV causes hepatocyte inflammation through the NF-kB pathway dysregulation, but it also inhibits antiviral immune responses.
Important downstream pathways of interferon (IFN) receptors that promote the synthesis of IFNstimulated genes (ISGs) include the Janus kinase (JAK)-signal transducer and activator of transcription(STAT) pathway.Numerous studies have shown that this pathway is often deregulated in cancer, including HCC.The pathway plays a vital role in IFN-mediated inhibition of HBV replication[53].According toYou et al.,the HBx protein can control JAK1, JAK2, and Tyrosine kinase 2 (TYK2), whereas the HBeAg protein can suppress AK-STAT signaling to promote HBV replication[53].Additionally, Choet al.showed that HBx could inhibit the activation of TYK2 by decreasing the IFN-α receptor 1 (IFNAR1) expression to inhibit extracellular IFN-mediated signal transduction[57].The activation of STAT3 in cancers leads to gene expression, resulting in cell proliferation and resistance to apoptosis.HBx also plays a vital role in STAT3 activation[58].
The Wnt/β-catenin signaling pathway plays a role in multiple developmental processes, such as migration,homeostasis, apoptosis, and cancer linked to inflammation[59].Alterations in the Wnt/β-catenin activity have been linked to liver damage, inflammation, and the development of HCC[60].The pathway activates cyclooxygenase (COX)-2, which enhances the inflammatory response[61].COX-2 plays a crucial role in producing matrix metalloproteinases (MMPs) by liver cells, which are essential for tumor progression and metastasis.Studies have shown that HBx is significant in modulating and inducing the canonical Wnt/βcatenin signaling pathway[62-64].
Transforming growth factor-beta (TGF-β) is a cytokine that controls immune cell function and plays a role in angiogenesis, immunoregulation, wound healing, and cancer[65].The dysregulation of TGF-β is a key factor in the development of HCC[66].Recent research has demonstrated that the coexistence of HBx and TGF-β1 is associated with the malignant transformation of hepatic progenitor cells (HPCs), and that the degradation of protein phosphatase magnesium-dependent 1A (PPM1A) induced by the HBx protein is a novel mechanism for the over-activation of the TGF-β pathway[67].Donget al.showed that HBx and TGF-β1 coexist in the malignant transformation of hepatic progenitor cells (HPCs)[68].The tumor suppressor protein P53 (TP53) plays a crucial role in maintaining genomic integrity[69].Yanget al.found that TP53 mutations were the most common gene change in HCC and HBV-related HCC patients using bioinformatics; this frequency can increase[70].Research has indicated that there are direct interactions between HBx and p53 bothin vivoandin vitro[69].Chanet al.found that HBx modulates p53 genes through post-translational modifications[71].According to a different study, p53 may function as an inhibitor of HBV replication[69][Figure 2].

Figure 2.Molecular mechanisms of HBV-related HCC.
Epigenetic and genetic alterations
Heritable modifications in gene expression that do not entail modifications to DNA sequence are referred to as epigenetics[72].The HBx protein induces hyper or hypo-methylation and histone acetylation or deacetylation of tumor-related genes, affecting epigenetic changes[73,74].These alterations may cause the genome of the host cell to become unstable, which would cause oncogenes and tumor suppressor genes to express abnormally[75].Furthermore, the modifications facilitate the virus’s evasion of immune monitoring and advance the course of the illness from inflammation to tumor development[76].Long noncoding RNAs(lncRNAs) are a class of noncoding RNA with a transcript length of > 200 nucleotides[77].Via particular cell signaling pathways, LncRNAs affect HCC invasion, migration, and proliferation.They also cause HCC therapy resistance[78].Several studies have shown that particular lncRNAs can be directly or indirectly disregulated by the HBx protein[79-81][Table 1].

Table 1.Role of lncRNAs in HBV-induced HCC
A family of small noncoding RNAs known as microRNAs (miRNAs) controls translational regulation of gene expression[82].Researchers have shown that in HBV-induced HCC, HBx dysregulated some miRNAs[Table 2][83-85].
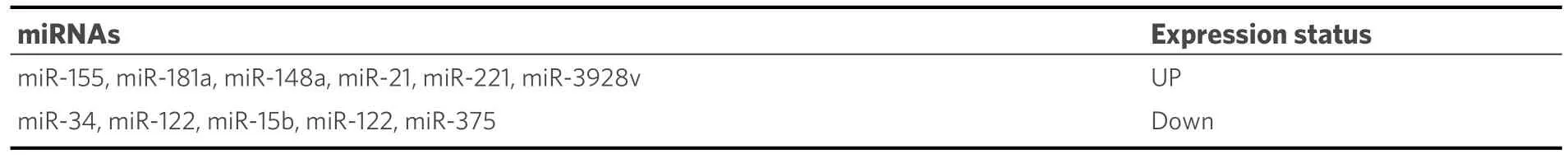
Table 2.Role of miRNAs in HBV-induced HCC
Autophagy
Autophagy (self-eating) is a lysosome-dependent cellular degradation program that acts as an adaptive cell response to stimuli or stresses to maintain homeostasis and prevent nutritional, metabolic, and infectionmediated stresses[86].However, autophagy plays a dual role in the development of liver cancer.On the one hand, it inhibits the expression of tumor suppressors and contributes to chemoresistance in HCC cells.On the other hand, it activates several signaling pathways, such as the PI3K-AKT-mTOR, AMPK-mTOR, EGF,MAPK, Wnt/β-catenin, p53, and NF-κB pathways, which can promote liver cancer development[87,88].HepG-2 cells’ autophagic lysosome pathway can be triggered by the HBV-encoded protein HBx via the PI3K-Akt-mTOR pathway[89]and in response to TLR4 stimulation[90].Luoet al.suggested that HBx induces autophagy to activate NF-κB and promote proinflammatory cytokine production, such as IL-6, IL-8, and CXCL2[91].
Reactive oxygen species
Reactive oxygen species (ROS)-induced activation of tumor suppressor genes and proto-oncogenes triggers the activation of signal transduction pathways, which in turn plays oncogenic roles[92].ROS is known to have a crucial role in the progression of liver disease, regardless of its underlying cause[93].Chronic viral infections can exacerbate ROS production, causing oxidative stress in host cells[94].HBV modifies mitochondrial function to generate ROS, and it has been demonstrated that the HBx protein increases mitochondrial ROS production[95,96].
MOLECULAR MECHANISMS OF HCV IN THE DEVELOPMENT OF HCC
Hepatitis C virus
A major cause of liver cirrhosis and hepatocellular carcinoma, affecting 71 million people globally, is the hepatitis C virus (HCV)[97].HCV can lead to acute and chronic infections, with acute infection affecting 20%-30% of adults, while chronic infection occurs in 70%-80% of infected individuals, causing prolonged liver damage[98].The genome of the virus codes for several different proteins, including structural proteins like core, E1, and E2, and non-structural proteins such as p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B[99].Presently, there are no vaccines available for HCV, but antiviral therapies are used to cure HCV-infected patients[100].The molecular mechanisms behind HCV-induced hepatocellular carcinoma are complex,involving several factors, such as oxidative stress, epigenetic and genetic alterations, steatosis, proliferation,and apoptosis[101].
HCV-induced HCC mechanisms
Oxidative stress
A major factor in the emergence of both chronic liver disease and liver cancer is oxidative stress.Oxidative stress can produce ROS, which can activate inflammatory genes and raise the risk of cancer[101].Patients with cirrhosis are more likely to develop liver cancer because chronic hepatitis C infection is linked to elevated levels of oxidative stress[102].Recent studies have shown that ROS production is initiated by the immune system in chronic hepatitis[103].Moreover, research by Farinatiet al.revealed that HCV infection leads to higher levels of ROS production compared to other hepatitis viruses[104].Additionally, research has shown that the HCV core protein raises oxidative stress levels in the liver[105].Several studies have shown that various proteins of HCV, including E1, E2, NS4B, NS5A, and NS3, are involved in inducing oxidative stress[106-108].The NS5A and NS3 proteins are known to cause oxidation of mitochondrial glutathione, which leads to an increase in ROS in the mitochondria.Oxidative stress is caused by the translocation of transcription factors into the nucleus of nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT-3)[109-111].Furthermore, p38 MAPK (mitogen-activated protein kinase), JNK (c-Jun N-terminal kinase), and AP-1 (activator protein-1) are activated by NS5A to cause oxidative stress[112].
Epigenetic and genetic alterations
Studies have shown that epigenetic alterations can contribute to initiating and promoting HCC.Researchers have reported that histone modifications, DNA methylation, and noncoding RNA (ncRNA) expression are associated with the progression of HCC[113].It is thought that HCV’s carcinogenic potential is indirectly related to epigenetic changes, which occur when oncogenic genes are activated by hypomethylation and tumor suppressor genes are inactivated by hypermethylation[114].Methylation disorders have the potential to cause HCC by inactivating tumor suppressor genes and activating carcinogenic genes.According to Manet al., there are different degrees of methylation in 18 genes related to HCV-induced HCC, including tumor suppressors and carcinogenic genes [Table 3][115].
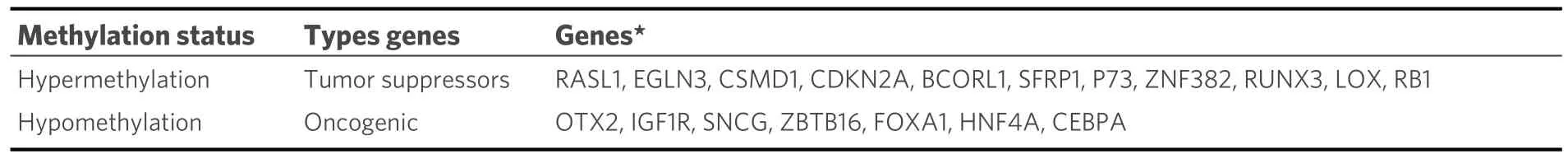
Table 3.Methylation disorders in HCV-related HCC
Moreover, the development of HCC is significantly influenced by histone modifications, including methylation, acetylation, phosphorylation, sumoylation, and ubiquitination.Overexpression of Jumonji AT-rich interactive domain 1B/lysine-specific demethylase 5B (JARID1B/KDM5B), for example, has been shown to promote HCC cell proliferation in HCV-induced HCC by controlling the expression of E2 factor 1 (E2F1) and E2 factor 2 (E2F2)[116].Similar to this, histone acetylation plays a major role in the development of HCV-induced HCC.For example, histone 3 is acetylated on lysine 27 (H3K27Ac), histone H3 is acetylated on lysine 14 (H3K14Ac), histone H3 is acetylated on lysine 9 (H3K9Ac), and histone H2A is acetylated on lysine 5 (H2AK5ac)[117,118].Additionally, noncoding RNAs (ncRNAs) have also been implicated in the progression of HCC.Dysregulation of ncRNA expression has been shown to affect HCC metastasis, invasion, dissemination, and recurrence.For example, Plissonnieret al.reported that the expression of lncRNA plays a crucial role in HCV-induced HCC [Table 4][119].
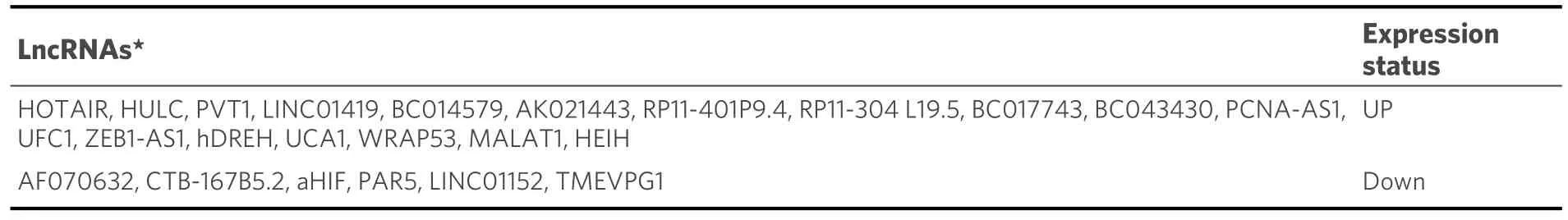
Table 4.Role of lncRNAs in HCV-induced HCC
Additionally, there is evidence suggesting a connection between hepatocellular carcinoma (HCC) and the disruption of microRNAs (miRNAs) regulation[120-122].Research indicates that HCV, miRNAs, and metabolic pathways in hepatocytes interact closely, leading to the development of liver disease.In a survey conducted by Diazet al., they identified 18 miRNAs that were specifically expressed in HCV-induced HCC[Table 5][120].

Table 5.Role of miRNAs in HCV-induced HCC
Steatosis and insulin resistance
An accumulation of lipids within hepatocytes, called hepatic steatosis, is a significant risk factor for HCC development[123].However, HCV infection frequently leads to hepatic steatosis[124].This condition is common in HCV patients (ranges from 40%-86%) and can be caused by various factors such as viral factors(HCV genotype3), host factors (overweight, insulin resistance, diabetes mellitus, hyperlipidemia, and alcohol consumption), and drug therapy (corticosteroids, amiodarone, methotrexate, among others)[125].Steatosis is strongly associated with higher fibrosis scores and severe histological injury in HCV patients.It is currently unknown exactly how HCV infection could lead to the development of parenchymal steatosis.
The enzyme known as microsomal triglyceride transfer protein (MTP) is essential to the formation of very low-density lipoprotein (VLDL), the blood-borne lipid-transporting protein.However, the activity of MTP can be inhibited by two proteins found in HCV - core protein and NS5A.This inhibition leads to the accumulation of triglycerides in cells, which causes steatosis[126,127].In addition, HCV core protein has been shown to cause mitochondrial dysfunction by inducing the overproduction of ROS and attenuating some of the antioxidant systems[128,129].This can be detrimental to the liver as it needs healthy mitochondria to function correctly.
Interestingly, peroxisome proliferator-activated receptor alpha (PPAR-α) can help alleviate steatosis[130,131].However, in the presence of mitochondrial dysfunction, PPAR-α may exacerbate steatosis instead ofLncRNAs★: HOTAIR: HOX transcript antisense intergenic RNA; HULC: highly upregulated in liver cancer; PVT1: plasmacytoma variant translocation 1; LINC01419: long Intergenic Non-Protein Coding RNA 1419; PCNA-AS1: PCNA Antisense RNA 1; ZEB1-AS1: ZEB1 Antisense RNA 1;hDREH: human ortholog RNA of Dreh; UCA1: urothelial carcinoma associated-1; WRAP53: WD repeat containing antisense to TP53; MALAT1:metastasis-associated lung adenocarcinoma transcript 1; HEIH: high expression in hepatocellular carcinoma; aHIF: hypoxia-inducing factor a;PAR5: prader willi/angelman region RNA 5.alleviating it.HCV NS5A and core protein can also induce insulin resistance, which further worsens steatosis.It is worth noting that the genotype of HCV can also play a role in inducing steatosis.Genotype 3,in particular, has the highest association with steatosis[126].
Proliferation and apoptosis
Normal development and tissue-size homeostasis depend on the coordination and balance of two fundamental cellular processes: cell proliferation and apoptosis.There is evidence that cancer may result from these processes being disrupted[132].Cyclin-dependent kinases (CDKs) and cyclins are one of the most important proteins involved in controlling cell division in response to extracellular and intracellular signals.Furthermore, inhibitors such as pRb, E2F-1, DP-1, p107, and p130 regulate the cell cycle[133].Since viruses alter CDK function to promote viral replication, CDKs play a crucial role in viral infections[134].
To maintain tissue-size homeostasis and normal development, cell proliferation and apoptosis must be balanced and coordinated.Evidence suggests that disrupting these processes can cause cancer[132].Cyclindependent kinases (CDKs) and cyclins are among the most important proteins involved in controlling cell division in response to extracellular and intracellular signals.Additionally, regulators such as pRb, E2F-1,DP-1, p107, and p130 manage the cell cycle[133].CDKs also play a significant role in viral infections, as viruses modify CDK function to favor viral replication[134].
Some viral infections can cause changes in cell proliferation due to the actions of viral proteins on CDKs or cyclins, which can lead to cancer development.Researchers have studied the effects of HCV proteins,particularly the core protein, on the cell cycle profile and related molecules.Evidence shows that HCV proteins can activate cyclin/Cdk complexes and stimulate G1/S transition.HCV core protein can increase cyclin E and Cdk2, while HCV NS2 protein can activate cyclin D/Cdk4 and stimulate the expression of cyclin E[135,136].The cyclin-dependent kinase inhibitor p21/Waf, a potential tumor suppressor, plays a critical role in cell cycle progression.HCV core protein can regulate the expression of p21/Waf by binding to p53 and Rb, which are both involved in cell cycle control[137].HCV proteins also target the retinoblastoma (Rb)protein.
One transcription factor that is essential for enabling cells to move into the S-phase of the cell cycle is called Rb.However, recent studies have shown that the HCV NS5B protein can negatively regulate Rb levels.This causes the activation of E2F-responsive genes, which can stimulate cell cycle progression[138].In order to keep tissues healthy, apoptosis is required to eliminate senescent cells that have been harmed by a variety of conditions, such as viral infections.However, it has been discovered that HCV proteins obstruct apoptosis signaling pathways[139].Bcl-2 and Bcl-xL proteins also play a crucial role in apoptosis, and changes in their expression can contribute to the development of cancer[140].HCV core protein can block apoptosis by promoting the expression of Bcl-xl[141].
Similarly, by inhibiting different caspase cascade components, HCV non-structural proteins such as NS4A/B, NS5A, and NS5B can also stop apoptosis[142].Moreover, the HCV proteins NS2 and NS3 can prevent apoptosis[142].Furthermore, HCV can prevent apoptosis indirectly by activating the PI3K-Akt survival pathway, which is facilitated by the HCV NS5A protein[143][Figure 3].
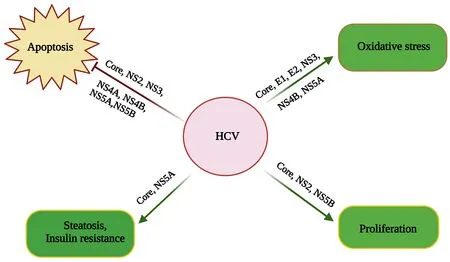
Figure 3.Molecular mechanisms of HCV-related HCC.
METFORMIN
HCC, a type of liver cancer, can be caused by several factors, such as alcohol consumption, obesity, and type 2 diabetes (T2D) in developed countries.Hepatitis B and C viruses, however, continue to pose a serious threat to international health since they can cause liver conditions like cirrhosis and HCC[144].Several cohort studies have shown that metformin, a medication for diabetes, can cause metabolic issues like insulin resistance and diabetes in people infected with HBV and HCV[145].Research on the effectiveness of metformin on viral hepatitis is still ongoing.HCC is a deadly condition with a dismal prognosis for cancer sufferers, and the treatments currently available are associated with several issues.Metformin is derived from a naturally occurring compound called galegine, found in the plantGalega officinalis[146].Since the 1950s, 1,1-dimethylbuguanide hydrochloride, or metformin, has been widely accepted as a first-line antidiabetic medication.However, it also seems to have anti-cancerous effects[147].
Metformin has been found to have anticancer effects by altering various signaling pathways responsible for cellular proliferation, apoptosis, and metabolism.It regulates the synergistic activity of AMPK, GSK-3, and PPAR, which leads to its anti-angiogenic, anti-invasive, and anti-proliferative properties.These effects have been observed in pancreatic cancer and glioblastoma multiforme.Metformin has a wide range of tissue effects, including on the liver, skeletal muscle, colon, pancreatic, breast, prostate, endometrium, and ovaries.Its use has been associated with reducing the incidence and mortality of various malignancies[148-151].Additionally, metformin is considered to be a useful drug in the treatment of polycystic ovarian syndrome,cardiovascular disease, and even anti-aging[152].In cultured and living cells, Metformin slows tumor growth by inducing apoptosis, stopping the cell cycle in some malignancies, and activating caspase 3.Scientists are now exploring how metformin can be used to prevent and treat HCC[153,154].
Worldwide, cirrhosis and CLD are highly prevalent.The incidence of chronic liver disease (CLD) has decreased globally as a result of hepatitis B and C vaccine, screening, and antiviral treatment programs;however, these advancements are at risk due to concurrent increases in injectable drug use, alcohol abuse,and metabolic syndrome.While encouraging recent developments have been made in the treatment of all stages of HCC, more needs to be done to slow the rise in HCC mortality, including better early detection,more use of HCC surveillance, and fair access to HCC medicines[155-157].
Metformin usage in cirrhotic patients has been found to reduce the occurrence of HCC.Metformin affects cancer cells by targeting the mitochondria and blocking mammalian targets of rapamycin (mTOR) and insulin-like growth factor (IGF) signaling, which reduces carcinogenesis[158].When metformin enters the cell, it inhibits the mitochondrial electron transport system, reducing cellular adenosine triphosphate (ATP)and increasing the adenosine monophosphate AMP/ATP ratio[159].Tregs are a subset of T lymphocytes that regulate the immune response by stopping the proliferation and cytokine production of effector T cells.In the liver and other organs, they are essential for peripheral self-tolerance and immunological homeostasis[160].One important component of the Tregs’ inhibitory effect is the expression of the transcription factor forkhead box protein 3 (FOXP3)[161].An increasing body of research indicates that this particular cell type is involved in the pathogenesis of several diseases, such as HCC, chronic viral liver problems, and autoimmune diseases[162].Consequently, innate immune cells (monocytes/macrophages, DC cells, NK cells) and adaptive immune cells (CD4+, CD8+ T cells) are reduced in chronic HBV infection.Therefore, HBV stimulates immunosuppressive cells including MDSC, NK-reg, and T-reg cells to establish an immunosuppressive cascade that aids in chronic and persistent viral infection through inhibitory substances such as PD-L1, PD-1, and IL-10[163].Similarly, a higher frequency of CD4+CD25+ Tregs in the blood and liver has been seen in patients with chronic HCV infection.These findings collectively demonstrate that Treg-driven inhibition of the host immune response is a hallmark of immunological chronic HBV and HCV infections[164,165].Thus, metformin enhances the immunological response of CD8 T cells to cancer and can stop the growth of tumors and eliminate cancer stem cells (CSCs)[166].In human HCC cells, metformin treatment can boost autophagy and autophagic flux while also triggering the signaling pathway.
Metformin activates the AMPK-mTOR signaling pathway and causes autophagy in human liver cancer cells[167].Compared to other medications, pharmacokinetics is a crucial aspect of metformin[168].It is more efficiently distributed in liver cells than Rosiglitazone and Simvastatin[165].Choi and Roberts claim that taking statins and metformin to prevent HCC may be extremely beneficial for high-risk people[169].Metformin use is independently linked to a lower incidence of HCC and liver-related death/transplantation in individuals with T2D who have HCV cirrhosis, according to a study by Nkontchouet al.[170].Del Campoet al.conducted a study to determine how metformin and simvastatin can protect against liver cancer[171].They exposed Huh7.5 cells to HCV particles before administering metformin and simvastatin.Both treatments reduced Huh7.5 cell growth and HCV infection.Combining metformin and simvastatin inhibited the TCTP and mTOR pathways, increased the tumor suppressor PTEN, and enhanced autophagy in HCV-infected Huh7.5 cells, which slowed down cell growth[171].This treatment reduces cell survival by interrupting the cell cycle, down-regulating several oncogene pathways, and decreasing viral particle generation.In human hepatocytes, metformin increased indicators of cell death[172].
Arterial chemoembolization (TACE) is the standard treatment for incurable HCC[173].When local ablation is not practical, TACE is frequently employed as a locoregional therapy for early HCC[174].TACE results in HCC cell death via ischemia and an increase in the local concentration of cytotoxic medications.However,neo-angiogenesis of residual HCC may result from TACE-induced ischemia because of elevated local concentrations of HIF-1α and vascular endothelial growth factor[175].Patients using metformin in addition to transarterial chemotherapy-embolization who have hepatocellular carcinoma have a better prognosis[176].According to a Chen’s study, exposure to metformin was associated with a favorable outcome when TACE was selected as the initial course of treatment for a single HCC[174].In actuality, metformin significantly lowers the risk of recurrence after TACE and extends long-term survival for HCC patients with T2 diabetes[174].However, not every patient reacts favorably to this process.Even in patients who respond well to TACE, refractoriness or failure affects the course of the disease[177].Thus, to improve the prognosis of patients with HCC following TACE, accessible and effective adjuvant therapies are required[173].Recent research indicates that metformin, aspirin, and statins may prevent HCC from spreading.The study findings demonstrate that co-treating HepG2 cells with metformin and aspirin is more effective at stopping the cell cycle in the G2/M phase and inducing apoptosis in a caspase-dependent manner by downregulating the expression of the pAMPK and mTOR proteins[178].Aspirin can help prevent malignancies such as hepatocellular carcinoma when taken in small quantities.According to Luca Ielasi’s observations, patients receiving aspirin and sorafenib concurrently as antineoplastic treatment had better results than those not receiving aspirin[179].Several mechanisms, both reliant and independent on cyclooxygenase (COX), have been postulated to explain this effect, given that HCC is a chronic inflammation-related carcinogenesis.It has been suggested that aspirin might be a viable treatment for HCC because it is marked by high levels of COX-2[179,180].
A cohort study involving 100 diabetic patients with HCV cirrhosis found that the use of metformin was significantly linked to a decrease in HCC incidence and a reduced need for liver transplantation or liverrelated death[170].In another study, Kasmariet al.analyzed the US insurance database.They discovered that even after excluding individuals with concurrent cirrhosis, NAFLD, and non-alcoholic steatohepatitis(NASH), the incidence of HCC in T2DM patients treated with metformin was still significantly lower[181].The research conducted by Laiet al.32 revealed that after accounting for factors such as sex, age, and comorbidities, individuals with T2D, HBV, and HCV who were on metformin exhibited the lowest HCC hazard ratio (HR) at 0.49 (95%CI: 0.37-0.66)[182].The study cohort included a total of 71,824 patients with HBV infection.The findings indicated that the use of either metformin or statin was linked to a decreased incidence of cancer.The most significant reduction was observed in patients taking both statin and metformin[183].Among 191 patients in the US diagnosed with T2D and histologically confirmed NASH along with fibrosis, the use of metformin showed a significant association with a decreased risk of HCC and a lower rate of all-cause mortality and transplantation[184].The use of metformin significantly lowered the risk of HCC following successful antiviral treatment in individuals with diabetes and chronic HC.A straightforward risk stratification model, which includes cirrhosis and non-metformin use in diabetes mellitus, could forecast long-term outcomes in individuals with CHC after achieving sustained virological response (SVR)[185].We have mentioned a summary of the studies in Table 4.
METFORMIN AND HBV/HCV-RELATED HCC
Metformin, as an antidiabetic agent, has been one of the main treatment options for type 2 diabetes patients[186].This drug is not only commonly used as the first line of treatment in these patients, but its potential anticancer properties in cancers such as the liver have also attracted the attention of researchers[186].This drug reduces the risk of liver cancer in these patients by treating their diabetes because it has been demonstrated in laboratory and clinical settings that diabetic patients are up to 7.1 times more likely than other people to develop liver cancer.Molecular target therapy is a new method for treating various cancers, including HCC.Researchers are interested in this method and hope it will reduce cancer progression and improve patient survival.Metformin’s anticancer molecular mechanisms involve various signaling pathways such as autophagy and apoptosis, hyperinsulinemia, and fatty liver accumulation, as well as cancer microenvironment factors, including cytokines and factors that contribute to cancer progression.This article discusses the roles of these factors in HCC, their interactions with hepatitis B and C viruses, and how metformin can help reduce HCC.
AMP kinase acts as a metabolic tumor suppressor and serves as an energy sensor in all mammalian cells.In cancer cells, this factor is activated by abnormal conditions like hypoxia and glucose deprivation, which cause an increase in the AMP/ATP or ADP/ATP ratio.By regulating cell energy levels, it interacts with mechanisms such as autophagy, apoptosis, and the cell cycle to play an important anticancer role[187].Its activities are mainly observed in tissues like muscle, fat, and liver.Some reports suggest the high significance of this factor in the survival of HCC patients, as the dysfunction of AMPK has been shown to cause cancer progression through an uncontrolled cell cycle, cell cycle progression, survival, migration, and invasion of cancer cells using various tumorigenic molecules and pathways[188].However, despite its inhibitory role in hepatitis C virus replication, activating AMPK via drug treatments is a novel strategy for treating HCVrelated diseases, including HCC cancer[189].
Metformin is the treatment of interest for researchers in stimulating AMPK activation in HCC patients via an LKB1-dependent mechanism[190].Furthermore, to increase its anticancer efficacy, this drug, through the activation of AMPK, prevents the activating of the NF-κB signaling pathway by stimulating the expression of IκBα.Because activation of this signaling pathway can reduce the anticancer effects of metformin by ectopic expression of P65 or overexpression of an undegradable mutant form of IkBa, respectively[191],treatment with metformin to activate AMPK, reportedin vivoandin vitro, can reveal the drug’s potential to treat HCC patients with HCV.Studies have also shown that metformin, when used in combination with other drugs such as celastrol, aspirin, and celecoxib, among others, can be more effective in preventing HCC[Table 6][178,186,192-194].

Table 6.The effects of various metformin and other treatment combinations on patients with HCC
Studies have mentioned various reasons for the role of NF-κB in cancer progression, highlighting the importance of metformin’s suppression of this factor.Although NF-κB[195]may have a dual role in suppressing tumorigenicity during the early stages of tumor development or contributing to tumorigenesis in the later stages due to mutations[195], inappropriate activation of this pathway has been reported in various diseases and cancers, including liver cancer[196].As a result, several studies have identified it as a crucial link between liver damage, fibrosis, and liver cancer.Therefore, targeting NF-κB can be effective in the prevention or treatment of liver fibrosis and liver cancer[197].
On the other hand, the up-regulation of this pathway by hepatitis B and C viruses has been reported as the main cause of liver cancer when damage and inflammation increase and eventually lead to cancer.As a result, this pathway, along with STAT3, can play a key role in the development of inflammation and liver cancer, which can be an important step in the development of liver cancer by affecting the production of inflammatory cytokines such as IL6[198]bTheIL-6/STAT3 signaling pathway is one of the critical pathways involved in HCC progression and plays a vital role in different stages of cancer, including HCC cell initiation, development, invasion and metastasis[199].
The mTOR protein is a type of serine protease that forms the main component of mtor1 and mtor2 complexes.These complexes are essential for controlling a number of biological functions, including autophagy, transcription, protein synthesis, growth and proliferation of cells, and survival[200,201].As a key signaling pathway, this protein, located upstream and downstream of other molecules, can significantly impact the metabolism and physiology of the mammalian body[202].However, its regulation is often disrupted in certain diseases, including cancer.Specifically, upregulating and activating mtor1 can contribute to cancer hallmarks such as cell growth, metabolism re-programming, proliferation, and apoptosis inhibition.This upregulation has also been observed in HCC cancer tissue samples compared to adjacent cirrhosis tissue, further confirming its role in HCC proliferation and spread[203].HCV and HBV are known to increase mTOR expression and activity by means of their NS5A (HCV) and pre-S1, HBx (HBV)proteins.This effect occurs via PI3K/Akt and Akt/mTOR signaling pathways, which ultimately leads to the progression of HCC cancer[204].
These oncogenic roles of mTOR have caused it to be targeted by metformin in the direction of the anticancer role of this drug, including in HCC cancer.This drug suppresses mTOR in HCC cancer in different ways, such as activating AMPK and preventing the phosphorylation of PI3K and AKT; the activation of AMPK located upstream of mTOR can have an inhibitory effect on mtorc1.By activating AMPK located upstream of mTOR, it inhibits mTORc1 activity.In addition, metformin activates autophagy through the AMPK/mTOR pathway.It also affects the PI3K/Akt pathway by preventing the phosphorylation of PI3K, AKT, and mTOR, leading to the prevention of their activity and thus activating autophagy.These effects of metformin on AMPK and PI3K/Akt pathways ultimately prevent the HCC process[194].
Most studies suggest that autophagy plays an anticancer role in liver cancer by preserving cellular homeostasis in normal hepatocyte cells despite its dual role in various cancers.HBV and HCV can activate autophagy to increase replication, but this process is incomplete.The viruses take over only the initial stages of autophagy while avoiding the final and destructive stages of this mechanism.Therefore, the increase in the level of lc3-ll, which indicates the number of phagosomes, and the decrease in the level of p62, a marker for autophagic activity that destroys protein aggregates, are indicators of metformin-induced autophagic activity and autophagic flux in HCC, particularly in the middle and late stages[167].
The PI3K, AKT, and mTOR pathways play a significant role in metformin-induced apoptosis in HCC.Metformin prevents the activity of this pathway, which is upstream apoptosis, thereby eliminating HCC cells[194].Disruption of apoptosis, a liver physiological mechanism that removes excess cells during growth and regeneration stages, paves the way for the development of liver and bile duct cancers.The regulation of many apoptotic factors, including p53 in HCC, is disrupted, leading to cancer cell resistance to apoptosis.The dysregulation of apoptosis factors in HCC cancer is often caused by HBV and HCV viruses.The NS5A protein of HCV and the HBx oncoprotein of HBV can disrupt the apoptosis signaling pathway, leading to HCC development[205,206].Although these two viruses affect different targets to suppress apoptosis, the most common ones are the apoptotic factor p53 and the PI3K/Akt signaling pathway[205,206].On the other hand,p53 is an essential tumor suppressor that is found to be mutated in most human cancers, including HCC.It is reported to be the most frequently mutated gene in liver cancer, with more than 45% of HBV-related liver cancers and 13% of HCV-related liver cancers showing mutations in this gene.Additionally, this gene has different isomers, such as p63 and p73, whose biological functions are similar to p53.In cases where p53 is mutated, especially p73, which is rarely mutated in human tumors, it can be a suitable anticancer replacement for p53.Various studies have shown that these genes can be important targets of metformin in reducing tumorigenesis in various cancers.The metformin/AMPK/p53, p63, and p73 pathways can serve as a new strategy in the treatment of HCC and prove to be vital in achieving this goal[207].Wnt/β-catenin is a signaling pathway that is activated in 40% to 60% of HCC cancers.This pathway regulates cellular processes such as initiation, growth, survival, migration, differentiation, and apoptosis of HCC.However, when mutations in Wnt signaling components and hypoxia inappropriately activate this pathway, it can also promote the progression of chronic HCV/HBV infection to cancer, making it one of the most common causes of HCC.In HCC tumors associated with HCV, an increase in CTNNB1 gene mutations occurs as one of the components of the Wnt/β-catenin signaling pathway.In tumors associated with HBV, an increase in CTNNB1 gene mutations in Axin1 as a negative regulator of the Wnt/β-Catenin cascade has also been reported[208].The activity of this pathway in chronic HCV infection promotes virus-induced cell proliferation and colony formation, which can be reduced by destroying β-Catenin, leading to reduced cell proliferation via an expanded G1 phase and apoptosis.A study by Linet al.showed that metformin can significantly reduce the growth of HCC tumor cells by destroying β-Catenin through the previously mentioned mechanism[209].T2D and hyperinsulinemia are common conditions in patients with hepatitis B and C virus-related liver cancer and cirrhosis.Increased insulin resistance in the liver, caused by the insulin/IGF-I signaling pathway or increased fat accumulation, plays a role in the development of liver cancer in these patients.Insulin-like growth factor (IGF)-1, which is part of the IGF signaling pathway, increases its level in hyperinsulinemic conditions.Through two signaling pathways, PI3K/Akt and RAS/Raf/ERK, it can contribute to the survival and growth of cancer cells[210].In general, IGF signaling, particularly the activation of the IGF-I receptor (IGF-IR)/IGF-II pathway, plays a vital role in malignant hepatocyte transformation and the development of HCC.Although it has been reported that the expression of these two genes is higher in tumor cells than in other liver cells, in the case of IGF-II, it has been shown that its expression is much higher in the early stages of the tumor and increases cancer cell proliferation via the effect on the phosphatidylinositol 3-kinase- and Ras/mitogen pathways[211].In addition, hypoxia-induced cancer conditions increase its expression, which in turn increases the expression of Vascular Endothelial Growth Factor A (VEGFA) and, as a result, angiogenesis[211].To improve these two conditions, metformin may be more effective than other antidiabetic drugs in preventing liver cancer progression and cancer-related mortality.
Anin vitrostudy conducted in 2019 showed that 400 µM of metformin can reduce the expression of IGF-II/IGF-IR, thereby reducing tumor cell proliferation and angiogenesis[211].Metformin is absorbed in the liver through the expression of the organic cation transporter 1 (OCT1) gene.It prevents glucose production by inhibiting gluconeogenesis in the liver, leading to a decrease in insulin resistance.Poor metformin absorption can occur in people with reduced functional polymorphisms in the OCT1 gene, causing their blood sugar levels to rise[190].Notably, the expression of the OCT1 gene plays a crucial role in the absorption and performance of metformin.
The most prevalent liver condition in the world, non-alcoholic fatty liver disease (NAFLD), is a major contributor to liver cancer in many nations.This disorder, which is closely related to type 2 diabetes, ranges from simple steatosis to NASH, which increases the risk of hepatocellular carcinoma (HCC)[212].NAFLD is also prevalent in patients who are positive for hepatitis C virus (HCV) and is considered a prominent feature of chronic HCV infection.HCV, particularly genotype 3, causes fat deposition in the liver to optimize the conditions for its survival, which accelerates the progression of liver fibrosis and contributes to the development of liver cancer[213].Unlike HCV, the relationship between hepatitis B virus (HBV) and NAFLD has yielded contradictory results.However, laboratory studies have demonstrated that the HBx protein can increase the formation of lipid droplets[214].
No specific drug has been approved by the FDA thus far for treating NAFLD/NASH, whether related or unrelated to HCC.Several pilot studies and clinical trials have investigated the effect of metformin on this condition.Still, so far, there has been no report on the drug’s protective effect in HCC-related NAFLD/NASH[215].However, various retrospective observational studies have reported beneficial effects of metformin in NAFLD/NASH patients associated with HCC[215].The drug’s positive effect can be attributed to several reasons, including the inhibition of glycogenesis and mitochondrial respiratory complex I,activation of NRF2 to reduce oxidative stress, activation of the AMPK signaling pathway to reduce fatty acid synthesis, and reduction in the number and activity of hepatic progenitor cells (HPCs) in NASH conditions.All of these cases are amplified in NAFLD/NASH conditions related to HCC, and metformin can lower the risk of HCC by acting on them[215].
The cancer microenvironment in HCC is complex and includes various factors, such as cytokines[216].Cytokines are known to play a significant role in the relationship between cancer cells and these factors,with interleukin 22, 12, 6, TNF, and IL-1b being the most important cytokines in HCC that are targeted by metformin[14].Active T lymphocytes secrete interleukin 22, and its overexpression and receptor are associated with promoting cell differentiation, proliferation, metastasis, progression, and poor overall survival in many cancers, including HCC.Its level also increases in liver fibrosis and advanced cirrhosis.Studies in mouse models have shown that knocking out IL22 significantly reduces the prevalence of HCC compared to wild-type mice.Zhaoet al.,2021 demonstrated that metformin suppresses IL22 in the diethylnitrosamine (DEN)-induced HCC mouse model[216].The results of the study’s whole transcriptome analysis and functional analysis indicated the inhibitory effect of metformin on IL22 through the stimulation of the Hippo signaling pathway[216].
Inflammation plays a significant role in the development and progression of HCC cancer.Proinflammatory cytokines such as IL-6, TNFα, and IL-1b are known to increase in HCC cancer, contributing further to inflammation and progression towards HCC[14].TNFα is a potent factor that stimulates the NFKB pathway,which, in turn, leads to an increase in proinflammatory factors (TNF-α, IL-1β, and IL-6).Metformin not only acts as an inhibitor of the NF-κB pathway but also suppresses TNF-α/NFKB/TNF-α, IL-1β, and IL-6 pathways, preventing inflammation and the progression of HCC by inhibiting TNFR gene expression[14].Apart from the signaling pathways discussed in the commentary, metformin targets several factors, such as opn, Rb, p21, ck19, and annexin A5, to reduce HCC.These factors play a crucial role in the development of HCC cancer and are often manipulated by HBV and HCV.Opn, a factor secreted by cancer cells, is responsible for the invasion of tumor cells and HCC cancer metastasis.Anin vitrostudy showed that 400 µM METF reduced the level of OPN expression in HepG2 cells.Ck19 is another factor that contributes to HCC recurrence and metastasis, causing drug resistance in HCC patients by affecting epithelialmesenchymal transition (EMT) and cancer stem cell (CSC) enrichment.By affecting Ck19 synthesis,metformin can increase cancer cell sensitivity to drugs, preventing recurrence and metastasis.
Annexin A5 is a protein that functions as an anticoagulant and has been suggested to have anticancer properties in certain cancers, such as uterine cervical cancer, by reducing cancer cell growth and increasing cell death.A study conducted by Hassan found that metformin could increase the levels of this protein in HepG2 cells, making it a potential new adjuvant therapy for HCC cancer.Metformin causes an increase in the p21 protein, which leads to hypophosphorylation of Rb and halts the cell cycle at the G1 phase.This cell cycle mechanism is important because it controls the transition from the G1 phase to the S phase[Figure 4][211].
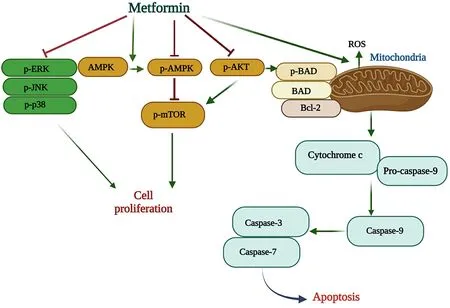
Figure 4.Molecular mechanisms of metformin signaling pathways in cell proliferation and apoptosis.
CONCLUSION
Metformin administration to cancer patients, including those with HCC, is associated with few complications in daily clinical practice.Patients at high risk of HCC may find it attractive to use metformin for chemoprevention due to its comparatively low cost and demonstrated benefits for lowering and controlling diabetes.Therefore, metformin is safe for cancer patients as well as diabetics.More clinical trials are needed to confirm metformin’s ability to combat cancer, which would be beneficial for many cancer patients with or without diabetes.
DECLARATIONS
Author’s contribution
Wrote the main manuscript text: Shojaeian A, Mahmoudvand S, Shokri S, Nakhaie M, Amjad ZS
Prepared figures: Shojaeian A
Edited the manuscript: Shojaeian A, Boroujeni AK
All authors read and approved the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
 Journal of Cancer Metastasis and Treatment2024年1期
Journal of Cancer Metastasis and Treatment2024年1期
- Journal of Cancer Metastasis and Treatment的其它文章
- Feature interview with Dr.William C.CHO -“Clarivate 2023 Highly Cited Researcher”
- Prognostic value of clonal evolution identified by sequential FISH in untreated chronic lymphocytic leukaemia
- Fast-tracking drug development with biomarkers and companion diagnostics
- Mechanical force-mediated interactions between cancer cells and fibroblasts and their role in the progression of hepatocellular carcinoma
- Regulation and function of the RSK family in colorectal cancer
