Expression and clinical significance of pattern recognition receptor-associated genes in hand,foot and mouth disease
Muqi Wang ,Huiling Deng ,Yuan Chen ,Yikai Wang ,Yufeng Zhang, Chenrui Liu ,Meng Zhang,Ting Li,Shuangsuo Dang✉,Yaping Li✉
1Department of Infectious Diseases,The Second Affiliated Hospital of Xi'an Jiaotong University,Xi'an 710004,China
2Department of Pediatrics,Xi'an Central Hospital,Xi'an 710004,China
3Department of Infectious Diseases,Xi'an Children's Hospital,Xi'an 710003,China
ABSTRACT Objective: To explore which pattern recognition receptors (PRRs)play a key role in the development of hand,foot,and mouth disease(HFMD) by analyzing PRR-associated genes.Methods: We conducted a comparative analysis of PRR-associated gene expression in human peripheral blood mononuclear cells(PBMCs) infected with enterovirus 71 (EV-A71) which were derived from patients with HFMD of different severities and at different stages.A total of 30 PRR-associated genes were identified as significantly upregulated both over time and across different EV-A71 isolates.Subsequently,ELISA was employed to quantify the expression of the six most prominent genes among these 30 identified genes,specifically,BST2,IRF7,IFI16,TRIM21,MX1,and DDX58.Results: Compared with those at the recovery stage,the expression levels of BST2 (P=0.027),IFI16 (P=0.016),MX1 (P=0.046) and DDX58 (P=0.008) in the acute stage of infection were significantly upregulated,while no significant difference in the expression levels of IRF7 (P=0.495) and TRIM21 (P=0.071) was found between different stages of the disease.The expression levels of BST2,IRF7,IFI16 and MX1 were significantly higher in children infected with single pathogen than those infected with mixed pathogens,and BST2,IRF7,IFI16 and MX1 expression levels were significantly lower in coxsackie B virus (COXB) positive patients than the negative patients.Expression levels of one or more of BST2,IRF7,IFI16,TRIM21,MX1 and DDX58 genes were correlated with PCT levels,various white blood cell counts,and serum antibody levels that reflect disease course of HFMD.Aspartate aminotransferase was correlated with BST2,MX1 and DDX58 expression levels.
1.Introduction
Since 2008,hand,foot,and mouth disease (HFMD) has spread across China,posing a serious threat to children's health.The incidence and mortality of HFMD rank first among class C infectious diseases in children,with heavy burdens on families and society.Hand,Foot and Mouth Disease Clinical Guidelines (2018 Edition) clearly state that the disease can be divided into five stages according to its occurrence and development: the eruption stage,nervous system involvement stage,precardiorespiratory failure stage,cardiopulmonary failure stage and recovery stage[1].The first four stages comprise the acute stage of the disease.It has been shown that staged management of patients with HFMD can effectively reduce acute mortality,possibly because the increased level of cytokines in the acute stage is helpful for the early initiation of specific and nonspecific immunity and the acceleration of self-limiting recovery from the disease[2,3].Moreover,HFMD caused by infection with enterovirus 71 (EV-A71) has a high mortality rate owing to the neurotropism of the VP1 protein;the pathological mechanism involves mainly the ability of the virus to directly damage and cause degeneration,necrosis or apoptosis of nerve cells through immune escape[4].EV-A71 invades the nervous system mainly through pattern recognition receptors (PRRs),scavenger receptor B2(SCARB2),P-selective glycoprotein ligand 1 (PSGL-1) and other neurons to produce cytokines and lead to nervous system injury[5,6].Our previous studies confirmed that after EV-A71 infection,PRRs play a key antiviral role by activating innate immunity[7-9].Indeed,the antiviral innate immune system is an important line of defense for the host to resist viral invasion.PRRs are important hubs of the whole innate immune system;they monitor and recognize a variety of different pathogen-associated molecular patterns (PAMPs) and serve as the first barrier against viral infection[10].After EV-A71 infection,the virus can be recognized by different PRRs,which subsequently activate downstream signal transduction pathways,promoting the production of cytokines and type Ⅰ interferons to inhibit and clear the virus[11].
This study aims to explore which PRRs play a key role in the development of HFMD by analyzing PRR-associated genes with significantly different expression levels at various time points after infection of human peripheral blood monocytes with clinical EVA71 isolates associated with differing levels of disease severity(mild,moderate,and severe) and detecting their expression levels in children with HFMD at different clinical stages.
2.Materials and methods
2.1.Data acquisition
The Gene Expression Omnibus (GEO) is an international public genomics repository with high capacity for storing high-throughput gene expression datasets.To identify the possible mediators responsible for the severity-dependent differences in EV-A71 infection,the GSE135964 dataset,which included 80 RNA-seq samples,was downloaded from the GEO.The 80 RNA-seq samples in the GSE135964 dataset included samples of EV-A71-infected PBMCs from four donors infected with clinical EV-A71 isolates at 0,6,12 and 24 hours post-infection (hpi) with differing severity levels(mild,moderate,and severe;mock-infected and heat-inactivated EV-A71 isolates were used as negative controls).To the best of our knowledge,this research is the first to describe the transcriptome profiles of primary human cells infected with EV-A71 and to analyze the cellular response after EV-A71 infection.We used part of the data from this study to analyze genes whose expression significantly changed in the PRR pathway during EV-A71 infection.
2.2.Acquisition of pattern recognition receptor (PRR)-associated genes
PRR-associated genes were obtained from GeneCards (https://www.genecards.org/),and their expression data were extracted from the GSE135964 dataset.When analyzing PRR gene expression levels in patients with different severities of infection and durations of infection,we applied two-way ANOVA to analyze whether there were significant differences in the independent variables (PRR pathway-associated genes) according to two independent variables(duration of infection and severity of infection).Genes with significantly different expression levels at the intersection of various times and severe viral infection were selected for the following analysis and research.
2.3.Ethical approval
This study was approved by the Medical Ethics Committee of Xi'an Jiaotong University Second Affiliated Hospital (grant No.2017-004)and Xi’an Children’s Hospital (grant No.2012-016),Xi’an,China.Informed consent was obtained from all participants.
2.4.Study participants
From September 2019 to May 2021,a total of 88 Chinese HFMD patients were included in this study.Those HFMD patients were included according to the diagnosis criteria in the Hand,Foot and Mouth Disease Clinical Guidelines (2018 edition) issued by the National Health and Family Planning Commission of the People’s Republic of China[1].Mild HFMD and severe HFMD at the clinical symptom stage were distinguished by clinical manifestations:children with mild HFMD had rashes on their hands,feet,mouth,and buttocks,with or without fever;and children with severe HFMD had neurological,respiratory,or circulatory manifestations.In the convalescent stage,the patient's body temperature gradually returned to normal,the patients’ dependence on vasoactive drugs gradually decreased,and symptoms of nervous system involvement and cardiopulmonary function gradually recovered.The HFMD pathogen status in all subjects was determined by detecting serumspecific IgM antibodies against CA16,Coxsackie B virus and EVA71 in peripheral blood,and pharyngeal/anal swabs were sent to the Xi'an Center for Disease Control and Prevention for viral RNA testing.The combination of these two methods is used to determine the pathogen that the patients have been infected.
2.5.Collection and preservation of specimens
Venous blood samples were collected from all participants on the first morning after admission.After blood collection,the samples were allowed to stand at room temperature for 1 hour and were subsequently centrifuged at 4 ℃ for 10 min at 2 500 rpm.The supernatant was used as the serum sample.
2.6.Significant PRR-associated gene detection in serum
In total,88 serum samples were collected from venous blood at room temperature and stored at −80 ℃ until use.An enzymelinked immunosorbent assay (ELISA) was used to determine PRRassociated genes with significant differences in expression (Shanghai Preferred Biotechnology,Shanghai,China) according to the manufacturer’s instructions.
2.7.Statistics
All the statistical analyses were performed with SPSS 22.0 software (SPSS,Inc.,Chicago,IL),and the figures were drawn with GraphPad Prism 9 (GraphPad,United States).Continuous variables with a normal distribution are expressed as the mean±standard deviation,and a t test was used to compare means.Variables with a skewed distribution are expressed as median (Q1,Q3),and comparisons were performed with the Mann-Whitney U test.Count variables are expressed as counts (%),and the Chi-square test was used to compare rates.The Pearson correlation coefficient test was applied for correlation analysis of two continuous variables with a normal distribution,otherwise the Spearman rank correlation test was used.P<0.05 indicated statistical significance.
3.Results
3.1.Significant PRR-associated genes in the GSE135964 dataset
In total,494 PRR-associated genes were obtained from GeneCards as of March 16,2022.According to two-way ANOVA,331 PRRassociated genes correlated significantly with infection duration,and 34 PRR-associated genes correlated significantly with infection severity,with a total of 30 genes at the intersection of the two(Table 1 and Figure 1).Among the 30 PRR-associated genes whose expression significantly differed according to disease severity and duration,the expression of most genes after virus infection was significantly greater than that in the negative control group and significantly greater than that at 0 hpi,except for 5 genes shown in Figure 1D whose expression was lower than that in the negative control group after virus infection.However,the upward trend of the expression of these genes slightly differed with increasing infection duration,and the upregulation of these genes was most obvious in the group with moderate severe infection.As shown in Figure 1A,the number of genes continued to increase with the increased infection time.Similarly,the number of genes in Figure 1B increased to the highest peak at 6 hpi and then plateaued,and the number of genes in Figure 1C increased to the highest peak after infection and then decreased.
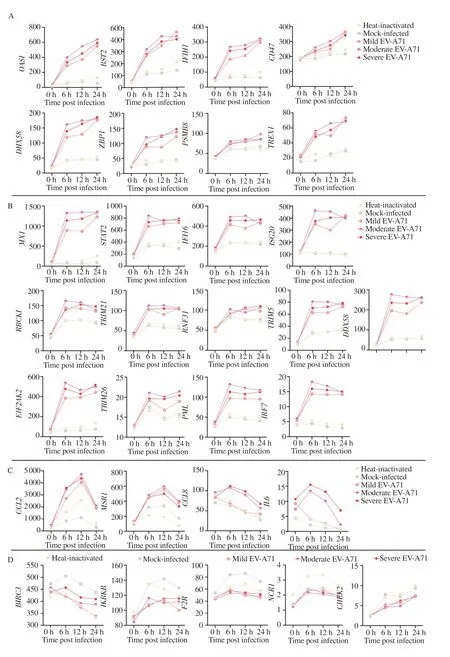
Figure 1.Expression of PRR-associated genes in relation to EV-A71 infection severity and duration.A.Gene expression gradually increased with increasing infection time;B.Gene expression reached a plateau after a certain period of infection;C.Gene expression was greatest at some time points during infection and then decreased;D.The gene expression level was lower in the infected group than in the control group;it was highest at some time point during infection and then decreased.

Table 1.Predictive analysis of PRR pathway-associated genes for enterovirus 71 infection severity and duration.
3.2.Correlation and potential interaction mechanism of PRR-associated genes in the GSE135964 dataset
Correlation analysis of the expression of these 30 PRR-associated genes was also conducted to construct a correlation heatmap(Supplementary Figure 1),which showed that most of the genes had strong correlations.Analysis of these 30 genes in the STRING protein interaction database revealed the protein interaction network illustrated in Supplementary Figure 2.The results in supplementary Figure 1 and Supplementary Figure 2 indicate that the expression of these 30 PRR-associated genes is strongly correlated and that there is a potential interaction between these proteins.These findings also indicate that the immune response triggered by EV-A71 infection is complex and involves not a single gene but rather a complex response coordinated by multiple genes.
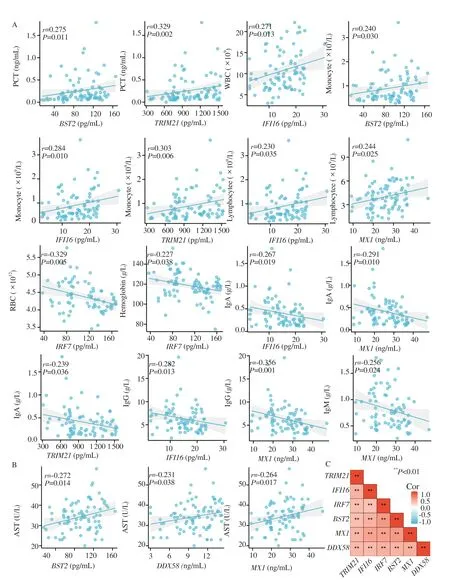
Figure 2.Correlations between the 6 PRR-associated genes and clinical characteristics.A.Associations between the 6 PRR-associated genes and clinical characteristics reflect the course of disease;B.Associations between the 6 PRR-associated genes and clinical characteristics associated with severity.C.Correlation heatmap of the 6 PRR-associated genes.
3.3.Clinicopathological characteristics
Eighty-eight patients with HFMD were included in the study;83 had mild disease and 5 had severe disease;among these patients,58 were in acute stage and in recovery stage.The detailed clinical features of these are shown in Table 2.

Table 2.The basic clinical features of hand,foot,and month disease patients.
3.4.Determination of BST2,IRF7,IFI16,TRIM21,MX1 and DDX58 expression
In the acute stage of HFMD patients,PCT,leukocytes and serum antibodies were also increased.Spearman rank correlation analysiswas performed on the expression of 6 genes in peripheral blood and the clinical features reflect the course of disease.The results(Figure 2A) showed that the expression level of PCT was positively correlated with BST2 and TRIM21 expression levels.Leukocyte count was positively correlated with IFI16 expression.The monocyte count was positively correlated with BST2,IFI16 and TRIM21 expression levels.The lymphocyte count was positively correlated with IFI16 and MX1 expression levels.Red blood cell count and hemoglobin were negatively correlated with IRF7 expression level.IFI16,TRIM21 and MX1 were negatively correlated with IgA levels.MX1 and IgM levels were negatively correlated.IFI16 and MX1 were negatively correlated with IgG levels.
Severe risk factors for HFMD include age≤≤ 3 years,premature infants,high fasting blood glucose,and high neutrophil to lymphocyte ratio.Children with severe HFMD may have abnormal liver function.Spearman rank correlation analysis between these clinical features and the expression of these six genes found that aspartate aminotransferase (AST) was correlated with BST2,MX1 and DDX58 (Figure 2B).In addition,TRIM21 was correlated with age ≤ 3 years (r=-0.322,P=0.002),MX1 was correlated with EV-A71 vaccination (r=0.244,P=0.022),BST2 (r=-0.214,P=0.045),IRF7 (r=-0.223,P=0.036),MX1 (r=-0.359,P=0.001) and DDX58 (r=0.357,P=0.001) were associated with preterm birth.TRIM21 expression in peripheral blood of HFMD patients ≤ 3 years old was significantly higher than that of patients > 3 years old (Supplementary Table 1).
3.5.Determination of BST2,IRF7,IFI16,TRIM21,MX1 and DDX58 expression
Among the 30 identified genes,BST2,IRF7,IFI16,TRIM21,MX1 and DDX58 were selected for validation,and BST2 (acute stage vs.recovery stage,99.11±35.07 vs.81.78±32.78,t=2.245,P=0.027),IFI16 (acute stage vs.recovery stage,15.94±5.82 vs.12.76±5.65,t=2.454,P=0.016),MX1 (acute stage vs.recovery stage,26.98±7.82 vs.23.56±6.82,t=2.023,P=0.046) and DDX58 [acute stage vs.recovery stage,10.62 (8.14,12.86) vs.9.31 (6.39,10.20),Z=-2.672,P=0.008] were found to be significantly overexpressed in patients at the acute stage compared with those at the recovery stage (Supplementary Table 1).However,there was no significant difference in the expression levels of these genes between mild and severe HFMD.Overall,the expression levels of these six genes correlated significantly (Figure 2C).
3.6.Correlations between the 6 PRR pathway-associated genes and different pathogens
The expression levels of BST2,IRF7,IFI16 and MX1 are significantly higher in children infected with single pathogen than those with mixed pathogens (Supplementary Table 1).In detail(Supplementary Table 1 and Figure 3),the expression levels of BST2,IRF7,IFI16 and MX1 are significantly lower in patients with coxsackie B virus (COXB) positive than negative;the expression levels of BST2 and MX1 are significantly lower in patients with EVA71 positive than negative;IFI16 expression is significantly higher in patients with coxsackie virus A6 (CA6) and coxsackie virus A10(CA10) positive than CA6 and CA10 negative;MX1 expression is significantly higher in patients with CA6 and coxsackie virus A16(CA16) positive.
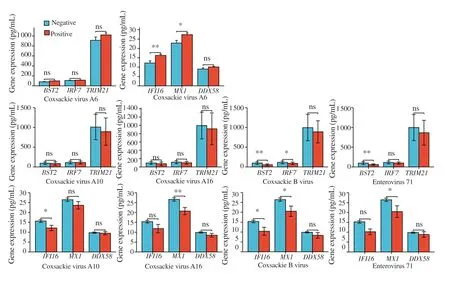
Figure 3.Correlations between the 6 PRR-associated genes and different pathogens.Positive vs.Negative *P<0.05,**P<0.01;ns: no significance.
4.Discussion
The innate immune system is the body’s main defense against EVA71 infection,and the outcome of HFMD patients is determined by the innate immune antiviral immune response and immune escape[12].Circulating T cells,B cells and the serum cytokines IL-1β,IL-6,IL-12 and TNF-α in patients at the acute stage of EVA71 infection are significantly greater than those in patients in the recovery stage,which suggests that the immune responses in these stages of HFMD differ significantly[13-15].After infection with EV-A71,nucleic acid is recognized by different PRRs,activating the antiviral innate immune response and promoting the production of cytokines and type Ⅰ interferons,thus achieving viral suppression and clearance[10].However,the viral proteins 2Apro and 3Cpro encoded by EV-A71 after infection can antagonize the innate immune response and block PRR pathways,such as retinoic acid-inducible geneⅠ(RIG-Ⅰ,encoded by the DDX58 gene),melanoma differentiation-associated gene 5 (MDA5),Tolllike receptor 3 (TLR3),NOD-like receptor thermal protein domain associated protein 3 (NLRP3) and type Ⅰ interferon (IFN).
Then,the production of IFN and the interferon-induced gene(ISG) is inhibited,resulting in innate immune escape by EV-A71,severe imbalance in the innate immune response,loss of type Ⅰinterferon production,abnormal increases in inflammatory cytokine production,and aggravation of the disease,causing severe damage to the nervous system[16].Although there is no specific therapy for severe HFMD,immunoglobulin is considered an effective method for treating this disease.Changes in immune mechanisms,especially the role of PRRs,during the acute and recovery stages of HFMD have not been fully elucidated.In general,a better understanding of the role of PRRs in virus recognition and antiviral activity in the acute stage of HFMD may reveal the immune mechanism involved in innate immune initiation after EV-A71 infection and identify PRR targets for the early diagnosis and treatment of severe HFMD.
In this study,we analyzed the expression of PRR-associated genes in mononuclear cells infected with clinical EV-A71 isolates associated with differing disease severities (mild,moderate,and severe) at different infection time points,and 30 PRR-associated genes were shown to be significantly related to both the duration of EV-A71 infection and the severity of the EV-A71 isolate.
The expression of most PRR-associated genes increased in EVA71-infected cells,and the change trend was more significant in patients with moderate EV-A71 infection than in those with severe or mild EV-A71 infection.Nonetheless,EV-A71,which has moderate virulence,caused the greatest increase in PRR-associated gene expression,which indicated that the expression of PRRassociated genes did not correlate positively with EV-A71 isolate severity.Immune depletion may explain this phenomenon,and it is also possible that these genes are the key genes that trigger the immune response after viral infection.An early increase initiates the response,and the negative feedback is reduced to the degree of maintaining the immune response but not severe inflammatory damage after high levels are reached.In addition,if these genes are not involved in increasing the degree of killing the virus or causing viral immune escape,severe illness may occur.PRR-associated genes are upregulated with time after infection and continue to increase,decrease or reach a plateau;hence,different PRRs play different roles in innate immunity following EV-A71 infection.Correlation and STRING online analyses revealed that 30 genes were significantly correlated,and most of these genes have potential interactions with each other,indicating that the mechanisms involved in initiating innate immunity are complex.
If the EV-A71 isolates that cells are infected are associated with differenet levels of HFMD severity (mild,moderate,or severe),then different duration of infection might correspond to the acute stage and the recovery stage.Among the 30 genes,the top 6 genes with the lowest adjusted P values were BST2,IRF7,IFI16,DHX58,TRIM21,and MX1.We found that DDX58 was expressed significantly differently in the serum of HFMD patients with mild and severe disease but that DHX58/LGP2 was not expressed (Supplementary Figure 3)[8].Therefore,we removed DHX58 and added DDX58 when measuring the expression of these six genes in the serum of patients in the acute and recovery stages and found that BST2,IFI16,MX1,and DDX58 were significantly differentially expressed.In addition,the expression levels of these genes were significantly different when patients were infected with different pathogens,suggesting that different pathogens may elicit different immune responses after infection.Research has revealed that polymorphisms in the MxA(protein encoded by MX1) promoter may play a role in mediating susceptibility to EV-A71 infection in the Chinese population[17],while the correlations between the other 5 genes and different pathogens (Figure 3) have not been studied.When we explored the correlation between the 6 genes and clinical features (Supplementary Table 1 and Figure 2A-2B,Figure 3),we found that the expression levels of these six genes differ in infection with different pathogens,and they are significantly associated with some of the risk factors that reflect the clinical characteristics of disease course and disease severity.Several studies have shown that EV-A71 infection,age <3 years,mixed-virus infection and premature birth are risk factors for severe HFMD[1,18,19].Furthermore,the number of white blood cells and neutrophils,the NLR,and the levels of red blood cells,hemoglobin,and platelets can be used to predict severe HFMD to a certain extent[20-23].Salivary EV-A71-specific IgA expression levels can also be used to diagnose EV-A71 infection[24].These results indicate that PRR-associated genes may be key targets for predicting and treating severe HFMD and are significantly related to immune function.However,single/mixed-virus infection status,age,white blood cell,neutrophil,and lymphocyte counts,the NLR,and IgA levels were significantly different between patients in the acute and recovery stages,possibly affecting the validity of the above correlation analysis results.Our data indeed suggest that the expression of MX1 is lower in children who have received the EVA71 vaccine than in healthy individuals,which seems to contrast with the findings that MX1 was upregulated in the early stages postvaccination in healthy children.Furthermore,MX1 expression was observed to increase following EV-A71 infection (Figure 1).This discrepancy could be attributed to the fact that only five out of the thirty-one vaccinated individuals were infected with the virus.It is plausible that the immunological response to the vaccine during the initial phase of administration differs from the response when the vaccinated host is subsequently exposed to the virus.
BST2 is a transmembrane protein expressed on a variety of cell types that can interact with posterior capsule viruses to bind viral particles to the membrane surface to inhibit their release;it can also be induced by NF-κB signaling,which activates the natural immune response to inhibit viral replication[25].Interferon gamma inducible protein 16 (IFI16) is a unique nucleic acid recognition receptor that helps the body recognize both nuclear and cytosolic viral DNA and is involved in the host antiviral immune response and inflammation[26].Interferon regulatory factor 7 (IRF7),myxovirusresistance protein 1 (MX1),is the most important transcription factor that regulates the production of IFN,especially IFN-α,and inhibits EV-A71 replication[27,28].RIG-I/DDX58 is activated by doublestranded RNA (dsRNA) and interacts with mitochondrial antiviral signaling (MAVS) proteins in cells,resulting in the activation and expression of type Ⅰ interferon genes;DDX58 expression and promoter methylation are associated with EV-A71 infection progression,especially in patients with severe EV-A71-induced HFMD[7,29].Tripartite motif-containing protein 21 (TRIM21)rapidly detects pathogens bound to antibodies in the cytoplasm.TRIM21 degrades pathogen/antibody complexes by recruiting proteasomes and activating innate immune signaling via pathways such as ubiquitin signaling,both of which can occur simultaneously,producing large amounts of proinflammatory cytokines[30].Although these 6 genes have rarely been studied in HFMD,their immune mechanism in this disease warrants further study.
Our study,while providing valuable insights,is subject to several limitations that warrant consideration.First,serum samples from the acute and convalescence stages were obtained from different patients.This introduces individual variability that cannot be reconciled,as the majority of parents were reluctant to authorize further blood sampling once their children transitioned to the convalescent stage of HFMD.Second,the limited number of samples from patients with severe HFMD might have skewed the comparative analysis of the serum expression levels of the six genes,preventing robust stratification and comparison across mild and severe cases in both the acute and recovery stages.Third,the expression levels of all 30 genes were not measured due to the logistical challenge of obtaining parental consent for additional blood collection from their children;therefore,we restricted our serum collection to only what was necessary to analyze the six genes.
In conclusion,PRR-associated genes,notably BST2,IFI16,MX1,and DDX58,may be instrumental in triggering the immune response at the onset of the acute phase of HFMD.These findings underscore the importance of PRRs in the body’s antiviral defense mechanism and may inform future research into therapeutic targets for HFMD treatment.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the doctors of the Department of Infectious Diseases of Xi’an Children’s Hospital and Xi'an Jiaotong University Second Affiliated Hospital for their help with sample collection.
Funding
This research was funded by the National Natural Science Foundation of China (grant no.81701632),Natural Science Basic Research Program of Shaanxi Province (Program No.2022JQ-916)and Key Industrial Innovation Chain-Social Development Field Project of Shaanxi Province (No.2022ZDLSF01-05).
Authors’contributions
SSD,YPL and HLD conceptualized and designed the study.MQW and YPL drafted and revised the manuscript.YC,HLD and YFZ collected clinical samples and data.MQW,YKW,CRL and TL completed all the experiments and statistical analysis.This study was funded by projects of HLD and YPL.All authors have read and agreed to the published version of the manuscript.
 Asian Pacific Journal of Tropical Medicine2024年4期
Asian Pacific Journal of Tropical Medicine2024年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Using X Social Networks (formerly Twitter) and web news mining to predict the measles outbreak
- A rare complication of measles infection presented with subacute sclerosing panencephalitis: Report of two cases in India
- Prognostic value of N-terminal pro B-type natriuretic peptide and troponin I in children with dengue shock syndrome
- Role of doxycycline in the treatment of dengue infection: An open-label,randomized,controlled,pilot trial
- Recent advances on vaccines against malaria: A review
