Role of doxycycline in the treatment of dengue infection: An open-label,randomized,controlled,pilot trial
Banothu Vinod Kumar ,Kajal Kamboj ,Ashok Kumar Pannu ,Ashok Kumar Yadav ,Mandip Bhatia ,Atul Saroch✉
1Department of Internal Medicine,PGIMER Chandigarh,India
2Department of Experimental Medicine &Biotechnology,PGIMER Chandigarh,India
ABSTRACT Objective: To measure the effect of doxycycline on inflammatory marker [IL-6,TNF-α,ferritin and C reactive protein (CRP)] levels in patients with dengue infection.Methods: A single-centre,open-label,parallel-group randomized controlled trial was done in PGIMER Chandigarh from June 2021 to October 2022.Patients were randomized using a simple randomization process into two groups: the doxycycline treatment group (n=35) and the control group (n=34).Patients in the treatment group were given oral doxycycline 100 mg twice daily for five days along with standard treatment,whereas patients in the control group received only standard treatment.The objective was to measure the effect of doxycycline on inflammatory markers in dengue infection.Results: On comparative analysis at day 5,there was a statistically significant reduction in the median values of ferritin and CRP in cases compared to the control group (ferritin: P=0.006 and CRP:P=0.006).No significant reduction was noted in the levels of IL-6 and TNF-α.Conclusions: Doxycycline treatment led to a reduction of inflammatory markers in dengue infection.
KEYWORDS: Dengue;Cytokine;Doxycycline;Serum ferritin;C reactive protein
1.Introduction
Dengue is the most rapidly spreading vector-borne viral disease(i.e.,an arboviral disease) affecting the mankind,prevalent in tropical and subtropical regions of the world,and in particular south east Asian region of the World Health Organization (WHO)[1].During the last five decades,there was 30-fold increase in dengue cases all over the world[2].In 2019,before COVID-19 pandemic,there were 20 000 deaths,264 disability-adjusted life years (DALY) per million of populations[3].
Dengue is the most common infectious cause of acute febrile illness (AFI) with thrombocytopenia[4].Dengue viral infection can have varied presentation starting from mild asymptomatic infection to severe dengue infection resulting in multiorgan dysfunction syndrome[5].Severe dengue infection is associated with marked elevation in pro-inflammatory cytokines level[6-8].Unfortunately,no effective vaccine exists for prevention of dengue infection,or no specific treatment for dengue or its severe dengue infection (DHF/DSS) (dengue haemorrhagic fever/dengue shock syndrome).So presently,management of dengue cases depends entirely on clinical symptoms and severity[7,8].
The pathophysiology of severe dengue infection involves an increase in the levels of the cytokines IL-6 and TNF[9].In various studies,it has been shown that,in patients with severe dengue infection,the increased levels of cytokines lead to profound shock and death[6-8].Drugs that modulate the cytokine/inflammatory markers may help in treating the dengue patients.Different classes of antibiotic possess the immunomodulatory property.The antibiotics belonging to the tetracycline family appear to have the most promising role in immunomodulation[6,10].It has also been shown that doxycycline or tetracycline down regulates the pro-inflammatory cytokines in dengue and tick-borne encephalitis[6,11,12].Doxycycline is a broad-spectrum antibiotic belonging to class tetracyclines.Inside the cell,it binds to the 30S ribosome and inhibits the protein synthesis by preventing the access of aminoacyl t-RNA to the acceptor (A) site on the mRNA-ribosome complex[13].The present study was conducted to measure the effect of doxycycline on cytokines levels in dengue patients.
2.Subjects and methods
2.1.Study setting and design
This is an open-label,parallel group randomized controlled trial conducted at Emergency Medical Unit,Department of Internal Medicine,at PGIMER Chandigarh,India.Patients were recruited from June 2021 to October 2022 (Figure 1).This trial was registered in Clinical Trials Registry-India (CTRI) by National Institute of Medical Statistics ICMR New Delhi (https://ctri.nic.in/Clinicaltrials/login.php).The registration number for this trial is CTRI/2021/09/036661.

Figure 1.Flowchart of the patient recruitment.
2.2.Participants
Patients of ≥12 years with acute febrile illness with thrombocytopenia of duration less than or equal to seven days with a positive laboratory test for dengue infection with warning sign or severe dengue infection (NS1 antigen or dengue IgM antibody or dengue virus RT PCR test was used) were recruited in this trial.Warning signs of dengue infection include recurrent vomiting,abdominal pain/tenderness,lethargy or restless,clinical fluid accumulation (ascites,pleural effusion),mucosal bleeding,hepatomegaly,increase in haematocrit concentration with rapid decrease in platelet count and hypotension/rapid pulse.
Patients with dengue infection without a warning sign and coinfection were excluded from the study.Exclusion criteria are: (1)Patients with dengue infection without a warning sign;(2) Patients with AFI with duration > 7 days;(3) Patient with an established infection (diagnostic test required),e.g.,acute malaria,scrub typhus,typhoid,leptospirosis,Japanese encephalitis;(4) Pregnancy or breastfeeding;(5) Known hypersensitivity to doxycycline;(6) Patient with oesophageal dysmotility.
2.3.Intervention
Patients recruited were randomized by simple randomization into two groups with the help of computer-generated random numbers.Control group was given standard treatment of dengue infection(intravenous fluids plus paracetamol 500 mg on SOS basis).Cases group was given standard treatment of dengue infection (intravenous fluids plus paracetamol 500 mg on SOS basis) and doxycycline 100 mg twice a day for 5 days.
We measured the effect of doxycycline on inflammatory markers in dengue infection as primary outcome.We measured the effect of doxycycline on recovery of platelet count and improvement of liver function test as the secondary objectives.
2.4.Cytokine assays
Serum was extracted from blood samples (on day 1 and day 5) and kept at -70 ℃.Following the collection of all samples,assays were run under blind conditions.Serum levels of IL-6 and TNF-α were analysed using commercially available ELISA kits (IL-6 and TNF-α Quantakine Elisa,R&D Systems,Minneapolis,MN,USA) per the manufacturer's instructions.IL-6 and TNF-α had minimum detection limits 0.7 pg/mL and 0.6 pg/mL,respectively.
2.5.Stastical analysis
Categorical data was represented as number and percentage.Depending on the normality of the data,continuous data were presented as mean and standard deviation or median and interquartile range.Chi-square/Fischer’s test applied for comparison of the categorical variables as appropriate.Mann-Whitney U test was used in the statistical analysis to compare the levels of IL-6 and TNF-α between standard and doxycycline treatment.Data was analysed using SPSS-IBM version 25 and P value <0.05 with two tailed was considered significant.
3.Results
A total of 69 patients were enrolled (35 patients in treatment group and 34 patients in control group) into the study.The most common age group affected in both groups was between 21 and 30 years and nearly half (n=34;49.3%) of the patients enrolled in the study were below 30 years of age.There were 62.3% male and 37.6% female patients in the study with male being more predominantly affected than female patients.77.1% (n=27) Of the patients in the treatment group were male as compared to 47.1% (n=16) in the control group(P=0.013).
Fever with chills was noted in all the patients in both groups.Other common symptoms noted were myalgia (73.9%),pain abdomen (59.4%) and headache (56.5%).Regarding their baseline symptomology,there was no statistically significant difference between the two groups.43.5% Patients presented with bleeding manifestation which included both minor and major bleeding (Table 1).Both groups are homogenous in terms of their baseline laboratory characters at the time of admission.Severe thrombocytopenia was present in both groups.
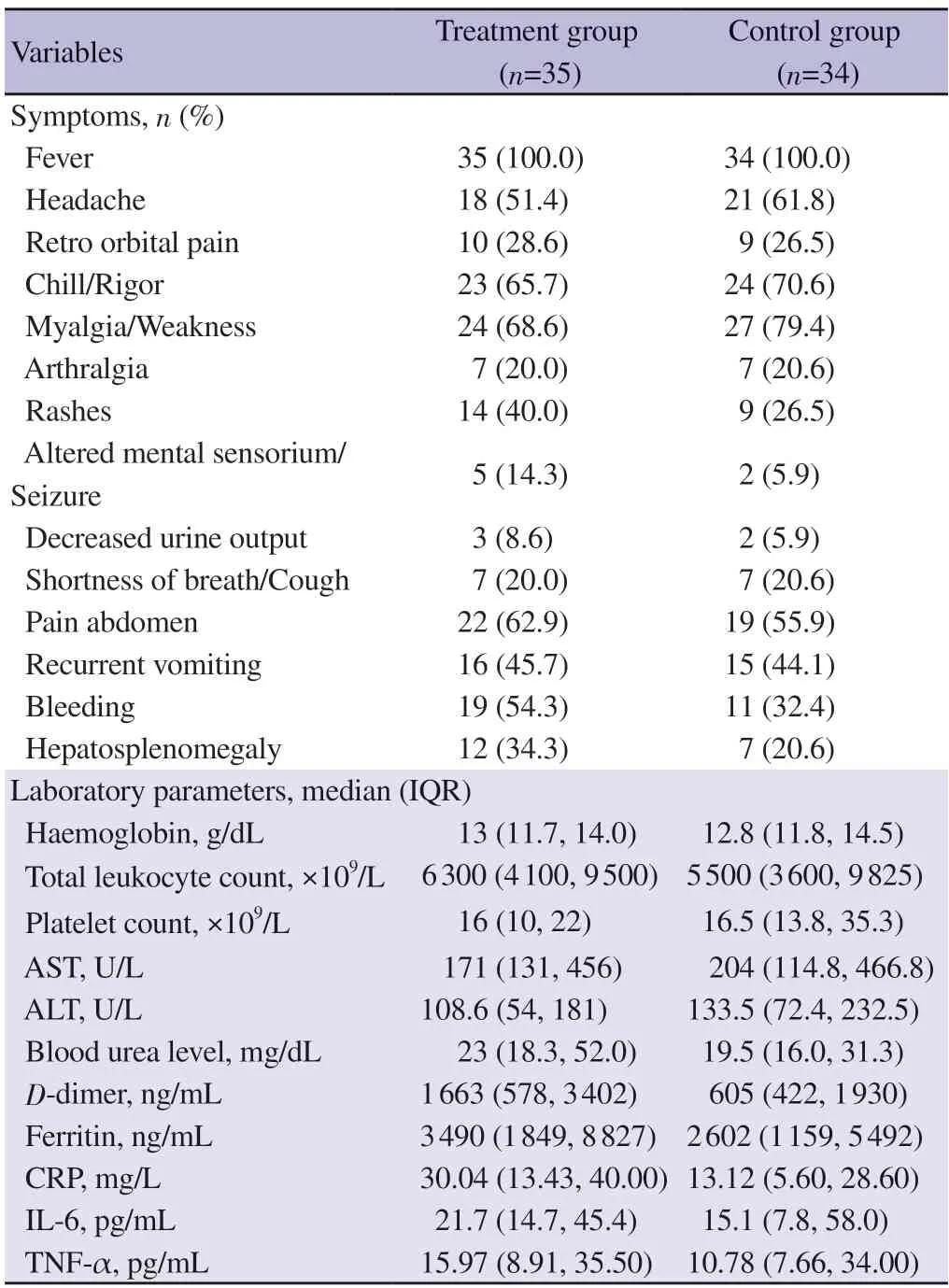
Table 1.Baseline characteristic and laboratory parameters at time of admission.
Overall,there was significant liver involvement in the form of elevated liver enzymes although no statistically significant difference noted between the two groups.There was no significant difference between baseline inflammatory markers value at the time of admission (Table 1).
On comparative analysis at day 5,there was statistically significant reduction in the values of ferritin and CRP in the treatment group compared to the control group (ferritin: P=0.006 and CRP: P=0.006).The median value of IL-6 was reduced at day 5 in both groups,but this reduction was not associated with any statistical significance(P=0.181).There was no statistically significant difference in the values of TNF-α in the treatment group compared to the control group at day 5 (P=0.094) (Table 2).
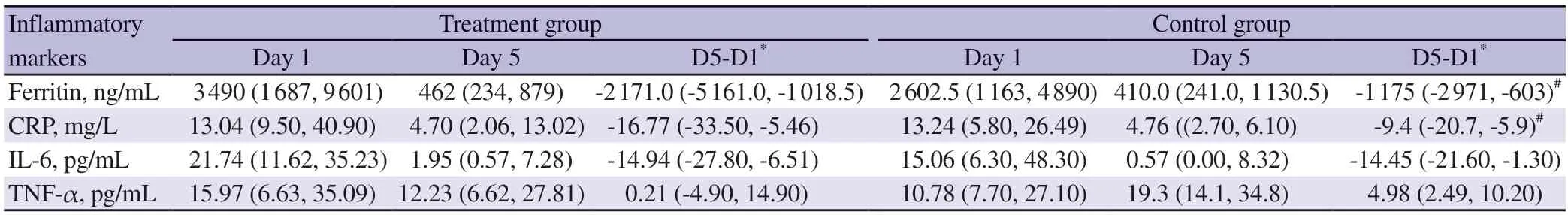
Table 2.Effect of doxycycline on inflammatory markers.
Table 3 showed the comparative analysis after intervention on day 1 and day 5 in both groups.There was fall in haemoglobincompared to baseline (less than 1 mg/dL) in both groups that was likely due to haemodilution during recovery period;however,this was not statistically significant (P=0.541).Similarly,improvement in platelet count and in liver enzymes in both groups were noted,but statistically significant difference was not seen.The control group had higher percentage increase in platelet count as compared to the treatment group;while favourable changes in D-dimer value were more in the treatment group in whom doxycycline was administered.

Table 3.Comparison of lab parameters between the two groups on day 1 and day 5.
4.Discussion
All the patients with dengue infection and fulfilling the WHO definition of “dengue fever with warning signs” presented from different states of North India were recruited in the study.Half of the patient recruited in this study were below 30 years of age consistent with study where higher seroprevalence (>50%) were reported among children aged 9-17 years or older individuals residing in the southern and northern regions[14].If we notice sex distribution,more percentage of male patients are affected statistically in the study which is like other studies.For example,a study by Anker et al.[15]on sex difference in six countries of Asia by analyzing the national surveillance data between 1995 and 2010 found that among dengue cases of more than and equal to fifteen years of age,male patients were reported in excess of female patients (Lao People‘s Democratic Republic 57.9%,the Philippines 57.2%,Singapore 60.7%,Sri Lanka 61.8% and Malaysia 51.6%).Predominantly affection of male patient is likely due to more outdoor activities and more exposed skin surfaces.
All the inflammatory marker (ferritin,CRP,IL-6 and TNF-α)were elevated more in the treatment group than the control group at baseline.However,statistically significant difference between the two groups was not noted at their baseline.This finding of elevated inflammatory markers at the baseline in our study was similar with results reported in other studies[6-9] on inflammatory markers in dengue fever indicating that severe dengue was associated with marked elevation in pro-inflammatory cytokines.There was a statistically significant reduction in the levels of ferritin and CRP at day 5 in the treatment group compared to the control group.There was decreasing trend in levels of IL-6 in both groups at day 5.Though the decrease in the IL-6 at day 5 was more in the treatment group than the control group,it was not statistically significant.The median value of TNF-α was decreased at day 5 in the treatment group,whereas,in the control group,it is increased;but significant difference was not observed.Higher CRP and ferritin level were established with increased severity of dengue infection and reduction in these values are associated with improved outcome[16,17].Decrease in CRP value was studied as response of therapy to doxycycline in various other rickettsial diseases[18].Doxycycline downregulating inflammatory markers were also demonstrated in COVID-19 infection[19,20].Evidence suggests that doxycycline downregulates the expression of dipeptidyl peptidase-4 through the inhibition of the NF-κB pathway[21].Apart from the effect on inflammatory cytokines,use of doxycycline in dengue fever associated with lower risk of bleeding[22].
A study by Fredeking et al.found that administration of doxycycline was associated with a significant decline in both IL-6 and TNF-α on day 3 when cytokine levels were compared with the control group[23].Meta-analysis of Lyn et al.showed that doxycycline was favoured in lowering the serum IL-6 and TNF-α,both on day 3 and day 7 post treatment in patients with dengue infection.In an in vitro study,the anti-viral effect of doxycycline was shown.Doxycycline inhibited the replication of dengue virus[24,25].These findings suggest that doxycycline may provide a clinical benefit to patients with severe dengue infection.Consistent to the above mentioned studies,the present study also highlights that doxycycline is helpful in reduction of inflammatory markers especially serum ferritin and CRP.Limitations of this study were small sample size,dengue patients without warning sign were not included and clinical outcomes were not studied.Piloting this study,a multicentric randomized controlled trial can be planned to assess the role of doxycycline in dengue treatment.
Conflict of interest statement
None of the author has conflict of interest in publication of this manuscript.
Funding
This trial was conducted by the thesis grant provided by Institute PGIMER Chandigarh.No financial support other than this from any Institute/Agency/Pharma company.
Acknowledgments
Authors acknowledge the help provided by Junior administrative assistant Mr Ajay Kumar in assistant provided for resource management.
Authors’contributions
B.V.K.and A.S.developed the theoretical formalism and data collection.A.K.P.and M.B.analysed the data and wrote the first draft of the manuscript.K.K.and A.K.Y helped in sample processing and laboratory work.All the authors were involved in the management of the study patients.
 Asian Pacific Journal of Tropical Medicine2024年4期
Asian Pacific Journal of Tropical Medicine2024年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Recent advances on vaccines against malaria: A review
- Prognostic value of N-terminal pro B-type natriuretic peptide and troponin I in children with dengue shock syndrome
- Expression and clinical significance of pattern recognition receptor-associated genes in hand,foot and mouth disease
- A rare complication of measles infection presented with subacute sclerosing panencephalitis: Report of two cases in India
- Using X Social Networks (formerly Twitter) and web news mining to predict the measles outbreak
