Non-alcoholic fatty liver disease and sleep disorders
Lu-Fang Bu, Chong-Yu Xiong, Jie-Yi Zhong, Yan Xiong, Dong-Ming Li, Fen-Fang Hong, Shu-Long Yang
Abstract Studies have shown that non-alcoholic fatty liver disease (NAFLD) may be associated with sleep disorders. In order to explore the explicit relationship between the two, we systematically reviewed the effects of sleep disorders, especially obstructive sleep apnea (OSA), on the incidence of NAFLD, and analyzed the possible mechanisms after adjusting for confounding factors. NAFLD is independently associated with sleep disorders. Different sleep disorders may be the cause of the onset and aggravation of NAFLD. An excessive or insufficient sleep duration, poor sleep quality, insomnia, sleep-wake disorders, and OSA may increase the incidence of NAFLD. Despite that some research suggests a unidirectional causal link between the two, specifically, the onset of NAFLD is identified as a result of changes in sleep characteristics, and the reverse relationship does not hold true. Nevertheless, there is still a lack of specific research elucidating the reasons behind the higher risk of developing sleep disorders in individuals with NAFLD. Further research is needed to establish a clear relationship between NAFLD and sleep disorders. This will lay the groundwork for earlier identification of potential patients, which is crucial for earlier monitoring, diagnosis, effective prevention, and treatment of NAFLD.
Key Words: Non-alcoholic fatty liver disease; Sleep duration; Sleep quality; Sleep disorders; Obstructive sleep apnea
INTRODUCTION
PATHOGENESlS OF NAFLD
The pathogenesis of NAFLD is complex and multi-factorial. Previous studies have confirmed its positive correlations with metabolic diseases such as obesity, IR, metabolic syndrome, and type 2 diabetes. The pathogenesis of NAFLD has frequently been probed and two hypotheses were successively proposed, namely the early proposed "two-hit" model and the current "multiple-hit theory". The "two-hit" model believes that IR and abnormal hepatic lipid accumulation is the first hit, while the oxidative stress and inflammation is the second hit[12]; however, because other alternative factors including glucose and lipid metabolism disorders, intestinal flora disorder and epigenetic regulation were confirmed to be involved in NAFLD development, the "multiple-hit theory" has been widely accepted nowadays[13]. In addition, a dysregulated circadian rhythm due to sleep mode changes have been implicated in the pathogenesis of NAFLD[14,15]. As one of the most reliable markers of the circadian rhythm, melatonin (MT) is also involved in the pathogenesis of NAFLD. It is known that MT promotes sleep, circadian rhythms, and neuroendocrine processes. Current evidence suggests that MT protects against liver damage by inhibiting oxidation, inflammation, hepatic stellate cell proliferation, and hepatocyte apoptosis, thus inhibiting the progression of NAFLD[16]. Renet al[17] observed that MT could ameliorate high-fat diet/chronic intermittent hypoxia-induced hepatocellular damage by activating sirtuin 1-mediated autophagy signaling.
CORRELATlONS BETWEEN SLEEP AND NAFLD
In this review, we see sleep duration, daytime napping, daytime sleepiness, sleep quality and sleep habits as sleep-related traits (Table 1). A randomized controlled trial indicates a causal relationship between sleep characteristics and NAFLD. The onset of NAFLD is the result of changes in sleep patterns, whereas alterations in sleep characteristics are not the cause of NAFLD. The causal relationship between the two is unidirectional[18]. Recent studies concerning the relationship between sleep duration and NAFLD suggest that short sleep duration and long daytime naps are risk factors for NAFLD[19-21]. A cohort study has shown that in young adults, short sleep duration is independently associated with an increased risk of incident NAFLD, regardless of the presence of intermediate/high fibrosis scores[22]. Furthermore, a cross-sectional study found a decreasing trend in the proportion of NAFLD in pace with increased sleep duration in men, whereas in women, the proportion of NAFLD displayed a U-shaped distribution, with the lowest in the group (6-7 h of sleep) and the highest in the group (≤ 6 h or ≥ 8 h of sleep)[23]. Similarly, a meta-analysis of the relationship between sleep duration (or quality) and NAFLD incidence showed that both short sleep duration (≤ 6 h) and long sleep duration (≥ 8 h) may increase the risk of NAFLD, and the incidence of NAFLD increases as the sleep duration decreased[24,25]. Accordingly, a case-control study on NAFLD demonstrated that optimal sleep duration (7-9 h/d) is negatively associated with IR and liver stiffness in patients with NAFLD[26]. Taken together, too short or too long sleep duration may both increase the risk of NAFLD in both men and women.
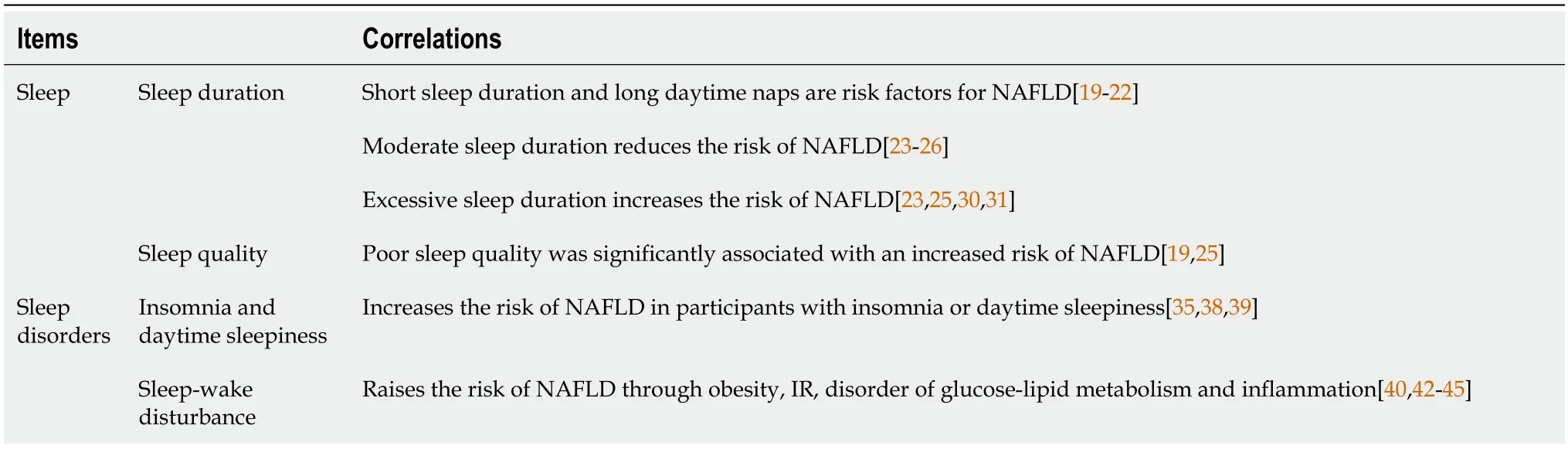
Table 1 Correlations between non-alcoholic fatty liver disease and sleep disorders
In addition, there were differences in the association between sleep duration and NAFLD in different populations: (1) Taking gender into account, a community-based longitudinal cohort study concluded that short sleep duration reduced the risk of NAFLD in men but had no risk in women[27]. Liuet al[28] found that sleep duration is an independent influencing factor for male NAFLD. The risk of NAFLD decreases with an increase in sleep duration in males, but there is also no significant correlation observed in females. A cross-sectional survey involving 4828 participants suggested that sleep quality was associated with NAFLD, and there were also gender differences[29]; and (2) Taking age into account, excessive nighttime sleep duration was associated with a moderately increased risk of NAFLD in a retrospective study targeted at middle-aged or elderly men in China[30]. In addition, in another cohort study of middle-aged or elderly people in South Korea, a positive correlation was also found between excessive sleep duration and elevated NAFLD scores[31].
SLEEP DlSORDERS AFFECT NAFLD
A population-based study showed that NAFLD is independently associated with sleep disorders after the adjustment of age, gender, and ethnicity[32]. Sleep disorders are present in NAFLD regardless of underlying cirrhosis[33]. The prevalence of sleep disorders was significantly higher in individuals with NAFLD compared to controls; while the prevalence of NAFLD was higher in individuals with sleep disorders compared to good sleepers[34]. Common sleep disorders associated with NAFLD include insomnia, daytime sleepiness, sleep-wake disorders and sleep-disordered breathing such as OSA (Table 2).
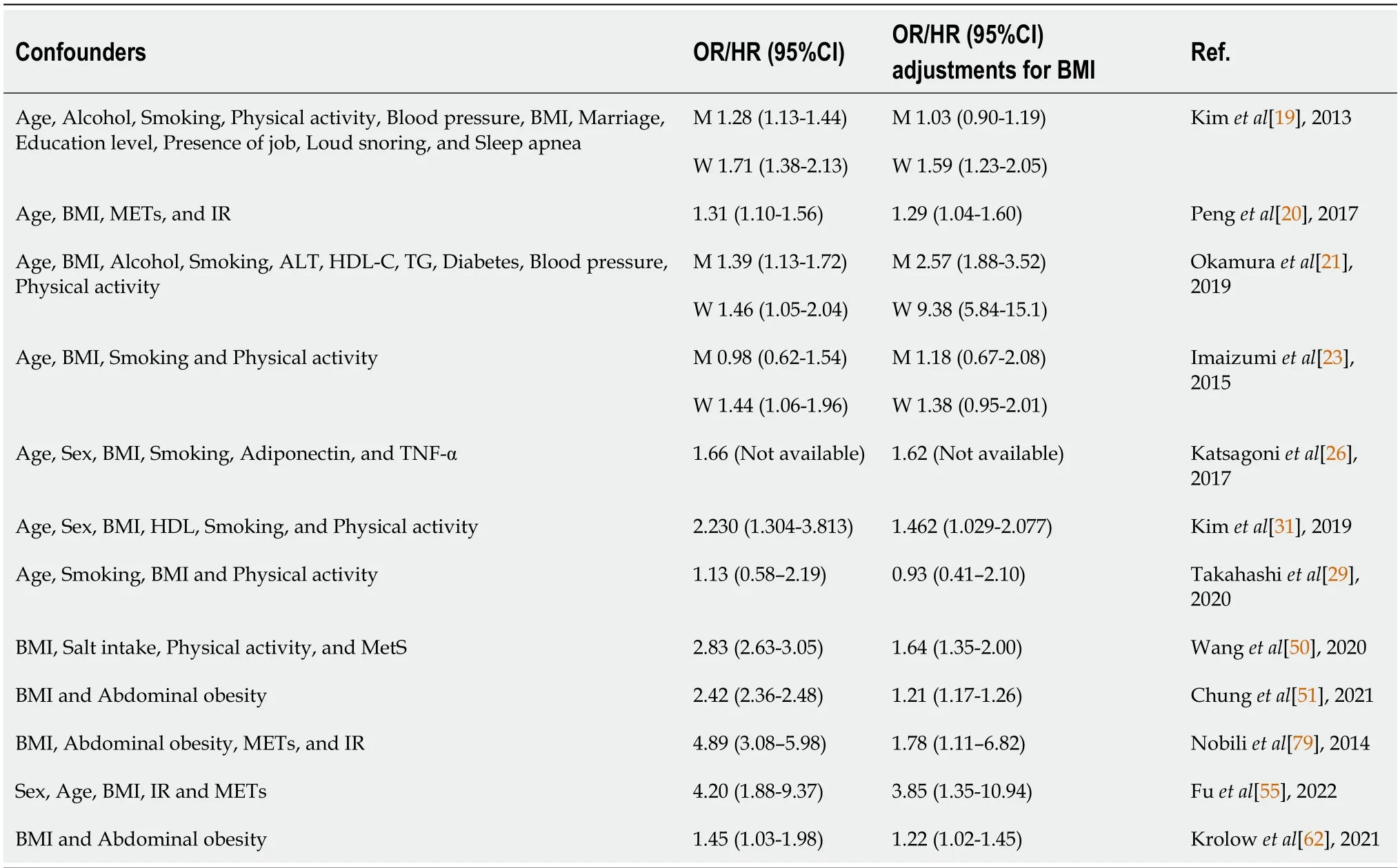
Table 2 Selected studies investigating associations between sleep disorders and non-alcoholic fatty liver disease
Insomnia and daytime sleepiness
A meta-analysis of seven studies showed that people with insomnia or excessive daytime sleepiness have an increased risk of NAFLD[35]. Moreover, patients with NAFLD may have more severe daytime sleepiness and shorter sleep duration[36]. A mendelian randomization demonstrated that trouble getting up in the morning and insomnia were associated with an increased risk of NAFLD[37]. Similarly, a case-control study found that nearly 30% of patients with biopsy-proven NAFLD confirmed insomnia, and the prevalence of NAFLD in insomnia patients was significantly higher than that in non-insomnia patients[38]. Furthermore, daytime sleepiness is significantly linked to the biochemical and histologic surrogates of NAFLD severity. It is not only positively correlated with liver enzymes and IR, independent of cirrhosis, but also positively correlated with the degree of fibrosis[39].
Suddenly the door opened, and in stepped a tiny little man13 and said: Good-evening, Miss Miller-maid; why are you crying so bitterly? Oh! answered the girl, I have to spin straw into gold, and haven t a notion how it s done
Sleep-wake disorders
Sleep-wake disorder, also known as non-24-h sleep-wake rhythm disorder, is a circadian rhythm sleep-wake disorder characterized by an inability to entrain to the 24-h environment. Sleep-wake disorders may increase the risk of NAFLD in patients suffered from obesity, IR, inflammation, and disorders in glucose or lipid metabolism, resulting in weight gain by increasing the food-sensitive dopaminergic activity[40] and the circulating concentration of growth hormone-releasing peptide[41]. It is well-known that IR plays a central role in the progression of hepatic steatosis and fibrosis. Therefore, IR may be a major intersection between sleep-wake disorders and NAFLD[42]. In addition, sleep-wake disorders can also facilitate glycometabolism, promote lipid mobilization in adipose tissue by increasing cortisol hormone concentrations and weakening the tissue response to insulin, and accelerate the transport of free fatty acids to the liver[43]. Increased sympathetic nervous system and adrenal cortical activity may lead to the adverse metabolic effects of sleep-wake disorders. In a comparative study, the sleep of healthy volunteers was experimentally fragmented at all stages using auditory and mechanical stimuli. After two nights of sleep fragmentation, the results indicated that insulin sensitivity and glucose effectiveness,i.e., the ability of glucose to mobilize itself was independent of the insulin response, were both decreased. In addition, morning cortisol levels were elevated, and the sympathetic nervous system was excited[44]. Sleepwake disorders are also associated with elevated pro-inflammatory factors such as interleukin (IL)-1β, which are involved in the development of liver inflammation promoting NAFLD[45].
Sleep-disordered breathing
OSA is the most common sleep breathing disorder. A general population-based polysomnography study showed that the incidence of mild OSA was estimated to be 59% in men but 33% in women, while the incidence of moderate to severe OSA was estimated to be 30% in men but 13% in women[46]. It is characterized by episodes of apnea, hypopnea and sleep fragmentation (SF) due to restricted airflow in the collapsed upper airway during sleep[47]. It has been shown that SFinduced intermittent hypoxia (IH) and sleep deprivation are associated with IR and metabolic dysfunction, as well as adipose tissue dysfunction which are thought to play key roles in the metabolic abnormalities of OSA[48,49]. Snoring is the direct consequence of airway collapse in OSA patients, which is independently and positively associated with a higher incidence of NAFLD[50].
There is growing evidence that OSA is involved in the development of NAFLD with IH acting as the most important connecting factor[51,52]. The IH of OSA may also be involved in the progression of NAFLD by affecting the level of liver enzymes. It increased hepatic production of lysyl oxidase, an enzyme that cross-links collagen, and may serve as a biomarker of liver fibrosis in patients with severe obesity and NAFLD[53]. In animal models, IH can directly induce hepatic steatosis by repeating brief hypoxia and reoxygenation simulating OSA[54]. Fuet al[55] found that IH caused by OSA may aggravate NAFLD and lead to a higher risk of NASH in patients with obesity.
OSA affects NAFLD
There are many studies on the aspects of OSA affecting NAFLD. Severe OSA is more likely to be associated with significant liver disease, one possible reason being its independent correlation with increased liver stiffness[56]. A systematic review and meta-analysis demonstrated that OSA is associated with an increased risk of NAFLD, NASH and fibrosis[57]. Jinet al[58] found significant correlations between OSA and NAFLD in terms of hepatic steatosis, lobular inflammation and fibrosis, suggesting that OSA may be involved in the progression of NAFLD through elevated liver enzyme levels and hepatic histological changes. In the presence of obesity, patients with OSA may potentially contribute to liver injury in NAFLD through IR and systemic inflammation[59]. Another case-control study showed that in the absence of considering obesity and metabolic syndrome, patients with OSA have a significantly high incidence of NAFLD and exhibit notable hepatic fibrosis[60]. After excluding the confounding factor of obesity, the severity of OSA emerges as an independent risk factor for both NAFLD and liver fibrosis[61]. Krolowet al[62] found that patients with moderate to severe OSA had an increased risk of hepatic fibrosis after adjusting for obesity level. Kimet al[63] demonstrated that the severity of OSA increased with the prevalence of NAFLD regardless of the gender. Also, compared to non-obese OSA patients, obese patients with OSA were more prone to developing NAFLD. In addition, regarding hepatic steatosis, there was no association between liver fibrosis and the severity of OSA. A retrospective analysis suggested that age and obesity predicted high liver fibrosis risk as assessed by noninvasive scoring systems, but not OSA severity[64]. In a crosssectional study of human subjects, the risk of hepatic steatosis increased along with the severity of OSA and sleep-related hypoxemia after the adjustment of confounding factors including centripetal obesity[65].
Recent studies have been devoted to determining the influence of IH and OSA-related parameters on NAFLD severity. A multivariate analysis showed that the apnea-hypopnea index (AHI), oxygen desaturation index (ODI), lowest desaturation values, and percentage of sleep duration with mean nocturnal oxygen saturation (SpO2) were independent predictors of NAFLD after adjustment for body mass index (BMI), weight, and IR (it was found that the most correlated parameter for the severity of NAFLD was the duration of IH during sleep)[66]. Furthermore, decreasing SpO2 during sleep was also associated independently with a higher risk of liver cytolysis[65]. Benottiet al[67] found that OSA severity (as measured by the AHI) and hypoxia parameters were positively correlated with NAFLD severity in subjects without metabolic syndrome. Cakmaket al[68] reported that AHI and ODI values were significantly higher in the moderate and severe NAFLD groups compared to counterparts in the non-NAFLD group, SpO2 and lowest O2 saturation (LaSO2) were significantly lower in the mild and severe NAFLD groups. These results revealed that the parameters AHI, ODI, LaSO2, and SpO2 levels play pivotal roles in the association between NAFLD and OSA. The severity of OSA was also associated with a decrease in high-density lipoprotein-cholesterol and an increase in BMI, triglycerides (TG), homeostasis model assessment IR index, transaminases, and FIB-4 index (a noninvasive score for liver fibrosis)[69]. Human subjects with OSA had significantly higher levels of alanine transaminase (ALT) and aspartate transaminase (AST) than those without OSA[70]. A single-center, cross-sectional study indicated that OSA may be an independent risk factor for dyslipidemia, and that OSA and obesity have a synergistic effect on ALT elevation[71]. A cross-sectional study showed that the risk of developing NAFLD increases in older patients with OSA, and high TG is an important factor leading to the development of liver injury[72]. Given that the pathological mechanism of OSA promotes the development of NAFLD, there are three aspects included, as shown in Figure 1.
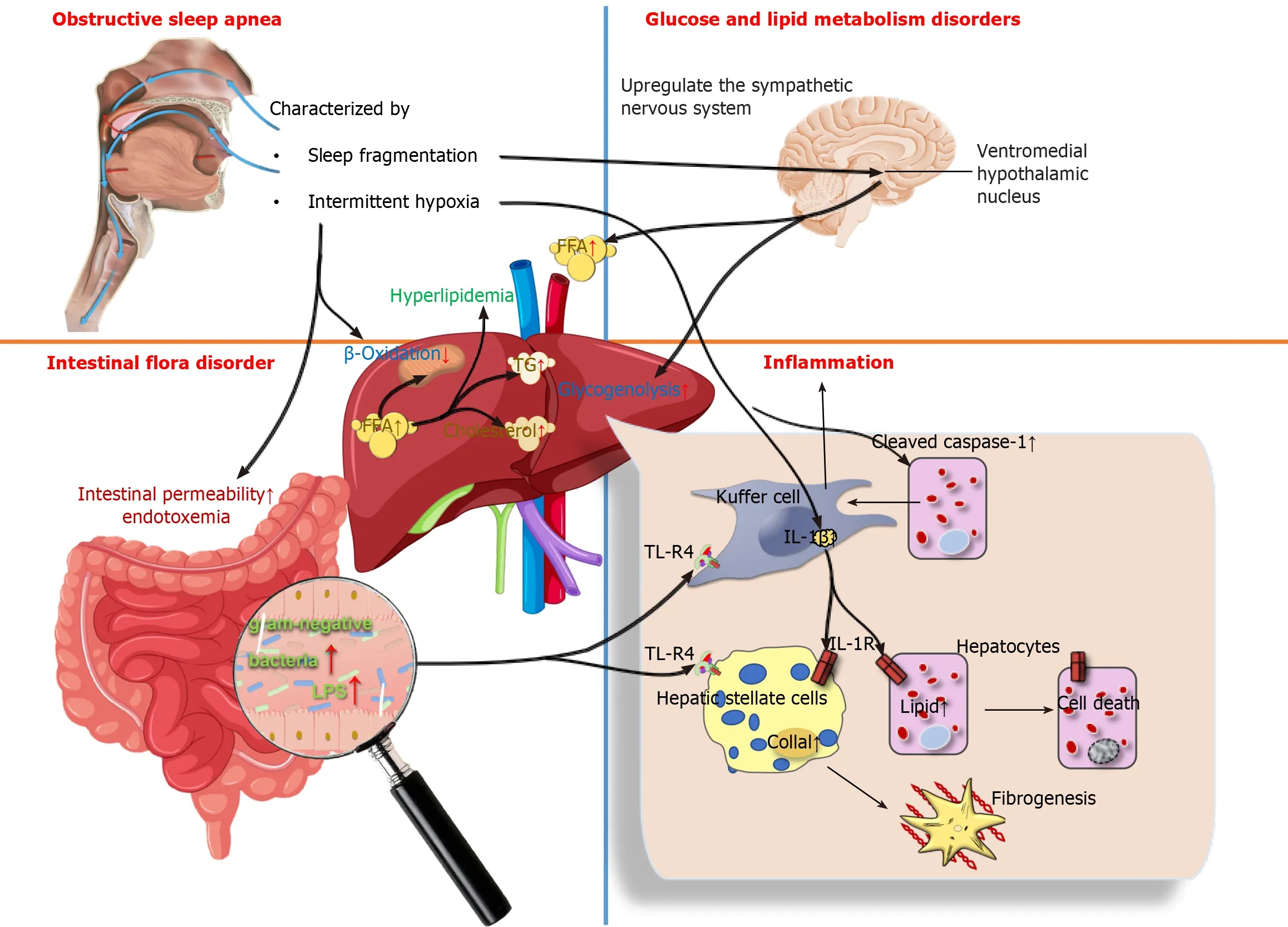
Figure 1 The pathological mechanism of obstructive sleep apnea promotes the development of non-alcoholic fatty liver disease. Obstructive sleep apnea causes glucose and lipid metabolism disorders, intestinal flora disorder and hepatic inflammation through the sympathetic nervous system, endotoxemia and hepatic toll-like receptor-4. TL-R4: Toll-like receptor-4; lL-1R: Interleukin-1 receptor; IL-1β: Interleukin-1 β.
Metabolism disorders in glucose and lipid:OSA is independently associated with metabolic dysfunction, including dyslipidemia and IR. Yokoeet al[73] found that IH impaired glucose homeostasis and stimulated pancreatic β-cell replication only during periods of hypoxic exposure, but the presence of hyperglycemia may increase the hypoxic susceptibility of β-cells. The mechanism of systemic glucoregulation by glucose-sensing neurons in the ventromedial hypothalamic nucleus is also involved in the process of IH inducing the occurrence of IR by up-regulating the sympathetic nervous system, increasing circulating free fatty acids (FFAs) and hepatic glycogenolysis[74]. In addition, IH induces the occurrence of hyperlipidemia by inhibiting the clearance of TG-rich lipoproteins. Drageret al[75] observed that, in male C57BL/6J mice on a high-cholesterol diet under exposure to IH air for 4 weeks, the clearance of lipoprotein lipase, a key enzyme for lipoprotein clearance, was inhibited; resulting in a significant increase in total cholesterol and TG levels. IH-induced hyperlipidemia is also associated with the up-regulation of sterol regulatory element binding protein-1 and the over-expression of stearoyl coenzyme A desaturase 1[76,77]. In conclusion, the mechanism by which OSA promotes the development of NAFLD may be IH-reduced utilization of FFAs by limiting β-oxidation in mitochondria, and excessive FFAs are diverted to the synthesis of TG and cholesterol to trigger hyperlipidemia, which ultimately leads to the development of NAFLD.
Inflammation:The roles of IH in the progression of NAFLD are related to inflammation[78]. IH in OSA patients affects liver histology and activation of inflammatory cells in NAFLD regardless of obesity or IR[79]. In NAFLD animal models, IH has been shown to modulate inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-6 to produce proinflammatory effects[80,81]. Savranskyet al[82] found that the levels of IL-1β, IL-6 and TNF-α were elevated in mice following exposure to IH, lobular inflammation and fibrosis were documented in the liver. Similarly, comparable results were observed in humans. Schaeferet al[83] usedin vitromodels of NASH to study the impacts of IH on the liver, they found that IH contributed to a significant induction of IL-6 expression in both hepatocytes and macrophages. Furthermore,in vitroandin vivomodels of NAFLD have shown that IH promotes the production of inflammatory signals by activating inflammatory bodies or caspase-1 in fat-laden hepatocytes, as well as promoting crosstalk between hepatocytes and Kupffer cells by releasing extracellular vesicles to induce hepatocellular damage. This is followed by increased cell mortality through a variety of mechanisms, including apoptosis and pyroptosis[84]. Notably, Tayloret al[85] discovered that human adipocytes are highly sensitive to IH, which enhances inflammatory gene expression in adipose tissue and the release of inflammatory cytokines involved in the development of NAFLD.
Intestinal flora disorder:There is a wide range of microorganisms in the human intestine, in which various microorganisms interact with each other to form a dynamic ecosystem called the gut microbial ecology. It has been shown that IH in OSA may affect the ecology of the gut microbiota and mediate a variety of cardiovascular diseases that coexist with OSA[86]. OSA is a risk factor for intestinal injury. Regardless of the metabolic status, intestinal permeability may be a possible factor leading to the susceptibility of OSA patients to NAFLD[87]. For example, Nobiliet al[88] found that a novel correlation exists between OSA and NAFLD, namely that IH may disrupt the intestinal-liver axis in pediatric NAFLD by increasing the number of gram-negative bacteria in the intestine and intestinal permeability, with increased endotoxemia coupled with toll-like receptor-4 (TLR-4) up-regulation in hepatocytes, Kupffer cells and hepatic stellate cells[88,89]. In addition, one of the characteristic manifestations of OSA-SF, induces metabolic alterations in the organism that may be mediated in part by concurrent changes in gut microbiota, which was confirmed using SF-derived microbiota routinized in germ-free mice[90]. Chronic SF-induced reversible gut microbiota changes led to systemic and visceral white adipose tissue inflammation in addition to altered insulin sensitivity in mice, most likelyviaenhanced colonic epithelium barrier disruption.
CPAP treatment on OSA and NAFLD
Currently, CPAP is the globally accepted gold standard for the treatment of OSA. It can keep the airway open and reduce daytime sleepiness, improving cognition and sleep quality in OSA patients[91]. There have been many studies performed to explore the effects of CPAP therapy on OSA patients suffering from NAFLD, but the results obtained were varied. Some observational data suggested that CPAP treatment improves hepatic biochemistry of NAFLD in OSA patients; and that CPAP treatment is statistically significantly associated with improvement of hepatic injury in OSA patients, but a sufficiently long duration of treatment (greater than or equal to 3 months) may be required to achieve a positive effect. Chenet al[92] enrolled 160 patients with OSA and measured serum transaminases before and after CPAP treatment. After 3 months of treatment, both ALT and AST levels decreased significantly. A recent meta-analysis also showed that, compared to controls, ALT and AST levels were significantly lower in OSA patients after CPAP treatment, and was more effective in OSA patients treated with CPAP for more than 3 months[93]. Hironoet al[94] found a significant reduction in AST and ALT levels and significant improvement in liver injury after 6 months of CPAP treatment in 50 patients with OSA suffering from NAFLD. In addition, the effect of CPAP treatment on NAFLD in OSA patients was also related to OSA patients’ adherence. Patients with good adherence to CPAP showed significantly decreased levels in AST and ALT than those with poor adherence[95]. Sundaramet al[96] also found that treatment of OSA with CPAP may reverse liver injury parameters and reduce oxidative stress, indicating that CPAP could be a new therapy for preventing NAFLD progression in obese children with OSA.
However, some randomized controlled trials did not show a benefit of CPAP treatment on liver injury in OSA patients. For instance, Jullian-Desayeset al[97] found that six to twelve weeks of effective CPAP did not show any impact on reducing steatosis, NASH or liver fibrosis even after adjustment for gender, BMI, baseline AHI and severity of liver injury. Also, in the randomized controlled trial by Kohleret al[98], 94 patients with moderate to severe OSA were randomized to therapeutic or subtherapeutic CPAP treatment. Plasma ALT and AST levels were measured before and after treatment. The results showed that 4 wk of active CPAP treatment did not show any beneficial effect on transaminase levels compared to subtherapeutic CPAP in patients with OSA. Nget al[99] showed that 6 months of CPAP treatment did not lead to improvement in hepatic steatosis and liver fibrosis, despite a significant correlation between hepatic steatosis and markers of OSA severity. Labarcaet al[100] performed a systematic evaluation and meta-analysis of 5 randomized controlled trials involving patients with OSA and NASH who were treated with CPAP, but did not find obvious changes in hepatic steatosis, liver fibrosis and transaminase levels (ALT and AST) in OSA patients. Differences regarding the effect of CPAP treatment in OSA patients on NAFLD may be related to the duration of CPAP treatment, compliance of OSA patients and the severity of NAFLD progression.
NAFLD AFFECTS SLEEP DlSORDERS
The effects of NAFLD on sleep can be observed from some observational studies, although there are no animal experiments to explain the specific mechanism by which NAFLD affects sleep. NAFLD patients have altered sleep status, namely in NAFLD, sleep duration was shortened, sleep onset was delayed and sleep quality poorer[39,101]. Moreover, NAFLD may increase the risk of developing OSA. A study showed that OSA is common in adults who have biopsyproven NAFLD[102]. Similarly, in a 6-month prospective study, Romdhaneet al[103] found that the incidence of OSA was relatively higher in patients with NAFLD in comparison with controls. In a nationwide population-based study, Chunget al[51] found that NAFLD was significantly associated with an increased risk of OSA after adjusting for multiple metabolic variables. Specifically, in younger, male or obese patients with NAFLD, there is a higher risk of OSA than that in older, female or non-obese patients.
The mechanism by which NAFLD affects OSA may be related to MT metabolism disorder. It is known that sleep is closely related to the metabolism of MT, which is metabolized by the liver. Liver metabolic dysfunction in NAFLD patients increases escalates as disease progresses. Currently, it has been found that key factors in NAFLD-induced sleep disorders include hepatic encephalopathy and circadian rhythm imbalance due to altered MT metabolism. Moreover, in the advanced stages of NAFLD, cirrhosis has an effect on circadian sleep regulation by a delay in the 24-h MT rhythm, which is likely to be related to reduced sensitivity to light signals[104]. The core feature of NAFLD is a discoordination between central and peripheral circadian rhythms[105]. This phenomenon has also been observed in db/db (hereditary obesity) mice[106], and the main circadian rhythm defect lies in the peripheral liver oscillator rather than the behavioral rhythm or master clock, but the mechanism by which peripheral circadian rhythm disorder affects the central circadian rhythm remains to be explored.
CONCLUSlON
This paper provides some significant insights into the correlations between sleep disorders and the occurrence or development of NAFLD. Excessive or short sleep duration and poor sleep quality may increase the risk of NAFLD. Similarly, insomnia, daytime sleepiness, sleep-wake disorders and OSA have been associated with the development of NAFLD. NAFLD is also a risk factor for OSA; thus, it is necessary to screen and monitor the occurrence and development of NAFLD in OSA patients. Moreover, CPAP treatment can stabilize and slow down the progression of NAFLD under certain circumstances. Sleep factors can be added to the list of changeable lifestyle behaviors to reduce the risk of NAFLD. This includes maintaining proper sleep duration, improving sleep quality, and addressing sleep disorders.
FOOTNOTES
Author contributions:Bu LF wrote the manuscript and designed the table; Xiong CY revised the manuscript and designed the figure; Zhong JY, Xiong Y were responsible for data collection; Li DM, Hong FF and Yang SL are co-corresponding authors who contributed equally to this work, and are responsible for improving the grammar and language, conceptualizing the idea, and obtaining funding; All authors have read and approved the final manuscript.
Supported byNational Natural Science Foundation of China, No. 82360880, and 82060661; Jiangxi Provincial Natural Science Foundation of China, No. 20232ACB206057; Key project of Jiangxi Provincial Department of Education, No. GJJ218104; Teaching reform research project of Jiangxi Province of China, No. JXJG-22-130-1; National Natural Science Foundation of China, No. 81660151; and Jiangxi Provincial Natural Science Foundation of China, No. 20212BAB206092.
Conflict-of-interest statement:All authors have no relevant conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Fen-Fang Hong 0000-0001-6440-1311; Shu-Long Yang 0000-0002-1407-6255.
S-Editor:Liu JH
L-Editor:Webster JR
P-Editor:Zheng XM
 World Journal of Hepatology2024年3期
World Journal of Hepatology2024年3期
- World Journal of Hepatology的其它文章
- Update in lean metabolic dysfunction-associated steatotic liver disease
- Retrospective study of the incidence, risk factors, treatment outcomes of bacterial infections at uncommon sites in cirrhotic patients
- Palliative long-term abdominal drains vs large volume paracenteses for the management of refractory ascites in end-stage liver disease
- Comprehensive prognostic and immune analysis of sterol Oacyltransferase 1 in patients with hepatocellular carcinoma
- Prediction model for hepatitis B e antigen seroconversion in chronic hepatitis B with peginterferon-alfa treated based on a responseguided therapy strategy
- lnfluence of nonalcoholic fatty liver disease on response to antiviral treatment in patients with chronic hepatitis B: A meta-analysis
