Development and formation of wing cuticle based on transcriptomic analysis in Locusta migratoria during metamorphosis
Jing Zhang ,Zhaochen Wu ,Shuo Li ,He Huang ,Suning Liu,Weimin LiuXiaoming Zhao#,Jianzhen Zhang#
1 Shanxi Key Laboratory of Nucleic Acid Biopesticides,Institute of Applied Biology,Shanxi University,Taiyuan 030006,China
2 College of Life Science,Shanxi University,Taiyuan 030006,China
3 Guangmeiyuan R&D Center/Guangdong Provincial Key Laboratory of Insect Developmental Biology and Applied Technology,South China Normal University,Meizhou 514779,China
Abstract Wings are an important flight organ of insects.Wing development is a complex process controlled by a series of genes.The flightless wing pad transforms into a mature wing with the function of migratory flight during the nymphto-adult metamorphosis.However,the mechanism of wing morphogenesis in locusts is still unclear.This study analyzed the microstructures of the locust wing pads at pre-eclosion and the wings after eclosion and performed the comparative transcriptome analysis.RNA-seq identified 25,334 unigenes and 3,430 differentially expressed genes (DEGs) (1,907 up-regulated and 1,523 down-regulated).The DEGs mainly included cuticle development (LmACPs),chitin metabolism (LmIdgf4),lipid metabolism-related genes,cell adhesion (Integrin),zinc finger transcription factors (LmSalm,LmZF593 and LmZF521),and others.Functional analysis based on RNA interference and hematoxylin and eosin (H&E) staining showed that the three genes encoded zinc finger transcription factors are essential for forming wing cuticle and maintaining morphology in Locusta migratoria.Finally,the study found that the LmSalm regulates the expression of LmACPs in the wing pads at pre-eclosion,and LmZF593 and LmZF521 regulate the expression of LmIntegrin/LmIdgf4/LmHMT420 in the wings after eclosion.This study revealed that the molecular regulatory axis controls wing morphology in nymphal and adult stages of locusts,offering a theoretical basis for the study of wing development mechanisms in hemimetabolous insects.
Keywords: Locusta migratoria,wing development,metamorphosis,RNA-seq
1.lntroduction
As the flying organ of insects,wings play an important role in finding mates,food,and suitable habitat (Lewin 1985;Engel and Grimaldi 2004).Insect wings,developing from ectoderm,are membranous structures composed of wing membranes and wing veins (Chapman et al.2013).There are significant differences in the development and metamorphosis processes of wings between the holometabolous and hemimetabolous insects.In the larval stage of holometabolous insects,the wing disc invaginates without visible functional wings.After pupation,the wing disc cells rearrange and assemble,and the wings with flight ability appear only after emerging into adulthood.InDrosophila melanogaster,the wing disc is wrapped in the body and is composed of a notum,hinge,and wing pouch.As the dominant area of the wing disc,the wing pouch folds and bonds together in the pupal stage to form a double-layer epidermal structure,which becomes the wing after eversion (García-bellido 1975).Similarly,the morphology of wing primordium dramatically changes after larvae develop into pupae,transforming into pupal wings after eversion in the silkwormBombyx mori(Kawasakiet al.2004;Oteet al.2004).In hemimetabolous insects,wing pads are visible at the nymphal stage.For example,the wing pads ofLocusta migratoriaare visible in the second instar nymphs.Consistent withD.melanogaster,the wing pads are also double-layered cell structures and gradually grow larger with the increase of larva instar.During the nymph-adult transition,the new cuticle of wing pads folds violently and stretches into wings with flight function after molting.
The control of insect wing development is a very precise and complex process.The insect wing has a bilateral cuticle structure secreted by two layers of cells.The wing cuticle is composed of cuticle proteins,the polysaccharide chitin,and a small number of lipids (Vincent and Wegst 2004).The pupal wing morphology ofDrosophilaat different stages was regulated by cuticlerelated genes,ZP domain protein-encoding genes,and transcription factors.For example,the cuticle protein gene (Cpr76Bc) and ZPD genes (dusky-like(dyl),dusky(dy),andminiature(m)) were highly abundant in the pupal wing around 42 h after white prepupae (awp).However,the abundance of the above genes was low in other phases,and these genes were key factors controlling the deposition of the envelope of the wing cuticle (Sobala and Adler 2016).InLasioderma serricorne,theβ-Nacetylglucosaminidase 2 gene (LsNAG2),encoding a chitin-degrading enzyme,was highly expressed in late larvae and late pupae and was involved in wing development during the larva-pupa transition and the pupa-adult transition (Yanget al.2019).As a regulator of lipolysis,lipid storage droplet 1 (Lsd1) knockdown in the dorsal wing disc ofD.melanogasterwould interrupt normal wing development (Menet al.2016).During the metamorphosis from larva to pupa,the wing cuticle protein genes (WCP1-9),chitin degradation,andde novosynthesis-related genes were significantly up-regulated to promote wing disc metamorphosis and pupal wing development inB.mori(Ouet al.2014).At present,the morphogenesis mechanism of wings in holometabolous insects has been systematically studied,but it is still unclear in hemimetabolous insects.
Locusta migratoriahas powerful wings for longdistance migration to find sufficient food resources and avoid natural enemies (Woottonet al.2003;Lovejoyet al.2006).The existence of wings is crucial to the survival of locusts,and the developmental mechanism of wings has also been widely studied.In the early fourth instar nymphs,the interference of theE93gene,a major response gene of 20-hydroxyecdysone (20E),led to the emergence of the overage nymphs of locusts and wing pads of these nymphs were similar to adult wings shape (Liuet al.2022).It is reported that a BTB domain gene (BTBD6) is a specific target gene that controls adult wing development inL.migratoria.Knockdown ofBTBD6at the nymphal stage did not affect the development of the nymph-to-nymph of locusts,but it suppressed the transition from wing pad to wing during the nymph-toadult metamorphosis (Zhao X Met al.2020).However,the determinative factors of wing morphology during the nymph-to-adult metamorphosis are currently unknown.
In the present study,we focused on the stage of wing morphogenesis of locusts and specifically analyzed the difference between nymphal wing pads and adult wings.Based on the difference in wing morphology,the main control genes in the two stages were screened by RNA-seq.The key genes controlling wing development in nymphal and adult stages were associated with morphogenesis.Among these genes,Spalt-major-like(LmSalm),LmZF593,andLmZF521,encoding three Cys2His2 (C2H2)-type zinc finger transcription factors,were identified among differentially expressed genes.Among them,LmSalmhas a high expression level in nymphal wing pads at pre-eclosion (PE) that maintained the morphological structure of wing pads by regulating the mRNA levels of two wing cuticle-specific genes (LmACP7andLmACP19).LmZF593andLmZF521were more abundant in adult wings after eclosion (AE) and regulated the expression of genes such asLmIdgf4,LmHMT420,andLmIntegrinto determine the adult wing morphology.The results of this study revealed the main controlling factors for the difference in wing morphology between the nymphal stages and adult stages of locusts.Moreover,the study provided new insights into the formation of functional wings of locusts and their molecular mechanisms.
2.Materials and methods
2.1.lnsects rearing
The eggs ofL.migratoriawere purchased from the Cangzhou Locust Breeding Center in Hebei,China,and were incubated at (30±3)°C under a light/dark cycle of 14 h/10 h,with 50% relative humidity.The hatched nymphs were fed with fresh and tender wheat sprouts under the same conditions and were fed with wheat bran to ensure their growth when reached the third instar stage.The fifth instar nymphs and adults were selected for morphological observation,total RNA isolation,and RNAi in this study.
2.2.Semithin section and transmission electron microscope (TEM)
According to the development of mature wings from nymph-to-adult locusts (Zhaoet al.2019a),we selected the wing pads at PE (about 144 h of fifth instar nymphs) and the adult wings at AE (about 24 h in adult) as the target tissues.The wing cuticle structures of the fifth instar nymphs at PE and the adults at AE were observed further by semithin sections and TEM as described previously (Liuet al.2009;Zhaoet al.2019a).The tissues were fixed,washed,and embedded as the method described previously (Zhaoet al.2019a).First,semithin sections were prepared that localized for the ultrathin sections.For semithin sections (1 μm) observation,slides were stained with 1% (v/v) toluidine blue.Images were captured by an optical microscope (OLYMPUS,Tokyo,Japan).For TEM,ultrathin sections (80 nm) obtained were counterstained with uranyl acetate and lead citrate.Images were captured by a JEM-1200EX transmission electron microscope (JEOL,Tokyo,Japan).
2.3.Total RNA isolation
The wing pads at PE and the wings at AE were separated and collected.Three biological replicates were dissected in each period,and the wing tissues of three locusts were collected in each biological replicate.Total RNA was extracted from the collected tissues using RNAiso Plus Reagent (TaKaRa,Tokyo,Japan) according to the manufacturer’s instructions.The extracted total RNA was qualitatively and quantitatively analyzed by 1% agarose gel electrophoresis and NanoDrop2000 (Thermo Fisher,Waltham,MA,USA),respectively.
2.4.RNA-seq
For RNA-seq,magnetic beads with Oligo (dT) were used to enrich the mRNA to construct a cDNA library.After the library was constructed,the cDNA library was sequenced on an Illumina HiSeq2000 platform by Biomarker Technologies (Beijing,China) to generate a large amount of raw data.Raw datawere filtered to remove joint sequences and low-quality Reads to obtain clean data.Unigenes were obtained by assembling clean data using Trinity software.For functional annotation of unigenes,the Unigenes sequences were aligned with the COG (Cluster of Orthologous Groups of proteins),GO (Gene Ontology),KEGG (Kyoto Encyclopedia of Genes and Genomes),KOG (clusters of euKaryotic Orthologous Groups),NR (non-redundant),Pfam,Swiss-Prot,and eggNOG databases using BLAST software with a cut-off E-value of 10-5.In terms of unigene abundance,FPKM (Fragments Per Kilobase of transcript per Million fragments mapped) was used to measure the transcription or gene expression level,and the DESeq2 software was used to obtain the differentially expressed genes (DEGs) (Loveet al.2014),the parameters were a false discovery rate (FDR)≤0.01 and |log2fold change| (log2FC)≥2.
2.5.Reverse-transcription quantitative PCR (RT-qPCR)
The DEGs in the transcriptome were detected by RTqPCR.A total of 1 μg total RNA was used to synthesize the cDNA using the M-MLV Reverse Transcriptase Kit (TaKaRa,Tokyo,Japan) according to the manufacturer’s instructions.The cDNA was diluted 10-fold for RTqPCR.RT-qPCR was performed in a 15 μL reaction volume containing 7.5 μL of SYBR®PremixEx Taq™ (#RR820A;TaKaRa,Tokyo,Japan),3 μL template cDNA,3 μL of specific primers (2 μmol L-1) and 1.5 μL of double distilled water using a BIO-RAD CFX ConnectTMReal-Time System (Bio-Rad,Hercules,CA,USA).The relative expression levels of the target genes were normalized to the expression of the internal reference gene,RPL32(Liuet al.2013;Zhaoet al.2018).The primers used are listed in Appendix A.
2.6.Tissue-specific expression analysis
For tissue-specific expression analysis,seven tissues (wing pads (WP),integument (IN),Fat body (FB),foregut (FG),midgut (MG),hindgut (HG),and gastric caecum (GC)) were dissected from the fifth instar nymphs.Tissues of three locusts were collected in each biological replicate,with four biological replicates.Total RNA extraction and RT-qPCR analysis were performed as described above.The primers are listed in Appendix A.
2.7.RNA interference (RNAi)
To determine whether DEGs play important roles in locust wing morphogenesis,RNAi was performed.Double-stranded RNA (dsRNA) ofLmIdgf4(dsLmIdgf4),LmHMT420(dsLmHMT420),LmSalm(dsLmSalm),LmZF593(dsLmZF593),LmZF521(dsLmZF521) andGFP(dsGFP,control) were synthesized by the T7 RiboMAX™ Express RNAi System Kit (#P1700; Promega,Madison,WI,USA) according to the manufacturer’s instructions.A total of 10 μg dsRNA was injected into the fifth instar nymphs (day 2) by a microsyringe as the method described previously (Zhaoet al.2018),and the same amount of dsGFPwas injected as the control group.After 120 h,the wing pads were dissected to detect the silencing efficiency for the corresponding genes and the expression of other genes (LmACP7,LmACP19,LmIdgf4,LmHMT420,LmIntegrinα-PS2,andLmIntegrinβ-PS) by RT-qPCR as described above.The primers are listed in Appendix A.Furthermore,the phenotype of locust wings was observed during wing development.
2.8.Microsection and hematoxylin and eosin (H&E) staining
To further explore the effects of the DEGs on wing cuticle structure between the wing pads at PE and the wings at AE,we carried out microsections and H&E staining.On day 2,the fifth instar nymphs were treated with dsLmIdgf4,dsLmSalm,dsLmZF593,dsLmZF521,and dsGFP,respectively.The hindwings dissected from these nymphs on day 7 were fixed in 3% glutaraldehyde and embedded in paraffin.Paraffin sections (5 μm) were prepared,deparaffinized,and then stained with hematoxylin and eosin as described previously (Songet al.2016).The Olympus BX51 microscope (Olympus,Tokyo,Japan) was used to observe the slides,and the pictures were taken by an Olympus digital camera (Olympus,Tokyo,Japan).
2.9.Statistical analysis
All data were statistically analyzed using an independent sample Student'st-test.In the figures,asterisks indicate significant differences (*,P<0.05;**,P<0.01;***,P<0.001).
3.Results
3.1.Microstructure and ultrastructure of the nymphal wing pads at PE and the adult wings at AE in L.migratoria
Based on our previous studies (Zhaoet al.2019a),we observed the microstructure of the wing pads at PE (about 144 h of fifth instar nymphs) and the adult wings at AE (about 24 h in adult) by semithin sections during the development and metamorphosis of the locust wing from nymph to adult (Fig.1-A-C).As shown in Fig.1-B,the wing pads consist of the lower and upper cuticular layers,and the apolysis of wing pad had already occurred at PE.Meanwhile,we observed that the old cuticle was degraded,and the new cuticle was formed at PE (Fig.1-B).In AE,the newly formed adult wings consist of wing veins and wing membranes with the lower and upper cuticular layers (Fig.1-C).Furthermore,the TEM analysis results showed that the old cuticle of wing pads was degraded (Fig.1-D),and the new adult cuticle was concomitantly synthesized at PE (Fig.1-D’).After molting,the adult wing cuticle gradually thickened to form a complete procuticle structure consisting of exocuticle and endocuticle (Fig.1-E).
3.2.ldentification of the DEGs in locust wings during the nymph-adult transition
To further explore the developmental mechanism of locust wings,RNA-seq was performed at the two stages.A total of 49.17 Gb Clean Data were obtained from the cDNA library,and the percentage of Q30 bases in each sample was not less than 89.46%.Trinity software was used to assemble 25,334 unigenes from Clean Data.The obtained unigenes sequences were aligned with COG,GO,KEGG,KOG,NR,Pfam,Swiss-Prot,and eggNOG databases for functional annotation.The results showed that 4,036 (15.9%),12,980 (51.2%),11,786 (46.5%),9,625 (38%),12,092 (47.7%),7,829 (30.9%),12,623 (49.8%) and 15,278 (60.3%) unigenes were annotated to COG,GO,KEGG,KOG,Pfam,Swiss-Prot,eggNOG,and NR databases,respectively.Although 15,706 (62%) unigenes could be annotated to the above databases,there were still 9,628 (38%) unigenes that did not match the above databases and were not annotated into any of the databases,which might be due to many potential new genes with unknown functions in the locust transcriptomic database.
A total of 3,430 DEGs were identified by RNA-seq,of which 1,907 unigenes were up-regulated and 1,523 unigenes were down-regulated (Fig.2-A).The DEGs were divided into three categories: biological process,cellular component,and molecular function by GO analysis (Fig.2-B).The three GO categories could be subdivided into 54 subcategories.Among them,the biological process category contained the most subcategories,consisting of 23 subcategories,of which the two subcategories with the highest abundance were cellular process and metabolic process,indicating the importance of cell activities and metabolic activities in the wing metamorphosis stage.The cellular component category could be divided into 16 subcategories in which cell,cell part,membrane,and membrane part were the most abundant.The molecular function category could be subdivided into 15 subcategories,with binding and catalytic activity accounting for the largest proportions,consistent with previously reported insect transcriptomes (Badiscoet al.2011;Baiet al.2011).In addition,as shown in Fig.2-C-E,DEGs were assigned to subcategories such as translation,cuticle development,reproductive behavior,chitin-based cuticle development,cuticle pigmentation,and melanin biosynthetic process in the biological process category.Under the cellular component category,the first three subcategories were integral components of the membrane,extracellular region,and ribosome.In the molecular function category,DEGs were mainly related to the structural constituent of ribosome,structural constituent of cuticle,ATPase activity-coupled to transmembrane movement of substances,translation initiation factor activity,transmembrane transporter activity,receptor binding,fatty-acyl-CoA reductase (alcohol-forming) activity,chitinase activity,ATPase activity,imaginal disc growth factor receptor binding,iron ion binding,ion transmembrane transporter activity,structural constituent of chitin-based larval cuticle,and other subcategories.
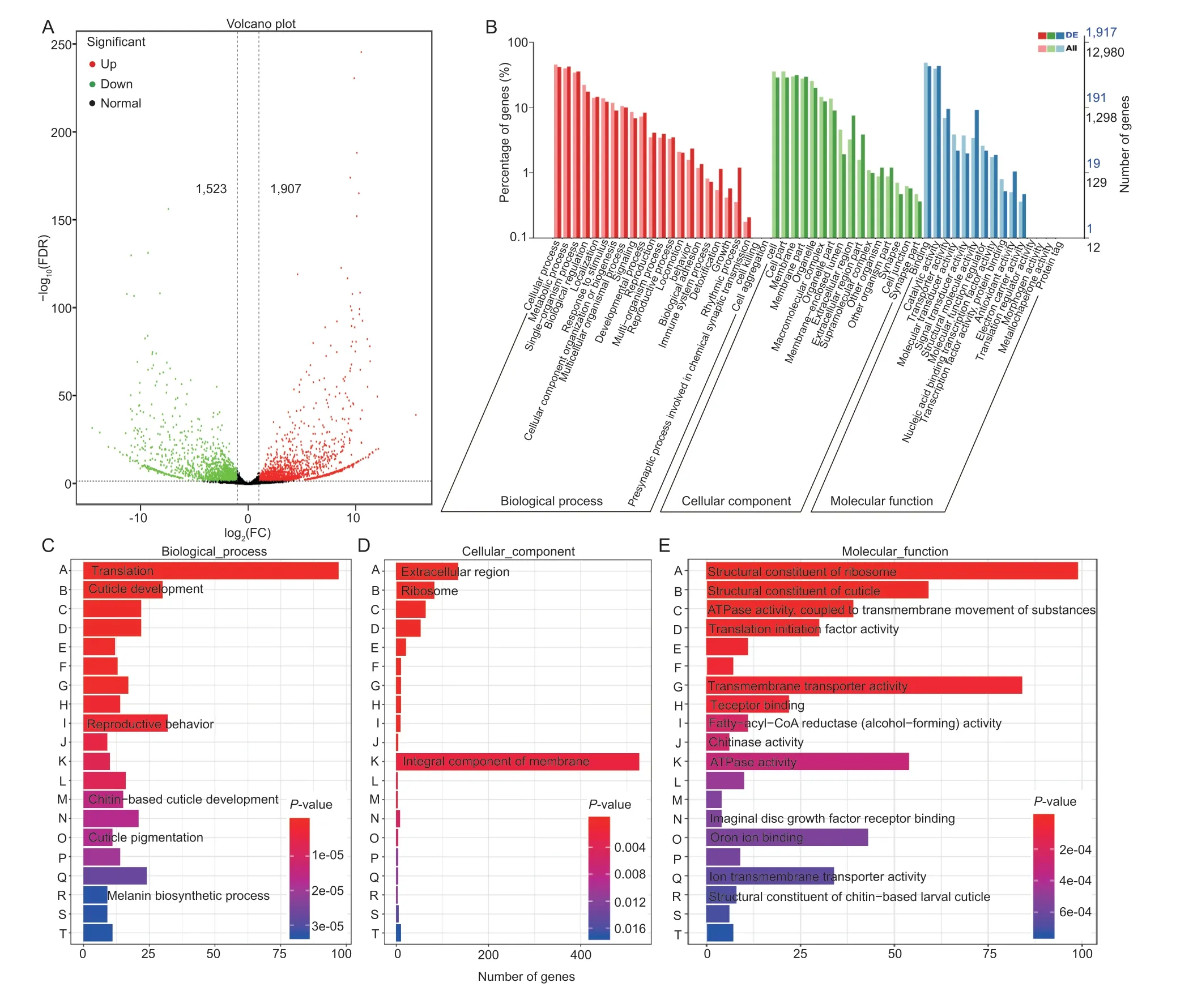
Fig.2 Transcriptomics analysis of the nymphal wing pad at pre-eclosion (PE) and the adult wing after eclosion (AE) in Locusta migratoria.A,volcano plot of differentially expressed genes (DEGs).The red pots represent significantly up-regulated genes,the green pots represent significantly down-regulated genes,and the black pots represent normal genes.B,Gene Ontology (GO) classification of DEG unigenes and all unigenes.GO annotation system includes three main branches,namely,biological process,cellular component,and molecular function.C-E,the enriched bar plot of biological process,cellular component,and molecular function.
These DEGs were further compared with COG to analyze and infer gene function (Appendix B).The locust transcriptome assigned DEGs to 26 COG categories,of which Carbohydrate transport and metabolism (122 DEGs) accounted for the largest,implying that chitin (polysaccharide) metabolism-related genes might be the main genes affecting the developmental transition of locust wing.Followed by translation,ribosomal structure,and biogenesis (114 DEGs),secondary metabolites biosynthesis,transport and catabolism and general function prediction only contained 82 DEGs and 81 DEGs,respectively.Lipid transport and metabolism contained 75 DEGs,ranking the fifth in the COG classification of the locust transcriptome.Based on the functional classification of DEGs in the transcriptome,we further analyzed the cuticle development-related genes,chitin metabolism-related genes,lipid metabolism-related genes,and transcription factors related to wing development.
3.3.Screening and verification of DEGs involved in the formation of cuticle structure
CPR family proteins are the largest cuticle protein family with the Rebers &Riddiford (R&R) motif,including RR-1,RR-2,and RR-3 (Andersen 2000).Based on the data of our transcriptome,we found that adult cuticle protein genes (LmACP7(LOCMI16851),LmACP19(LOCMI16600)) containing the RR-2 motif andLmCP66D(LOCMI02963) had higher abundance in the wing pads at PE.However,in the wings at AE,the annotated endocuticle structural glycoprotein gene (SgAbd-2(LOCMI17211),LmAbd-4(LOCMI17206) andLmAbd-6(LOCMI17141)),CfACP20-like(LOCMI16638) andPxCP7(LOCMI16637) were more abundant (Fig.3-A).The results of RT-qPCR showed that bothLmACP7andLmACP19had higher mRNA levels at PE (Fig.3-B and B’),whereasLmAbd-2andLmAbd-4had higher expression levels in the wings at AE (Fig.3-B and B’),which were consistent with transcriptome data.According to our previous studies,LmACP7andLmACP19were the potential genes involved in the formation of the exocuticle of the wing (Zhaoet al.2019a,2022),whereas an endocuticle structural glycoprotein gene was involved in the formation of endocuticle in locusts (Zhaoet al.2019b).The newly formed locust wings only have the epicuticle and exocuticle but not the endocuticle,and the endocuticle gradually forms after eclosion,so we speculated that the formation of the endocuticle of the locust wing is related to the expression of theAbdsgene.In a word,CPR family genes might play different functions in the formation of the locust wing cuticle.
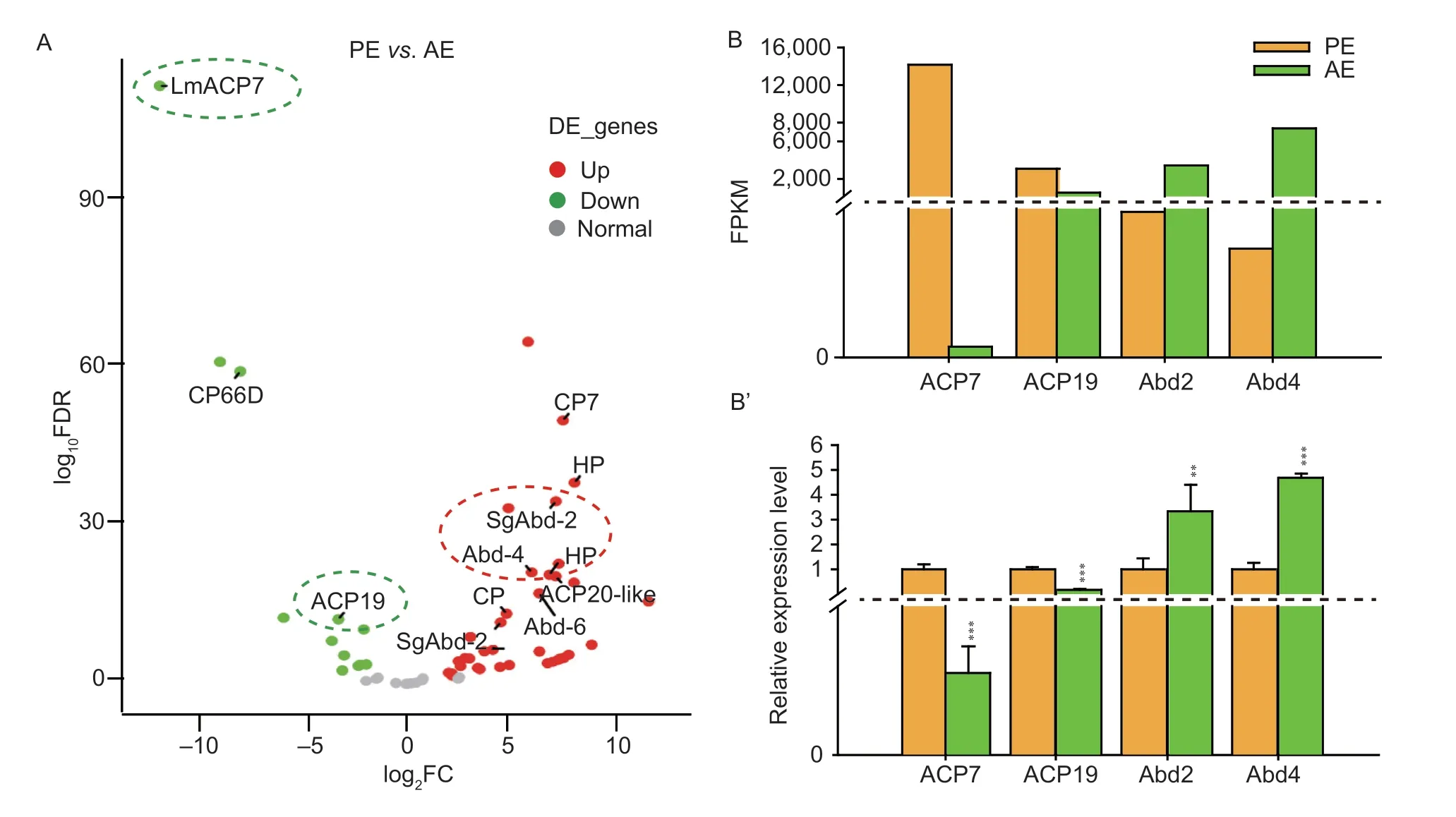
Fig.3 Screening and analysis of differentially expressed genes (DEGs) encoding cuticle proteins from the transcriptome data.A,Volcano map of DEGs encoding cuticle proteins in the nymphal wing pad at pre-eclosion (PE) and the adult wing after eclosion in Locusta migratoria.The red pots represent significantly up-regulated genes,the green pots represent significantly down-regulated genes,and the gray pots represent normal genes.B,the FPKM levels of DEGs encoding cuticle proteins.B’,the relative expression levels of DEGs encoding cuticle proteins detected by RT-qPCR.The data are shown as fold changes as compared with the expression of these genes in PE,which is ascribed an arbitrary value of 1.RPL-32 was used as the reference control.All data are reported as mean±SD of three independent biological replications.Asterisks indicate significant differences,**,P<0.01;***,P<0.001.
For the development of locust wings,theintegringene is also an extremely important factor.Depending onintegrin,epidermal cells of the locust wing attach to the basement membrane to maintain the normal shape of the wings (Zhaoet al.2021a).Fourintegringenes were screened in the transcriptome,which were all up-regulated in the wings at AE (Fig.4-A).Among them,integrinβ-PSandIntegrinα-PS2genes were concentratedly expressed in adult wings at AE from both RNA-seq level and RT-qPCR level (Fig.4-B and B’).These results suggested that theintegringenes mainly act in the adulthood stage of locusts and may be involved in maintaining the tight connection between the wing epidermal cell layers and the basement membrane in the early adult stage.
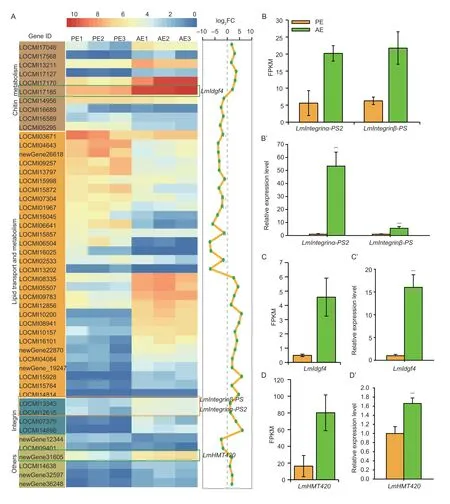
Fig.4 Screening and analysis of differentially expressed genes (DEGs) involved in chitin metabolism,lipid metabolism,integrin,and others based on transcriptomics.A,heat map of DEGs involved in chitin metabolism,lipid metabolism,integrin,and others in the nymphal wing pad at pre-eclosion (PE) and the adult wing after eclosion in Locusta migratoria.B and B’,the FPKM levels and the relative expression levels of the LmIntegrinβ-PS gene and LmIntegrinα-PS2 gene from the transcriptomics data and RT-qPCR.C and C’,the FPKM level and the relative expression level of the LmIdgf4 B-D gene based on transcriptomics data and RT-qPCR.D and D’,the FPKM level and the relative expression level of the LmHMT420 gene based on transcriptomics data and RT-qPCR.The data are shown as fold changes as compared with the expression of these genes in PE,which is ascribed an arbitrary value of 1.RPL-32 was used as the reference control.All data are reported as mean±SD of three independent biological replications.Asterisks indicate significant differences,***,P<0.001.
3.4.Screening and verification of DEGs involved in chitin metabolism and lipid metabolism
The molting process of insects involves the catabolism and anabolism of a series of important substances,such as chitin and lipids.These biological processes are controlled by chitin metabolism-related genes and lipid metabolism-related genes.In the locust transcriptome,there were 10 DEGs involved in the chitin metabolism pathway,6 of which were up-regulated and 4 of which were down-regulated (Fig.4-A).Among them,we found a glycoprotein gene,which encoded imaginal disc growth factors4 (Idgf4) belonging to the glycoside hydrolase 18 (GH18) family of chitinase-related secretory protein (Peschet al.2016),had a high expression level in the adult wings at AE from transcriptome data and results of RT-qPCR (Fig.4-C and C’).The results of RNAi by injecting dsLmIdgf4showed that after successfully silencingLmIdgf4(Appendix C-a),94% of the adult wings were severely curled and could not be fully stretched in the dsLmIdgf4-treated insects,compared with the dsGFP-treated controls (Appendix C-c and d).To explore the effect ofLmIdgf4on the microstructure of locust wings,H&E staining was performed.It was found that the old cuticle of dsLmIdgf4-treated insects was thicker and not fully degraded compared to that of dsGFP-treated insects (Appendix D-a and b).On the other hand,the structure of the new wing cuticle of locusts injected with dsLmIdgf4was seriously damaged,compared with the intact wing cuticle of locusts injected with dsGFP(Appendix D-a and b).This observation suggests thatLmIdgf4not only plays a role in the degradation of nymph wing pad cuticle but also promotes the formation and development of adult wing cuticle,thus maintaining the basic wing morphology.
Another class of metabolism genes,histone lysine methyltransferases(HMTs),induces histone lysine methylation and plays an important role in chromatin remodeling,DNA replication,maintenance of genome integrity,and other processes (Greer and Shi 2012).The results of transcriptome and RT-qPCR showed thatHMT420had a high expression level at AE (Fig.4-D and D’),and the reduced expression ofHMT420resulted in the failure of adult wings to close,with both forewings and hindwings curling (Appendix C-b,c and e).
In the metabolic activities of insects,the lipid metabolism of cuticles also accounts for a large proportion.There were 30 DEGs involved in lipid transport and metabolism both in PE and AE,among which 16 genes were down-regulated,for instance,fatty acyl-CoA reductase(LOCMI06504),fatty acid elongase 3(LOCMI13202),andlong-chain fatty acid CoA ligase 5(LOCMI16025).In contrast,the remaining 14 genes were significantly up-regulated,such asacyl-CoA synthetase(LOCMI15928) andfatty acid elongase 2(LOCMI10200) (Fig.4-A).
3.5.Screening and functional analysis of DEGs encoding C2H2-type zinc finger transcription factors
C2H2-type zinc finger proteins are the largest family of transcription factors and participate in various biological processes through transcriptional regulation (Vaquerizaset al.2009;Weirauch and Hughes 2011).By analyzing the transcriptome,we found that the expression ofZF521(LOCMI11297),ZF-MYM-type1(newGene31884),basonuclin-2-like(newGene33533),andKruppel-like(LOCMI06132) was more abundant in adult wings at AE,compared with the wing pads at PE.However,the expression ofZF706(LOCMI09493),ZF593(LOCMI14734),Spalt-major-like(Salm) (newGene10127),andHR3(LOCMI16491) was greatly reduced in AE (Fig.5-A).Based on our previous results and RNAi screening,we found that silencing ofZF521,ZF593,Salm,andHR3caused abnormalities in locust development during the nymph-to-adult transition,in whichHR3deletion resulted in a lethal phenotype of locusts (Zhaoet al.2018).Therefore,we selectedZF521,ZF593,andSalmas the target genes for subsequent studies.Among them,the quantitative analysis showed that theSalmgene was more abundant in the wing pads at PE from both RNA-seq level and RT-qPCR level.The expression level ofLmZF521was distinctly increased in AE compared with those in PE both at RNA-seq level and RT-qPCR level,whereasLmZF593was increased in AE by RT-qPCR analysis,which was inconsistent with the transcriptome results (Fig.5-B and B’).In order to determine the tissuespecific expression of these genes (LmSalm,LmZF521,andLmZF593),we analyzed their expression in seven tissues from fifth instar nymphs by RT-qPCR.The results showed thatLmSalmandLmZF521were highly expressed in the wing pads but showed low expression in other tested tissues.LmZF593was expressed in all tested tissues,implying thatLmZF593may have multiple functions in locusts (Appendix E).
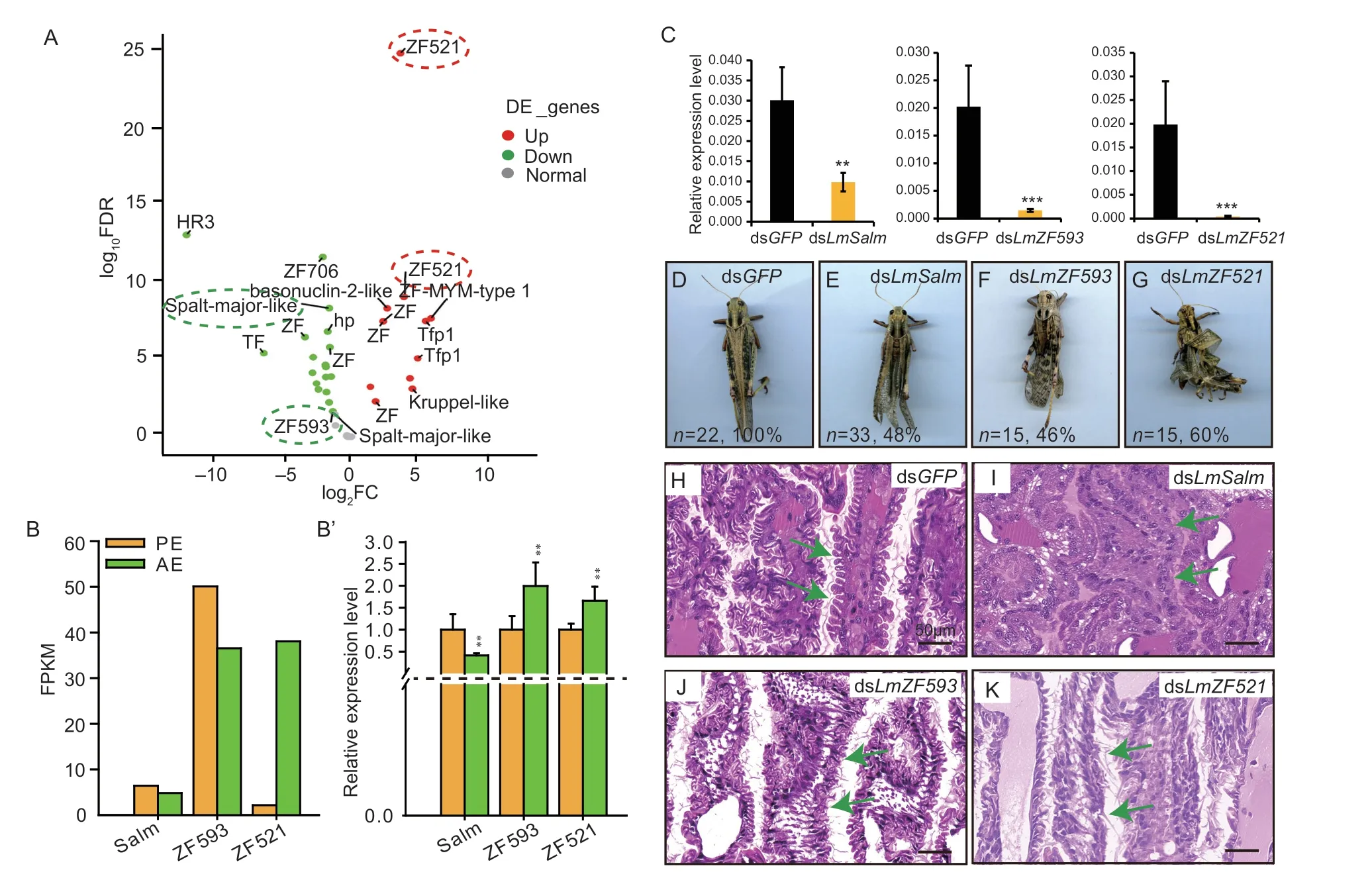
Fig.5 Screening and functional analysis of differentially expressed genes (DEGs) encoding zinc finger transcription factors based on transcriptomics.A,Volcano map of DEGs encoding zinc finger transcription factors in the nymphal wing pad at preeclosion (PE) and the adult wing after eclosion in Locusta migratoria.The red pots represent significantly up-regulated genes,the green pots represent significantly down-regulated genes,and the gray pots represent normal genes.B,the FPKM levels of DEGs encoding zinc finger transcription factors.B’,the relative expression levels of DEGs encoding zinc finger transcription factors were detected by RT-qPCR.The data are shown as fold changes as compared with the expression of these genes in PE,which is ascribed an arbitrary value of 1.RPL-32 was used as the reference control.All data are reported as mean±SD of three independent biological replications.Asterisks indicate significant differences,**,P<0.01;***,P<0.001.C-G,the silencing efficiency and phenotypes of wings in adults after injection of dsGFP,dsLmSalm,dsLmZF593,or dsLmZF521,respectively.H-K,effect on the ridge structure inside the wing pads by H&E staining after injection with dsGFP or dsLmSalm,or dsLmZF593,or dsLmZF521,respectively.Scale bar=50 μm.
For their specific physiological functions,we synthesized the dsRNAs of these three genes invitroand injected them into the blood cavity on day 2 of fifth instar nymphs,respectively.The results showed that the wings of locusts could not close properly,with the forewings and hindwings curling after silencingLmSalmorLmZF593,with phenotypic rates of 48 and 46%,respectively (Fig.5-C-F).The injection of dsLmZF521resulted in locust wings being bent and unable to fully unfold,with phenotypic rates of 60%,compared with the control group injected with dsGFP(Fig.5-C,D and G).
To further explain the above phenotypes,paraffin section and H&E staining were performed.The results showed that the old cuticle of the hindwing of the nymphs treated with dsGFPwas degraded,and the secreted new cuticle folded into ridges in an orderly manner (Fig.5-H).However,the ridge cuticle (new cuticle) of the hindwing of nymphs injected with dsLmSalmcould not be formed completely,and it was the defect of the ridge structure that caused the adult wings to curl (Fig.5-I).After silencingLmZF593,although the two layers of epidermal cells of the wing pads could be normally arranged on both sides of the dorsal and ventral cuticle,the connection between cell and cell was lost,resulting in abnormal cuticle structure (Fig.5-J).Similarly,after silencingLmZF521,the normal connection between the two layers of epidermal cells was also damaged,and the formation of the wing cuticle and the ridge was blocked (Fig.5-K).
3.6.Determining the mechanism of wing morphology before and after eclosion
To investigate the mechanism of wing development regulated by C2H2-type zinc finger transcription factors,we determined the expression level of wing developmentrelated genes based on our transcriptome data by RTqPCR analysis.The results showed that the decline of theLmSalmgene by RNAi resulted in the decreased expression ofLmACP7andLmACP19at PE compared with the control group,but it had no such impact onLmIdgf4,LmHMT420,LmIntegrinα-PS2,andLmIntegrinβ-PShighly expressed in AE (Fig.6-A).In turn,the expression levels ofLmIdgf4,LmHMT420,LmIntegrinα-PS2,andLmIntegrinβ-PSwere significantly decreased in the dsLmZF593-treated insects compared with the dsGFPtreated controls,butLmACP7was highly expressed in PE (Fig.6-B).Similarly,the expression levels ofLmIdgf4,LmHMT420,andLmIntegrinα-PS2were also downregulated in the dsLmZF521-treated insects,without such impact forLmACP7andLmACP19in PE (Fig.6-C).
In summary,we speculate that two gene circuits determined the morphology of nymphal wing pads and adult wings in locusts,respectively.In PE,LmSalmpromoted the formation of the new cuticle (epicuticle and exocuticle) and maintained the basic morphological structure of wing pads by regulating the wing cuticle protein genes (LmACP7andLmACP19).In AE,LmZF593andLmZF521regulated the transcription ofLmIdgf4,LmHMT420,LmIntegrin,and other genes to maintain the normal connection between double-layered epidermal cells of wing and the complete deposition of wing cuticle,determining the adult wing morphology (Fig.6-D).
4.Discussion
The wing tissue undergoes several dramatic morphogenetic transitions during development in holometabolous insects.InDrosophila,around 42 h awp,the wing cells flatten and expand while the volume of the pupal sac is unchanged,resulting in curved pupal wings.Around 80 h awp,the wing cuticle begins to deposit until the eclosion around 96 h awp (Sobala and Adler 2016).In locust,the cuticulin layer was first synthesized,followed by the deposition of the epicuticle and exocuticle in PE,whereas the endocuticle gradually formed in AE (Fig.1-D and E),which is consistent with the reported deposition sequence ofDrosophilawing cuticle (Sobala and Adler 2016).The locust is a typical hemimetabolous insect,one of the global destructive migratory agricultural pests.In this study,comparative transcriptome analysis was performed to identify the wing development-related genes during locust metamorphosis.From transcriptome data,3,430 DEGs were identified between the locust wing pads and the adult wings.Similarly,inD.melanogaster,a total of 17,243 unigenes were obtained from the transcriptome of the formation of wing cuticle,of which 5,097 genes showed different expressions (Sobala and Adler 2016).InB.mori,12,254 transcripts and 5,287 DEGs were obtained during the transition of the wing disc to the pupal wing (Ouet al.2014).These reported results suggest that the mechanism of wing morphology at different stages in both holometabolous and hemimetabolous insects may be regulated by a series of genes.
In this work,we further analyzed the key genes related to the morphological structures of the nymphal wing pads and the adult wings,including cuticle structurerelated genes,metabolism-related genes,and zinc finger genes from the transcriptome.Cuticle proteins are important components of the cuticle in insects and play a decisive role in cuticle formation and development (Vincent and Wegst 2004).InB.mori,13 wing cuticle protein genes have been identified in the wing disc,among whichWCP1a,WCP1b,WCP2,WCP3,WCP4,WCP5,WCP8,andWCP9were significantly up-regulated at the beginning of pupa,whereasWCP10andWCP11were mainly expressed at the end of fifth instar larvae.These genes were involved in wing development during metamorphosis (Takedaet al.2001;Nojiet al.2003;Futahashiet al.2008;Nitaet al.2009;Denget al.2011;Denget al.2016).InL.migratoria,previous studies identified three wing-specific cuticular protein genes (LmACP7,LmACP8,andLmACP19) in the locust wing (Zhaoet al.2017,2019a,2021b,2022).Among them,LmACP7andLmACP19were highly expressed in the wing pads at PE,and their encoded proteins were located in the exocuticle of locust wings,which contributes to the morphogenesis of functional wings in the migratory locust (Zhaoet al.2019a,2022).In addition,we searched for two endocuticle structural glycoprotein genes (LmAbd-2andLmAbd-4) based on transcriptome data.According to our previous study,LmAbd-2andLmAbd-9were highly expressed after molting at each instar (the period of endocuticle formation),and after silencingLmAbd-2andLmAbd-9,the integument of locusts became thinner and lamellae structure of endocuticle was significantly reduced (Zhaoet al.2019b).The locust transcriptome showed that bothLmAbd-2andLmAbd-4were specifically expressed in the wings at AE,compared with the nymphs at PE.This finding implies that the Abds family genes not only affect the development of the body wall of locusts but also may be the key factor determining endocuticle deposition in locust wings.
In addition to cuticle protein,chitin is another major structural component of the insect exoskeleton.Chitin filaments are embedded into the matrix of cuticle protein to form a helical structure (Togawaet al.2004).Chitin metabolism pathway genes play a key role in promoting insect wing cuticle assembly and maintaining the normal development of insect wings.Inhibition ofDmCht10(Chitinase 10) expression in the wing pouch led to a loss of the procuticle lamellar structure of the wing vein and damage of cuticle deposition (Donget al.2020).After injecting dsSfCht7(Chitinase 7) intoSogatella furcifera,some nymphs had difficulty in molting because chitin could not be degraded;the wings of other adults could not spread,although they could molt normally (Chenet al.2017).Imaginal disk growth factors (IDGFs) are an important member of the glycoside hydrolase 18 (GH18) family of chitinases (Žurovcová and Ayala 2002).Idgf4gene inBactrocera dorsalisandIdgf6gene inB.correctawere both highly expressed in the pupal stage and adult stage,and the silencing ofBdIdgf4andBcIdgf6caused larval death and adult wing malformation (Guet al.2019;Zhao Yet al.2020).In this study,we identified anIdgfgene (LmIdgf4),which mainly played a role in the late development of locusts and significantly affected the normal stretch of adult wings (Appendices C and D).InD.melanogaster,the down-regulation ofDmIdgfs(idgf1,3,4,5,6) resulted in the thinning of epidermal chitin-ECM (extracellular-matrix) and the shrinkage and degeneration of procuticle (Peschet al.2016).InL.migratoria,we also found that the old cuticle of locust was thicker and not fully degraded in the dsLmIdgf4-treated insects,compared to that of dsGFPtreated insects;moreover,the structure of the new wing cuticle of locusts injected with dsLmIdgf4was seriously damaged (Appendix D-a and b).These findings indicate thatLmIdgf4was essential for the maintenance of the wing cuticle of locusts.However,whetherLmIdgf4affects the formation of chitin-ECM at the apical cell surface needs further exploration.In addition to the chitin metabolism pathway genes mentioned above,HMT420is also an important metabolism gene.HMTsare closely related to many human cancers,such as leukemia,breast cancer,bladder cancer,and squamous cell carcinoma (Vougiouklakiset al.2015).Studies have shown that Suv4-20h1,the vertebrate homolog of HMT420,suppressed the expression of thecyclindependent kinase inhibitor(p21) to accelerate the G1/S transition,thereby promoting the proliferation and growth of leukemic K562 cells (Wuet al.2018).In locusts,the silencing of theHMT420gene led to the disorder of adult wings.It is speculated thatHMT420may maintain the basic morphology of adult wings by affecting the wing cell division,but the specific mechanism is still unclear.
As the largest transcription factor family,C2H2-type zinc finger genes also participate in the wing morphogenesis of insects.In our studies,the expression ofLmSalmwas higher in the wing pads at PE.Furthermore,it has been reported that the wing pad size of nymph would decrease by 69 and 45% by interfering with C2H2-type transcription factors (Lmsal468andLmsal411),respectively (Wanget al.2017).This result indicates thatsalis required for locust wing growth,and this function is conserved during wing development inD.melanogaster(Wanget al.2017).InNilaparvata lugens,another hemimetabolous insect,Nlsal-RNAi caused the wings of the long-winged brown planthopper to twist (Liet al.2019).Although knockdown ofLmSalmcaused a disorder of adult wing development,which was due to the abnormal internal structure of nymphal wing pads (Fig.5).The silencing of theLmSalmgene induced the decreased expression ofLmACP7andLmACP19at PE,but not the genes such asLmIntegrins,LmIdgf4,and LmHMT420at AE (Fig.6-A).Previous studies indicated thatBmBlimp-1,a C2H2-ZF protein containing a SET (Su(var)3-9,Enhancer-of-zeste,and Trithorax) domain and five C2H2-type zinc finger domains,regulated the wing cuticle protein genes (WCP1a,WCP1b,WCP2,WCP3,WCP4,WCP5,WCP6,WCP9,WCP10,andWCP11),positively or negatively (Wuet al.2019).In this way,zinc finger translation factors may be involved in regulating the expression of wing cuticle protein genes,thus controlling wing development during metamorphosis.The developmental patterns of wings between hemimetabolous insects (e.g.,L.migritoraandN.lugens) and holometabolous insects (e.g.,D.melanogasterandB.Mori) are different,but the regulatory mechanism for wing development seems to be similar.In this work,another two C2H2-type zinc finger genes,LmZF593andLmZF521,were searched in the locust transcriptome and have more abundant expression in the adult wings of locusts,indicating that they may play a dominant role in the wing development.After silencingLmZF593andLmZF521,respectively,the junctional components between the two layers of epidermal cells in the wing pads were greatly reduced,which seriously affected the secretion and formation of wing cuticle.Further results indicated thatLmZF593andLmZF521regulated the expression ofintegrinfamily genes.Generally,the epidermal cells attach to the basement membrane in the wing of locusts (Zhaoet al.2021a),so we speculated that the deficient wing phenotype caused by the deletion ofLmZF593andLmZF521may be due to the destruction of the tight connection between the epidermal cells and the basement membrane.However,we also noted that the mRNA level ofLmACP19was significantly reduced by silencingLmZF593,suggesting thatLmZF593may directly or indirectly regulate the expression ofLmACP19.Thus,their function in locust and other insects need further exploration.
5.Conclusion
There were apparent differences in the internal microstructure and ultrastructure of the nymphal wing pads and the adult wings.RNA-seq was used to identify candidate genes controlling the nymphal wing pads and adult wing development,including the wing cuticle development genes,the chitin metabolism pathway genes,the lipid metabolism pathway genes,and the genes encoding C2H2-type zinc finger proteins.Silencing the above genes caused the malformation of adult wings to different degrees,significantly reducing locusts’ flying ability.Finally,the gene regulation axis controlling the wings (or wing pads) morphology of locusts was speculated: theLmSalm-LmACPsaxis promoted the formation of new cuticle and determined the morphological structure of wing pads;theLmZF593/LmZF521-LmIntegrin/LmIdgf4/LmHMT420axis maintained the adult wing morphology by controlling the effective connection of wing epidermal cells and the normal deposition of adult wing cuticle.This study preliminarily reveals wing morphology’s regulated mechanism during the nymph-to-adult transition in locusts.It provides a theoretical basis for studying the mechanism of wing development in hemimetabolous insects and new molecular targets for pest control.
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFD1700200),the National Natural Science Foundation of China (31970469),earmarked fund for Modern Agro-industry Technology Research System,China (2023CYJSTX01-20),the Fund for Shanxi “1331 Project”,China,and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi,China (2022Y032).
Declaration of competing interests
The authors declare that they have no conflict of interest.
Ethical statement
All applicable international,national and institutional guidelines for the care and use of animals were followed.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.10.022
 Journal of Integrative Agriculture2024年4期
Journal of Integrative Agriculture2024年4期
- Journal of Integrative Agriculture的其它文章
- OsNPF3.1,a nitrate,abscisic acid and gibberellin transporter gene,is essential for rice tillering and nitrogen utilization efficiency
- Fine mapping and cloning of the sterility gene Bra2Ms in nonheading Chinese cabbage (Brassica rapa ssp.chinensis)
- Basal defense is enhanced in a wheat cultivar resistant to Fusarium head blight
- Optimized tillage methods increase mechanically transplanted rice yield and reduce the greenhouse gas emissions
- A phenology-based vegetation index for improving ratoon rice mapping using harmonized Landsat and Sentinel-2 data
- Combined application of organic fertilizer and chemical fertilizer alleviates the kernel position effect in summer maize by promoting post-silking nitrogen uptake and dry matter accumulation
