Fine mapping and cloning of the sterility gene Bra2Ms in nonheading Chinese cabbage (Brassica rapa ssp.chinensis)
Liping Song ,Xia Li ,Liguang Tang ,Chuying Yu ,Bincai Wang ,Changbin Gao ,Yanfeng Xie,Xueli Zhang,Junliang Wang,Chufa Lin,Aihua Wang#
1 Wuhan Vegetable Research Institute,Wuhan Academy of Agricultural Sciences,Wuhan 430345,China
2 Biotechnology and Genetic Resources Institute,Yunnan Academy of Agricultural Sciences,Kunming 650000,China
Abstract The application of a male-sterile line is an ideal approach for hybrid seed production in non-heading Chinese cabbage (Brassica rapa ssp.chinensis).However,the molecular mechanisms underlying male sterility in B.rapa are still largely unclear.We previously obtained the natural male sterile line WS24-3 of non-heading Chinese cabbage and located the male sterile locus,Bra2Ms,on the A2 chromosome.Cytological observations revealed that the male sterility of WS24-3 resulted from disruption of the meiosis process during pollen formation.Fine mapping of Bra2Ms delimited the locus within a physical distance of about 129 kb on the A2 chromosome of B.rapa.The Bra039753 gene encodes a plant homeodomain (PHD)-finger protein and is considered a potential candidate gene for Bra2Ms.Bra039753 was significantly downregulated in sterile line WS24-3 compared to the fertile line at the meiotic anther stage.Sequence analysis of Bra039753 identified a 369 bp fragment insertion in the first exon in male sterile plants,which led to an amino acid insertion in the Bra039753 protein.In addition,the 369 bp fragment insertion was found to cosegregate with the male sterility trait.This study identified a novel locus related to male sterility in non-heading Chinese cabbage,and the molecular marker obtained in this study will be beneficial for the marker-assisted selection of excellent sterile lines in non-heading Chinese cabbage and other Brassica crops.
Keywords: non-heading Chinese cabbage,male sterility,Bra2Ms,fine mapping,PHD-finger protein
1.Introduction
Non-heading Chinese cabbage (Brassica rapassp.chinensis,AA,2n=20) is an important leafy vegetable of the Brassicaceae family (Li X 2016).Non-heading Chinese cabbage is one of the most extensively sown vegetables in China because of its short growing period,high yield,elegant taste,and convenient cooking.In addition,non-heading Chinese cabbage has become an indispensable part of people’s diet because it is rich in vitamins (A,C,B6 and K) and minerals such as iron and calcium (Quet al.2015).In recent years,Japan,South Korea,and the United States have also widely introduced this vegetable,and it has gradually become one of the world’s most popular vegetables (Li H 2016;Xiaoet al.2016).With increasing market demand,it is particularly important to cultivate high-quality and multi-resistance varieties of non-heading Chinese cabbage.
Male sterility is a tool used by breeders for creating hybrid varieties (Huet al.2021).Hybrid varieties always show heterotic vigor of high uniformity,tolerance to environmental challenges,and high yield (Bahaduret al.2015).Chinese cabbage is a typical cross-pollinated crop with strong heterosis (Wanget al.2005).At present,the most commonly used hybrid seed production technology in Chinese cabbage is the self-incompatible lines and male-sterile lines.Compared with cytoplasmic male sterility (CMS),genic male sterility (GMS) has the advantages of complete abortion,no adverse cytoplasmic effects,and restoration of the original range.Hence,it has great application prospects in production.
Currently,the molecular mechanism and regulatory network of GMS in the model plantsArabidopsis thalianaandOryza sativahave been studied in detail and good progress has been made (Wilson and Zhang 2009;Shiet al.2015).Since the successful cultivation of the Chinese cabbage genic sterility line in the 1970s,it has played an important role in the production of F1hybrids.However,the genetic rule of male sterility was largely unknown for many years.With the development of molecular marker technology,molecular mechanism research on the male sterility of Chinese cabbage began to develop.Over the years,molecular markers of genes related to fertility and male sterility in Chinese cabbage have been developed by many researchers.After constructing near-isogenic lines of Chinese cabbage GMS plants,Yinget al.(2003) selected the backcross offspring of the Chinese cabbage recessive male sterile line and corresponding maintainer line as the research materials.They used the bulked segregation analysis (BSA) method and AFLP technology to analyze the genetic linkage map of the recessive male sterile gene.Four specific fragments were found to be closely linked to the restorer gene,and the corresponding STS markers were obtained (Yinget al.2003).Zhanget al.(2008) obtained RAPD markers of sterility genes in the dominant GMS lines of Chinese cabbage and transformed them into SCAR markers.Fenget al.(2009) identified SSR markers linked to multiple allelic male sterility genes and localized them to the R07 linkage groups.In addition,Shiet al.(2020) used the Chinese cabbage genetic male sterile lines AB03 and AB04 as parents to locate theMsgene.The results showed that this gene was located between two SSR (simple sequence repeat) markers (SSRY17 and SSRY22) on chromosome A07,andBra015018encoding an FHA structural protein was predicted to be anMscandidate gene.Tanet al.(2019) mapped the candidate region of Chinese cabbage male sterile mutant ftms on chromosome A05 by using BSR-seq,and predictedBra010198as a candidate gene for ftms by using linkage analysis and resequencing methods (Tanet al.2019).However,these studies mainly focused on Chinese cabbage,while molecular marker development for nonheading Chinese cabbage GMS has rarely been reported.Thus far,only the gene loci associated with non-heading Chinese cabbage recessive GMS have been identified in the R07 linkage group (Fenget al.2009).There are few reports on the mapping and cloning of recessive male sterile genes in non-heading Chinese cabbage.
In this study,fine mapping of the sterility related geneBra2Ms(Li X 2016) was conducted using the non-heading Chinese cabbage sterile line WS24-3 and the fertile line WS-135 as experimental materials.Combined with our previous transcriptome data (Songet al.2019),the geneBra039753encoding a PHD-finger protein was considered the best potential candidate gene for Bra2Ms.Our findings provide a new gene resource for crossbreeding and heterosis utilization in non-heading Chinese cabbage.
2.Materials and methods
2.1.Plant materials and growth conditions
WS24-3 is a GMS line that originated from a spontaneous mutant of the inbred line WS24 (Li X 2016).WS-135 is a male-fertile line conserved by the Wuhan Academy of Agricultural Sciences,China.A BC1 backcross population was constructed using WS-135 as the recurrent parent.The materials were planted in Wuhu Base in Wuhan City of Hubei Province,China.
2.2.Scanning electron microscopy (SEM) obser-vations of mature anthers
Mature anthers of sterile and fertile plants were collected and fixed in 2.5% glutaraldehyde solution for 24 h at 4°C.The samples were dehydrated through a series of graded ethanol solutions (30,50,70,85,95,100,and 100% again),with each grade processed for 10 min.After the samples were soaked in a mixture of isoamyl acetate:ethanol=1:1 for 10 min,they were soaked in pure isoamyl acetate for 10 min.Then,the samples were dried,glued and sprayed with gold at the critical point.The samples were observed using SEM (S570,Hitachi,Tokyo,Japan).
2.3.Cytologic observations of the meiosis process
For observing meiosis in sterile and fertile materials,young flower buds were dipped in Carnoy’s solution (ethanol:glacial acetic acid=3:1),and the solution was replaced several times until the bud faded to white.The samples were soaked in 70% ethanol and stored at 4°C.The anthers from the young buds were bathed in 1 mol L-1hydrochloric acid at 60°C for 1-2 min.After washing with distilled water,the anthers were stained with 10% carbo magenta for 30 s.Finally,the anthers were squashed on slides and the meiotic behaviors were observed with a Nikon Eclipse 80i microscope (Nikon,Japan).
2.4.Development of molecular markers and fine mapping of Bra2Ms
Our previous study delimited the Bra2Ms locus to a 1.2 cM region on the A2 chromosome (Li X 2016).For fine mapping of the Bra2Ms locus,we collected previously reported SSR markers located in the Bra2Ms candidate region.The sequences of the SSR markers were searched in the Brassica Database (BRAD,http://www.brassicadb.cn) to identify polymorphism loci,and then the primers of the SSR markers were redesigned through WebSat (http://wsmartins.net/websat/).The scaffold sequence of the A2 linkage group of the Chinese cabbage genome for IP marker development was downloaded from BRAD (http://www.brassicadb.cn).Primers were designed using Seqman software,and the amplification length was generally less than 1 kb.The primer sequences of the molecular markers are listed in Appendix A.
Genomic DNA was extracted from young leaves using the cetyltrimethylammonium bromide (CTAB) method (Murray and Thompson 1980).First,three fertile bulks and three sterile bulks with 15 individuals for each bulk were used to screen for effective SSR and IP markers.Then,3,520 BC1 individuals were used for fine mapping of the Bra2Ms locus using the effective SSR and IP markers.
2.5.Candidate gene prediction
The primer sequences of the two flanking markers of fine mapping were mapped to the reference genome ofB.rapacv.Chiifu (V1.5) to confirm the candidate region of Bra2Ms.Functional annotation and sequence analysis of the genes in the fine-mapping region were based on Chiifu (V1.5).Differential expression analysis of the genes in the candidate region was performed using our previously published transcriptome sequencing data (Songet al.2019).
2.6.Cloning and sequence analysis of Bra2Ms
Genomic DNA sequences of the candidate genes (Bra039753andBra039757) were isolated from WS24-3 and WS-135 using PCR amplification.The primer sequences used for gene isolation are listed in Appendix A.The amplified fragments were cloned into the pMD18-T vector,and six monoclones were selected for each sample and sent to BGI (Beijing Genomics Institute) for sequencing.The sequence assembly was conducted using DNAStar SeqMen software (http://www.dnastar.com/).Hidden Markov Model (HMM)-based gene structure prediction of the candidate genes was performed using FGENESH of Softberry (http://www.softberry.com/berry.phtml?topic=fgenesh&group=programs&subgroup=gfind).
2.7.Quantitative real-time PCR (qRT-PCR) analysis
Flower buds at the stages of premeiotic anther (PMA) (<0.8 mm),meiotic anther (MA) (0.8-1.5 mm),and anthers with single-celled pollen (SCP) (1.5-2.5 mm) were collected from WS-135 and WS24-3 for qRT-PCR analysis.Total RNA was extracted using an RNAprep Pure Plant Kit (BioTeKe,CHN) according to the manufacturer’s instructions.The RNA concentration and purity were determined using the ultramicroscopic spectrophotometer Nanodrop2000 (Thermo,USA).The qRT-PCR analysis was conducted according to Songet al.(2016).BrActin7(XM_009127097) was used as an internal control to normalize gene expression.Significant differences in gene expression levels between male fertile and sterile flower buds were evaluated using Student’st-test.
2.8.Phylogenetic and conserved motif analysis
A phylogenetic tree was constructed using the PHD finger protein (MMD1) sequences ofB.rapa,B.oleracea,B.napus,and eight other species downloaded from the NCBI database:Raphanus sativus(accession no.XP_018456444.1),Eutrema salsugineum(accession no.XP_006391351.1),Arabidopsis suecica(accession no.KAG7589052.1),Arabidopsis lyratasubsp.lyrata(accession no.XP_002888484.1),Arabidopsis thaliana(accession no.AAG51303.1),Camelina sativa(accession no.XP_010511544.11),Juglans regia(accession no.XP_018816590.1),andCarya illinoinensis(accession no.XP_042970716.1).Protein sequence alignment was accomplished with ClustalW (Saitou and Nei 1987),and the neighbor-joining tree was constructed using MEGA 7.0 software (http://www.megasoftware.net/) with 1,000 bootstrap replicates (Kumaret al.2016).
3.Results
3.1.Characteristics of anther and pollen in sterile and fertile plants
SEM observations showed significant structural differences between the anthers and pollens from the sterile and fertile plants.The fertile anthers were larger than the sterile anthers (Fig.1-A and E).The pollen grains of fertile line WS-135 were plump and nearly spherical,with a clear and regular mesh structure on the surface (Fig.1-B-D).In contrast,the pollen grains from sterile plants were deformed and collapsed (Fig.1-F),with irregular nodular protuberances on the surface (Fig.1-G and H).These differences indicate that sterile pollens grains developed abnormally.

Fig.1 Scanning electron microscopy of pollen grains in fertile (A to D) and sterile (E to H) Chinese cabbage.A,normal anthers in fertile plants.B,normal pollen grains in fertile plants.C,scan showing the structure of the normal external surface of a single pollen grain.D,enlarged mesh scanning structure of a single normal pollen grain.E,anthers in sterile plants.F,malformed pollen grains in sterile plants.G,scan of a single malformed pollen grain from a sterile plant.H,magnification of a single deformable tuberous pollen grain from a sterile plant.
3.2.Meiosis in sterile and fertile plants
The meiosis processes of sterile and fertile anthers were observed at meiosis metaphase,first meiosis,metaphase of second meiosis,and telophase of second meiosis stage.The meiosis metaphase and first meiosis stages were normal in both fertile and sterile plants (Fig.2-A and B,F and G).The meiotic nuclear divisions of fertile plants proceeded normally and produced viable pollen (Fig.2-C-E).However,meiotic nuclear division stopped at the second meiosis stage in sterile plants,with failure to form tetrad and mononuclear pollen (Fig.2-H-I).Ultimately,the pollen grains of sterile plants were shriveled (Fig.2-J).

Fig.2 Cytological observations of the meiosis processes in fertile (A to E) and sterile (F to J) Chinese cabbage anthers.A and F,metaphase of meiosis;B and G,first meiosis;C and H,metaphase of meiosis II;D and I,telophase of meiosis II;E and J,stage 14,the release period of mature pollen grains.
3.3.Fine mapping of the Bra2Ms locus
Previously,BSA-seq analysis and preliminary mapping delimited theBra2Mslocus to a region of 1.2 cM on the A2 chromosome of theB.rapagenome (Li X 2016).According to the previously reported SSR markers in the A2 linkage group and the scaffold sequences of the preliminary mapping region,12 SSR and IP markers were developed successfully (Appendices A and B).Finally,three SSR markers (SSR82-8,SSR883,and SSR980) and three IP markers (IP10,IP18,and IP29) were used for fine mapping of the Bra2Ms locus.A total of 3,520 BC1 individuals were used for fine mapping of the Bra2Ms locus.The genotypes of all 3,520 individuals were first detected using the flanking markers IP29 and IP18 at the flowering period to screen recombinant plants.The IP29 molecular marker was used to verify the backcross population BC1,and a total of 33 exchange recombinant plants were screened.The IP18 molecular marker on the other side was used for population screening,and a total of 162 exchange recombinant plants were obtained.The recombinants were further genotyped using the SSR and IP markers.According to the phenotypes of recombinant plants L514,L2292,and L106 (Fig.3-B),the Bra2Ms locus was finally delimited between the markers IP10 and SSR883,with a physical distance of 129 kb (ChrA2: 8,704.7-8,833.7 kb) (Fig.3-A).

Fig.3 Fine mapping of the Bra2Ms locus.A,chromosome walking based mapping of the Bra2Ms locus using the BC1 segregated population.Numbers in brackets indicate the number of recombinant plants.B,candidate genes in the fine-mapping region.
3.4.Candidate gene prediction of Bra2Ms
According to theB.rapareference genome,the 129 kb region contains 17 genes (Table 1).In our previous study,anthers at the premeiotic (<0.8 mm),meiotic (0.8-1.5 mm),and single-celled pollen (1.5-2.5 mm) stages from the male-sterile line WS24-3A and its maintainer line WS24-3B were collected for transcriptome sequencing analysis (Songet al.2019).WS24-3A is a genic malesterile line generated from backcrossing by using the male fertile line WS-135 as the recurrent parent and WS24-3 as the donor parent.Differential expression analysis showed that two of the 17 genes in the candidate region,Bra039753andBra039757,were significantly downregulated in the sterile line WS24-3A at the meiotic stage (Table 1).Studies inArabidopsisshowed that theAT1G66170gene,a homolog of Bra039753,encodes a PHD-domain containing protein that is required for male meiosis (Yanget al.2003;Wanget al.2016).Further qRT-PCR analysis found that the transcript level ofBra039753in WS24-3 was significantly lower than in WS-135 at the meiotic stage (Fig.4).Thus,Bra039753was predicted to be the most likely candidate gene forBra2Ms.

Table 1 Differential expression analysis of genes in the candidate region of Bra2Ms1)
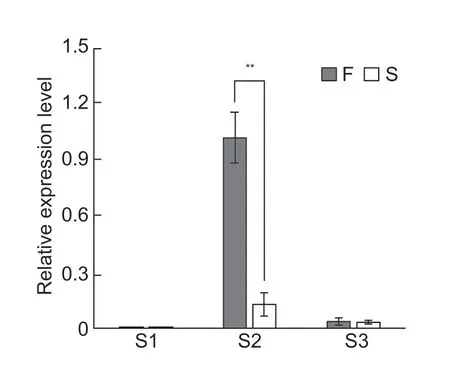
Fig.4 Quantitative real-time PCR of Bra039753 in WS-135 (F) and WS24-3 (S).S1,S2 and S3 are buds at the stages of premeiotic anther (PMA) (<0.8 mm),meiotic anther (MA) (0.8-1.5 mm) and anthers with single-celled pollen (SCP) (1.5-2.5 mm),respectively.Significant differences between the parents were assessed using Student’s t-test.**,P-value<0.05.
3.5.Isolation and sequence analysis of Bra039753
To confirm the candidate gene forBra2Ms,the genomic DNA sequence ofBra039753was isolated from the fertile line WS-135 and the sterile line WS24-3.Sanger sequencing identified a 369 bp fragment insertion and three synonymous mutations in theBra039753gene in the sterile line WS24-3 (Fig.5-A;Appendix C).The 369 bp insertion is located in the first exon ofBra039753and contains five terminators (Fig.5-A;Appendix C).HMM-based gene structure prediction identified an amino acid (N,glutamine) insertion at the 72nd position of the Bra039753 protein in the sterile line (Fig.5-B).A BrMs molecular marker (primer sequences: BrMS-F and BrMS-S369;Appendix A) was designed and the PCR results showed that the marker cosegregated with the target sterile gene (Appendix D).

Fig.5 Sequence and phylogenetic relationships of the candidate gene Bra039753 (Bra2Ms).A,structure and sequence variation in Bra039753 between WS-135 and WS24-3.The green boxes indicate exons,the inverted triangle indicates a fragment insertion in WS24-3.B,predicted amino acid sequence variation in Bra039753 between WS-135 and WS24-3.C,phylogenetic relationships of the candidate gene Bra039753 (Bra2Ms).On the left is the phylogenetic tree of the PHD finger protein in Brassica and its homologs in eight other species.On the right is the motif analysis of these proteins.
3.6.Phylogenetic relationships and motif analysis of Bra2Ms
The Bra2Ms protein sequences ofB.rapa,B.oleracea,B.napus,and the eight other species were used for phylogenetic tree construction.Two groups of these proteins were identified,and the proteins from theBrassicawere clustered in the same group (Fig.5-C).Structure analysis revealed four motifs for all 12 PHDdomain containing proteins,with motif one annotated as a zinc finger PHD-type signature,and the others are functionally unknown.
4.Discussion
Non-heading Chinese cabbage is a cruciferous vegetable native to China (Qiuet al.2009).Cruciferous vegetable has also become very popular and widely used in people’s diets because it contains high levels of several nutrients that are beneficial to health (Lewis and Fenwick 1987;Rybarczyk-Plonskaet al.2014).The male sterility used for hybrid seed production to increase crop yields can contribute to this non-heading Chinese cabbage consumption worldwide.However,male sterility has not been extensively applied to utilize hybrid vigor in nonheading Chinese cabbage because few male sterility genes have been identified in this species.In plants,reproductive development requires normal meiosis,and meiosis can affect pollen fertility (Tianet al.2022).Studies have shown that plants with most meiosis mutations can complete meiosis and cytoplasmic division to produce abnormal microspores,which degenerate during pollen development and eventually lead to infertility (Mercieret al.2015).The whole meiosis process has many important biological events,including chromosome adhesion and homologous chromosome synapsis (pairing) (Zickler and Kleckner 2015;Wang and Copenhaver 2018),and previous studies have found that many genes are involved in these events.For example,inA.thaliana,the REC8 homologous proteins SYN1/DIF1 and DYAD/SWITCHI (SWI1) are associated with chromosome adhesion (Baiet al.1999;Mercieret al.2001).Wheat Ph1 ensures the normal pairing and association of homologous chromosomes by inhibiting the pairing of some homologous chromosomes (Sears 1976;Martinez-Perezet al.2001).TheArabidopsisSPOROCYTELESS/NOZZLE gene,which encodes a nuclear protein with similarity to transcription factors,is critical for the formation of male and female meiocytes (Schiefthaleret al.1999;Yanget al.1999).In addition,the analysis of the male sterile mutant male meiocyte death1 (mmd1) inArabidopsisshowed that MMD1 may participate in chromatin remodeling and/or transcriptional events in meiosis (Yanget al.2003).Although these studies have elucidated the genes involved in meiosis,little is known about the roles of these genes in meiosis in nonheading Chinese cabbage.In this study,we identified and mapped a new male sterility gene,Bra2Ms,and revealed thatBra2Msmay be involved in meiosis in non-heading Chinese cabbage and play a critical role in fertility through cytological observations,mapping,cloning,and sequence analysis.
Cytological observation of pollen grains is an important research method for understanding male sterility in non-heading Chinese cabbage.It is also important for the breeding of male sterility in non-heading Chinese cabbage.In this study,the results of scanning electron microscopy showed that the pollen grains of fertile plants were plump and nearly spherical,with a clear and regular mesh structure on the surface (Fig.1-B-D).In contrast,the pollen grains of the sterile plants were abnormal and contracted into irregular shapes,and many pollen grains were aggregated together (Fig.1-F-H).The phenomenon of abnormally collapsing sterile pollen was also observed in Japanese apricot and poplar (Moriet al.2021).In addition,the anthers of the sterile plants were smaller than those of the fertile plants in this study,and this result is also consistent with a previous report in Chinese cabbage (Brassica rapaL.ssp.pekinensis) (Donget al.2022).These results indicate that the occurrence of male sterility is closely related to the later development of pollen grains.Pollen development has two major phases,the developmental phase and the functional phase (Hafidh and Honvs 2021;Zhouet al.2022).The developmental phase of pollen is initiated by the meiosis of diploid pollen mother cells (PMCs).The functional phase refers to the interaction between pollen and the stigma,in which the pollen grains rehydrate and germinate with pollen tubes to accomplish double fertilization (Johnsonet al.2019).The observations of meiosis in the anther in our study indicated that during the second division of meiosis,the microspore of the sterile plant remained a dicaryon.The division ended,and then withered pollen grains formed (Fig.2).In addition,our previous study showed that the anthers of the sterile material WS24-3 could not produce normal tetrads,and the abnormal tetrads will degrade with the continuous development of anthers,ultimately leading to sterility (Songet al.2019).In addition,callose is not degraded during the development of anthers in sterile plants.However,callose still surrounds the abnormal tetrad cells at the later stage of anther development,and the tapetum wall is not degraded in time (Songet al.2019).Combining these results,we speculate that the meiosis process may not be completed in sterile plants and that the signal is not released in a timely manner,which leads to the failure to secrete callose.Abnormal degradation of the outer wall and callose eventually leads to pollen abortion.
SSR markers can provide valuable genetic and genomic tools for genetic research in crops,such as marker-assisted selection of genes associated with genic male sterility (Houet al.2017;Liuet al.2018).In our previous study,Bra2Ms was localized to the A2 chromosome and genetically delimited to a region of 1.2 cM between the markers SSRa2-952 and SSRa2-980 (Li X 2016).To further clone and analyzeBra2Ms,SSR markers and IP markers were developed in the current study.A total of seven SSR markers and five IP codominant markers were developed (Appendix B).According to these SSR and IP markers,Bra2Mswas further mapped at a physical distance of about 129 kb on chromosome A2 of theB.rapagenome (Fig.3-A).To detectBra2Ms,we integrated the transcriptome data published in a previous study (Songet al.2019) and found that the number of differentially expressed genes was the lowest in the early stage of anther development,with little difference between the numbers of upregulated and downregulated genes.However,the numbers of downregulated genes increased significantly in both the meiosis stage and the tetrad stage of anther development,with the percentage of downregulated genes reaching 69% (Songet al.2019).These data suggest that the expression of key genes related to anther development is severely affected in sterile materials during the meiosis tetrad stage of anther development.According to these results,we analyzed the expression levels of the genes within the 129 kb fine-mapping region.Two genes,Bra039753andBra039757,were found to be significantly differentially expressed between fertile and sterile plants at the meiosis stage (Table 1).The qRT-PCR analysis in this study indicated that the transcript level ofBra039753in WS24-3 was significantly lower than that in WS-135 at the meiotic stage.Further sequence analysis showed that a 369 bp fragment insertion was located in the first exon of theBra039753gene in the sterile line WS24-3 (Fig.5-A).In addition,the 369 bp insertion contains five terminators (Appendix C);thus,the insertion of this fragment inhibited transcription of theBra039753gene,which then led to sterility.These results suggested thatBra039753encodes a PHD-domain containing protein and is the most likely candidate gene forBra2Ms(Figs.4 and 5-A).
PHD-type zinc finger proteins are located in the nucleus,and they are involved in chromatin-mediated transcriptional regulation (Aaslandet al.1995).They are proteins that are widely distributed in eukaryotes and play important roles in gene transcription and chromatin state regulation.PHD proteins have been proven to play important roles in plant growth and stress responses by regulating the recognition and binding of DNA,proteins,and RNA (Yaoet al.2020).Bra039753is orthologous toAT1G66170(MMD1),which is a PHD-finger protein encoding gene (Songet al.2019).InA.thaliana,MMD1(MALE MEIOCYTEDEATH1) is associated with microspore mother cell meiosis.It has a PHD domain and is specifically expressed in meiotic cells,and it may be involved in chromatin remodeling during the first meiosis (Wanget al.2016).Wheat PHD transcription factor (MALE STERILITY 1,MS1) functions in transcriptional activation,contains a leucine zinc finger domain and PHD domain,and plays an important role in pollen tapetum development and pollen wall biosynthesis (Wanget al.2017).OsTI-TANIA,a zinc finger protein with the PHD domain,regulates the expression of multiple metal ion transporter genes and plays a vital role in maintaining the normal growth and development of rice (Tanakaet al.2018).In the present study,sequence analysis of Bra2Ms showed that it contains a PHD domain,and the functional annotation of motif 1 shows a zinc finger PHD-type signature.Phylogenetic analysis showed that the PHD finger protein encoding gene MMD1 is highly conserved among species (Fig.5),implying a common function in regulating plant pollen development.Except for MMD1 inArabidopsis,no PHD finger has been reported to regulate fertility inBrassicacrops.
5.Conclusion
In summary,we mapped a novel male sterile locus,Bra2Ms,in non-heading Chinese cabbage.Bra2Msencodes a PHD-domain containing protein and acts as a crucial regulator of meiosis and microspore development.The findings of this study lay the foundation for further studies of the molecular mechanism of non-heading Chinese cabbage nuclear sterility and provide new clues for exploring the genetic mechanism of plant nuclear sterility.The molecular markers obtained in this study can assist in accelerating the breeding of excellent sterile lines of non-heading Chinese cabbage,and produce more high-quality,multi-resistant and competitive new varieties of non-heading Chinese cabbage.
Acknowledgements
We thank the Wuhan Major Project of Key Technologies in Biological Breeding and New Variety Cultivation,China (2022021302024852),the Science and Technology Support Project of Rural Vitalization in Hubei Province,China (2022BBA121),the Key Research and Development Project of Hubei Province,China (2021BBA097),and the Key Research and Development Project of Hubei Province,China (2021BBA102) for the financial support.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.08.008
 Journal of Integrative Agriculture2024年4期
Journal of Integrative Agriculture2024年4期
- Journal of Integrative Agriculture的其它文章
- OsNPF3.1,a nitrate,abscisic acid and gibberellin transporter gene,is essential for rice tillering and nitrogen utilization efficiency
- Basal defense is enhanced in a wheat cultivar resistant to Fusarium head blight
- Optimized tillage methods increase mechanically transplanted rice yield and reduce the greenhouse gas emissions
- A phenology-based vegetation index for improving ratoon rice mapping using harmonized Landsat and Sentinel-2 data
- Combined application of organic fertilizer and chemical fertilizer alleviates the kernel position effect in summer maize by promoting post-silking nitrogen uptake and dry matter accumulation
- miR-24-3p promotes proliferation and inhibits apoptosis of porcine granulosa cells by targeting P27
